Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
The Role of miR-1183: A Potential Suppressor in Hepatocellular Carcinoma via Regulating Splicing Factor SRSF1
Authors Yao Z, Liu N, Lin H, Zhou Y
Received 14 February 2023
Accepted for publication 7 July 2023
Published 21 July 2023 Volume 2023:10 Pages 1169—1180
DOI https://doi.org/10.2147/JHC.S408542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Manal Hassan
Zhilu Yao,1,2,* Ning Liu,3,* Hui Lin,1 Yingqun Zhou2,4
1Department of Gastroenterology, Jingan District Zhabei Central Hospital, Shanghai, 200072, People’s Republic of China; 2Clinical Medical College of Shanghai Tenth People’s Hospital, Nanjing Medical University, Nanjing, Jiangsu, 211166, People’s Republic of China; 3Department of Gastroenterology, Changzhou Maternal and Child Health Hospital, Changzhou, 213004, People’s Republic of China; 4Department of Gastroenterology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, 200072, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingqun Zhou; Hui Lin, Email [email protected]; [email protected]
Purpose: Hepatocellular carcinoma (HCC) is a severe global health problem, causing many deaths of patients all over the world. Serine and arginine-rich splicing factor 1 (SRSF1) functions as an important oncogenic role in tumorigenesis and progression in HCC. Therefore, therapies targeting SRSF1 may provide promising therapeutic approaches. MiRNAs are virtually involved at the post-transcriptional level and bind to 3’ untranslated region (3’-UTR) of their target messenger RNA (mRNA) to suppress expression.
Methods: Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to detect the expression of SRSF1 and miR-1183 in HCC cell lines. CCK8 assay, colony formation assay and wound healing assay were used to detect the function of miR-1183 in HCC cell lines in vitro. Luciferase reporter assay and Western blot were applied to detect the regulation of particular molecules. Xenograft tumor assay was used to detect the function of miR-1183 in HCC cell lines in vivo. Immunohistochemistry (IHC) was used to detect the expression of SRSF1 in HCC tissues and Xenograft tumors.
Results: In this study, we identified that miR-1183 was downregulated in HCC cell lines. Functional assays indicated that miR-1183-upregulation cells show weakened proliferation ability and migration ability in vitro and inhibit subcutaneous tumor formation in vivo. With respect to the underlying mechanism, we found that miR-1183 function as a tumor suppressor by specifically binding to SRSF1.
Conclusion: This study is the first to demonstrate that miR-1183 function as an important tumor-suppressing role by binding to the 3’-UTR of SRSF1 mRNA and suppressing its protein level in HCC cells in vitro and in vivo. Further, miR-1183 may be a potential target in the prognosis and treatment of HCC.
Keywords: SRSF1, miR-1183, HCC, proliferation, migration
Introduction
Hepatocellular carcinoma (HCC) is a severe global health problem, causing many deaths of patients all over the world.1 Despite significant advances in surgical operations and medicine treatment, there are still many HCC patients diagnosed at advanced stages by the time of initial diagnosis.2 The prognosis for unresectable HCC remains unsatisfactory. Therefore, it is urgent to deeply elucidate the potential molecular mechanism of HCC and identify promising novel targets for therapy.
In recent years, studies have shown that abnormal regulation of alternative splicing (AS) is usually accompanied by the occurrence and development of tumors.3 The serine/arginine (SR) splicing factor family consists of 12 members. SR protein can participate in RNA splicing, transcription, export, translation and degradation by the RNA recognition motifs (RRM) and an RS domain.4,5 For example, SRSF6 functions as an oncogene in mediating colorectal cancer (CRC) progression through regulating ZO-1 aberrant splicing.6 SRSF3 markedly promotes the proliferation and migration of CRC cells through upregulating its target gene DHCR24.7 As a member of SR splicing factor family, splicing factor SRSF1 is upregulated in various types of cancer and its expression level is related to the occurrence, development and treatment response of multiple tumors.8 SRSF1 could inhibit autophagy by regulating Bcl-x splicing in lung cancer.9 In addition, SRSF1 exerts oncogenic roles in breast cancer by regulating the AS of PTPMT1.10 Importantly, SRSF1 promotes tumor invasion and metastasis in HCC cells by interacting with the exon 3 of SRA1 pre-mRNA.11 Furthermore, SRSF1 could bind to RECQL4 mRNA and enhance its stability, thereby promoting the progression of HCC.12 Previous studies have shown the oncogenic role of SRSF1 in HCC. Therefore, therapies targeting SRSF1 may provide promising therapeutic approaches.
Many previous studies have demonstrated the roles of deregulated miRNAs in multiple stages of tumorigenesis and progression.13 Increasing evidence supports the roles of miRNAs in diverse biological processes, including tumor cellular proliferation, invasiveness, metastasis, and apoptosis.14,15 A single miRNA can direct entire cellular pathways via interacting with a broad spectrum of target genes, making them potential therapeutic targets of multiple diseases.16 MiRNAs are virtually involved at the post-transcriptional level and bind to 3’ untranslated region (3’-UTR) of their target messenger RNA (mRNA) to suppress expression.17,18 Therefore, therapies targeting miRNAs may provide promising therapeutic approaches. Using bioinformatics prediction database, miR-1183 is a potential binding molecule for SRSF1. Recently, several studies have shown that miR-1183 play important roles in several diseases. Higher expression levels of miR-1183 appear to affect myocardial apoptosis by regulation BCL-2.19 MiR‑1183 could inhibit proliferation, migration and glycolysis in non‑small‑cell lung carcinoma.20 In addition, miR-1183 inhibits PDK1/p-AKT-mediated cell proliferation and migration in triple-negative breast cancer.21 However, the involvement of miR-1183 in HCC progression remains unexplored. Further study of the regulation of miRNAs and SRSF1 might promote the development of promising therapies of HCC.
In this study, we demonstrate that miR-1183 is downregulated in HCC, and acts as a tumor suppressor by binding to the mRNA 3’UTR of the oncogenic splicing factor SRSF1 in HCC.
Materials and Methods
Cell Culture and Transfection
All animal protocols complied with the rule of the ethics committee of Shanghai Tenth People’s Hospital (SHDSYY-2022-1474) and the use of human tissues have been approved by the committee of Shanghai Tenth People’s Hospital (2021-KN167-01).
L02 (RRID: CVCL_6926), HCC-LM3 (RRID: CVCL_6832), SMMC-7721 (RRID: CVCL_0534), and BEL-7402 (RRID: CVCL_5492) cell lines were donated by Shanghai Tenth People’s Hospital. Huh7 (RRID: CVCL_0336) cell line was purchased from Shanghai Yuanchuang Biotechnology Co., Ltd. The use of the cell lines was approved by the committee of Shanghai Tenth People’s Hospital and all cell lines were authenticated by STR profile. We used Dulbecco’s modified Eagle’s medium (GBICO, USA) containing streptomycin/penicillin (100 U/mL) and 10% fetal bovine serum (GBICO, USA) to cultivate L02, HCC-LM3, SMMC-7721, and BEL-7402 cells and Roswell Park Memorial Institute medium 1640 to cultivate Huh7 cells. All cells were incubated in a humidified chamber with 5% CO2 at 37°C. The HCC-LM3 and SMMC-7721 cells were seeded in 6-well plates at a concentration of 0.5×106 cells per well the day before transfection. MiR-1183 mimics, miR-1183 inhibitor and negative control miR-NC were purchased from IBOSI (Shanghai, China). Each transfection was performed using Lipo8000™ Transfection Reagent (Beyotime, Shanghai, China) following manufacturer instructions. 24–48 hours after transfection, cells were harvested for the following experiments. Lentivirus with stable overexpression of miR-1183 (LV-miR-1183) and negative control (LV-NC) was purchased from KeyGEN BioTECH (Jiangsu, China). When LM3 cells seeded in 6-well plates reached 60%–70% confluency, LV-miR-1183 and LV-NC were transfected into LM3 cells at appropriate concentrations as multiplicity of infection reached 20 and positive cells were selected by puromycin resistance.
RNA Extraction and Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from cells using EZ-press RN purification kit (EZBioscience, USA) following the manufacturer’s instructions. Extracted RNA was transcribed into complementary DNA (cDNA) by using PrimeScript™ RT Master Mix (Takara, China). TB Green® Premix Ex Taq™ (Takara, China) was used to carry out qRT-PCR to measure the mRNA expression level in cells according to the manufacturer’s instructions. β-actin and U6 was used as the internal reference. Primers used in this study were shown in Table S1. Expression fold changes relative to the control group were determined by the 2−ΔΔt method.
CCK8 Assay
The cell counting kit-8 (CCK-8; Biosharp, Shanghai, China) was used to monitor cell proliferation. Transfected cells were seeded into 96-well plates in an amount of 2000 cells per well and cultured for indicated time. Prior to observation, 0.1 mL of 10% CCK-8 solution was added to each well and incubated for 2h at 37◦C. Finally, the optical density was measured at 450 nm using a microplate reader (SpectraMax iD5, Molecular Device, Shanghai, China).
Colony Formation Assay
6-well plates were used for seeding cells (1000 cells/well) following transfection. Every 3d, the authors replaced the culture medium and 2 weeks later, cells were washed gently by phosphate-buffered saline and underwent 10 min of fixation with absolute ethyl alcohol. At last, cells were stained for 15 min with 0.1% crystal violet. The colonies were photographed and counted by Image J.
Wound Healing Assay
Cells were seeded into 6-well plates (5*105 cells/well). Using a sterile 200μL micropipette tip to make the wound when the cells reached 90%. The wounded monolayers were washed with phosphate buffer solution to remove the cell debris. The gap between the two edges of the wound was measured after incubation at 0h and 36h. The rate of migration is obtained by the following formula: (initial area – final area)/initial area*100%.
Luciferase Reporter Assay
The 3′-UTR sequences of wild-type (WT) SRSF1 and mutated (MUT) SRSF1 were synthesized by IBOSI (Shanghai, China) and cloned into the luciferase reporter vector. For the luciferase reporter assay, 293T cells were plated at a density of 5 × 104 cells per well in 24-well plates. The next day, 293T cells were co-transfected with the following combinations: WT-SRSF1 plus miR-NC or miR-1183-mimics, MUT-SRSF1 plus miR-NC or miR-1183-mimics. 48h after transfection, luciferase assays were performed using a dual-luciferase reporter assay Kit (Yeasen, China). The level of luciferase activity was calculated as the normalized relative Firefly luciferase/Renilla ratio.
Western Blot
We used RIPA lysis buffer (Beyotime, Shanghai, China) to extract total protein from hepatic cells. In a nutshell, the equivalent amount of protein was separated by 10% SDS-PAGE, and then transferred to polyvinylidene fluoride membranes (Servicebio, Wuhan, China). After soaking in 5% skim milk at room temperature for 1h, the membranes were incubated with primary antibodies against ACTIN (1:1000, Abcam, ab8227, Cambridge, UK), proliferating cell nuclear antigen (PCNA, 1:1000, 60097-1-Ig, Proteintech, Chicago, USA), matrix metalloproteinase 2 (MMP2, 1:1000, 10373-2-AP, Proteintech), matrix metalloproteinase 9 (MMP9, 1:1000, 27306-1-AP, Proteintech), and serine and arginine rich splicing factor 1 (SRSF1, 1:1000, A4091, ABclonal, Wuhan, China) overnight at 4°C, and then incubated with the secondary antibody against rabbit IgG H&L (1:2000, ab6721, Abcam) at room temperature for 1h. Finally, we use Odyssey CLx image studio system (LI-COR, USA) to detect the results. β-actin was adopted as an internal control.
Xenograft Tumor Assay
The reporting of this study conforms to ARRIVE 2.0 guidelines.22 We followed the “Guide for the Care and Use of Laboratory Animals, 8th Edition”.23 Eight nude mice bought from Shanghai Sippe-Bk Lab Animal Co., Ltd. were housed in plastic cages and maintained under an alternating 12h:12h light–dark cycle at a constant temperature (22°C~25°C) with free access to food and water. They were randomly divided into two groups: LV-NC and LV-miR-1183 and injected with HCC-LM3 cells subcutaneously. Tumor tissues were collected 5 weeks after injection.
Immunohistochemistry (IHC)
The human HCC tissue and para-carcinoma tissue slices were purchased from Servicebio (Wuhan, China). The human and mouse tissue slices were baked in an oven at 60°C for 2h. After dewaxing and rehydration, sections were dipped in citrate buffer and incubated in a water bath at 90°C for 20 min to achieve antigen retrieval. The sections were combined with 3% hydrogen peroxide to prevent endogenous catalase activity, and then blocked with 5% BSA for 20 min to block non-specific binding. Then, sections were combined with SRSF1 (1:100) primary antibody and dilution at 4°C overnight. Then the secondary antibody was added and incubated at 37°C for 1h. After combined with diaminobenzidine, sections were observed under a light microscope (Olympus, CX23).
Statistical Analysis
Data were presented as mean ± SD, and all experiments were repeated at least three times. The data were analyzed by Student’s t-test. P-values of <0.05 were considered statistically significant. All statistical graphs were drawn by GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA).
Results
SRSF1 is Up-Regulated in HCC Tissues and Cell Lines
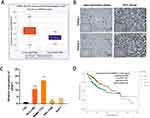
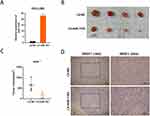
Using online tool starbase (starbase.sysu.edu.cn/) to analyze 374 HCC and 50 normal samples from TCGA database, we found that SRSF1 expression is higher in HCC than in normal samples (Figure 1A). Then we detected the protein level of SRSF1 in clinical HCC samples with IHC. The protein level of SRSF1 was significantly higher in HCC tissues that in para-carcinoma tissues (Figure 1B). The expression of SRSF1 in HCC cell lines was also upregulated, which is consistent with the results in HCC tissues (Figure 1C). Among the HCC cell lines, the expression level of SRSF1 in HCC-LM3 and SMMC-7721 cell lines was relatively higher; therefore, HCC-LM3 and SMMC-7721 cell lines were selected for further research. Furthermore, we observed longer OS in low SRSF1 expression group than in high SRSF1 expression group, indicating that high SRSF1 expression might lead to poor clinical prognosis (Figure 1D).
MiR-1183 Suppresses Cell Proliferation of HCC Cells
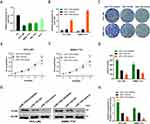
The results of qRT-PCR showed that the miR-1183 expression level in four HCC cell lines was lower than that in normal hepatic cell lines (Figure 2A). HCC cells were transfected with miR-1183-inhibitor or miR-1183-mimics to knockdown or overexpress miR-1183 in HCC (Figure 2B). As shown in Figure 2C and D, the inhibition of miR-1183 significantly promoted the clonogenicity of HCC cells. Consistent with the results of the colony formation assay, CCK-8 assay showed that downregulation of miR-1183 remarkably increase the cellular growth rate while overexpression of miR-1183 decreased it (Figure 2E and F). Protein expression of PCNA increased when miR-1183 was inhibited and declined when it was overexpressed (Figure 2G and H). In summary, miR-1183 suppresses cell proliferation of HCC cells.
MiR-1183 Suppresses Cell Migration of HCC Cells
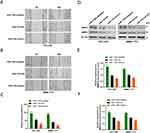
Wound healing assay was performed to detect the effect of miR-1183 on migration of HCC cells. Obviously, the consequence of 36 h after scratch revealed that inhibition of miR-1183 promotes the migration rate of HCC cells and overexpression of miR-1183 decreases the migration rate of HCC cells (Figure 3A-C). MMP2 and MMP9 are commonly used to measure the migration ability of tumor cells, and the results of Western blot also proved that miR-1183 restrains cell migration of HCC cells. MMP2 and MMP9 expressions were significantly higher in HCC cells treated with miR-1183-inhibitor than treated with miR-1183-mimics (Figure 3D-F).
SRSF1 is a Direct Target of miR-1183
According to the miRNA-mRNA target prediction online tool TargetScan (http://www.targetscan.org/vert_72/), three binding sites between miR-1183 and SRSF1 mRNA 3’UTR were found (Figure 4A). Therefore, we hypothesized that SRSF1 was the target of miR-1183. Three binding sites were used to conduct luciferase reporter assay. The results of the first two binding sites showed that the co-transfection of miR-1183-mimics inhibited the relative luciferase activity of WT-SRSF1-transfected cells, whereas it did not affect the activity of MUT-SRSF1-transfected cells (Figure 4B and C). The result of the last binding site showed no difference in relative luciferase activity between WT-SRSF1-transfected cells and MUT-SRSF1-transfected cells (Figure 4D). In another word, miR-1183 could directly bind to the 3’UTR of SFSF1 mRNA. Western blot results showed that compared with control group, miR-1183-inhibitor group exhibited increased SRSF1 protein level while miR-1183-mimics group showed decreased SRSF1 protein level (Figure 4E and F). Therefore, we draw a conclusion that SRSF1 was a direct target of miR-1183 and the binding of miR-1183 to the SRSF1 mRNA 3’ UTR could suppress SRSF1 protein expression.
Depletion of SRSF1 Rescues the Effect of miR-1183-Inhibitor on HCC Cells
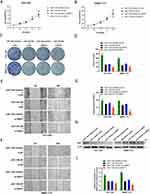
To verify the suppressive role of miR-1183 on HCC cells partially based on suppressing SRSF1 protein expression, miR-1183-inhibitor and si-SRSF1 were co-transfected in HCC cells. CCK8 and colony formation assays showed that depletion of SRSF1 could reverse the proliferation-promoting function of miR-1183-inhibitor on HCC cells (Figure 5A-D). What is more, the accelerative effect on cell migration of miR-1183-inhibitor can be rescued by SRSF1 suppression (Figure 5E-G). The results of Western blot emphasized that depletion of SRSF1 can disturb the upregulation of miR-1183-inhibitor on SRSF1 protein expression (Figure 5H and I). All the consequences above came to a conclusion that miR-1183 can regulate biological function of HCC cells by targeting SRSF1.
Overexpression of miR-1183 Inhibits HCC Growth in vivo
We injected HCC-LM3 cells into nude mouse models subcutaneously to confirm the role of miR-1183 in HCC in vivo. As shown in Figure 6A, HCC-LM3 cells were stably infected by lentiviral (LV-miR-1183 or LV-NC). Overexpression of miR-1183 notably reduced the average volume of tumor size (Figure 6B and C). In addition, the expression of SRSF1 was also suppressed by miR-1183 according to the results of IHC (Figure 6D). In a word, upregulation of miR-1183 could inhibit the growth of HCC in vivo.
Discussion
Abnormal alternative splicing is often detected in the occurrence and development of HCC.24 For example, DDX17 regulates alternative splicing that produces an oncogenic isoform of PXN-AS1 to promote HCC metastasis.25 MTR4 drives cancer metabolism by ensuring correct alternative splicing of pre-mRNAs of critical glycolytic genes such as GLUT1 and PKM2.26 SRSF1 is known to affect alternative splicing of genes involved in transformation, apoptosis, and cell cycle progression and to activate the oncogenic pathway. Abnormally high expression of SRSF1 has been observed in many cancers, such as breast cancer,27 gastric cancer28 and lung cancer.29 Most studies have shown that SRSF1 is a potential oncogene, causing tumorigenesis by regulating various tumor-related genes. For instance, SRSF1 regulates PTPMT1 alternative splicing to affect the development of lung cancer.30 SRSF1 can also regulate BIN1 alternative splicing to affect the tumorigenesis of non-small cell lung cancer.31 In that regard, this study has underscored the importance of SRSF1 as a novel HCC therapeutic target.
SRSF1 expression in HCC tissues and its correlation with the prognosis of HCC patients were analyzed using TCGA databases. Results suggested that SRSF1 was overexpressed in HCC tissues, and high SRSF1 expression in patients with HCC was correlated with poor prognosis. According to the results of TCGA databases, SRSF1 was highly expressed in HCC cell lines.
Considering the important regulatory role of miRNAs in genes, we sought to find a novel miRNA that could suppress SRSF1 expression. Using bioinformatics prediction database, we found that miR-1183 is a potential binding molecule for SRSF1. Three potential binding sites between miR-1183 and the 3’UTR region of SRSF1 mRNA were found by analyzing the network database. In this study, we identified miR-1183 was expressed at low level in HCC cell lines. Functional assays indicated that miR-1183-upregulation cells show weakened proliferation ability and migration ability in vitro and inhibit subcutaneous tumor formation in vivo. Therefore, miR-1183 was identified as a tumor suppressor in HCC. To verify whether miR-1183 could directly target SRSF1, luciferase reporter assay was performed and results showed that miR-1183 could modulate SRSF1 by binding to the 3’ UTR of SRSF1 mRNA. Further experiments found that miR-1183 reduced the protein level of SRSF1. Rescue assays indicated that depletion of SRSF1 crippled the promoting effects of miR-1183-inhibitor on the progression of HCC cells, further verifying that miR-1183 suppresses HCC via SRSF1.
Conclusion
Taken together, our data suggest that miR-1183 regulates the proliferation and migration of HCC cells through modulating SRSF1 by bind to the 3’ UTR of SRSF1 mRNA. This finding provides the first evidence of the interaction between SRSF1 and miR-1183 in HCC and improves the understanding of the mechanisms involved in the progression of HCC. The current findings provide a possible target for HCC treatment in the future.
Abbreviations
HCC, hepatocellular carcinoma; SRSF1, Serine and arginine-rich splicing factor 1; 3’-UTR, 3’ untranslated region; mRNA, messenger RNA; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; AS, alternative splicing; SR, serine/arginine; RRM, RNA recognition motifs; CRC, colorectal cancer; cDNA, complementary DNA; WT, wild-type; PCNA, proliferating cell nuclear antigen; MMP2, matrix metalloproteinase 2; MMP9, matrix metalloproteinase 9; IHC, Immunohistochemistry.
Acknowledgments
The authors would like to acknowledge the helpful comments on this paper received from the reviewers. This research was funded by the Natural Science Foundation of Shanghai, grant number 19ZR1439900 and Changzhou Science and Technology Bureau grant number CJ20200086.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Sperandio RC, Pestana RC, Miyamura BV, Kaseb AO. Hepatocellular carcinoma immunotherapy. Annu Rev Med. 2022;73:267–278. doi:10.1146/annurev-med-042220-021121
3. Zhang Y, Qian J, Gu C, Yang Y. Alternative splicing and cancer: a systematic review. Signal Transduction Targeted Therapy. 2021;6(1):78. doi:10.1038/s41392-021-00486-7
4. Howard JM, Sanford JR. The RNAissance family: SR proteins as multifaceted regulators of gene expression. Wiley Interdiscip Rev RNA. 2015;6(1):93–110. doi:10.1002/wrna.1260
5. Shepard PJ, Hertel KJ. The SR protein family. Genome Biol. 2009;10(10):242. doi:10.1186/gb-2009-10-10-242
6. Wan L, Yu W, Shen E, et al. SRSF6-regulated alternative splicing that promotes tumour progression offers a therapy target for colorectal cancer. Gut. 2019;68(1):118–129. doi:10.1136/gutjnl-2017-314983
7. Zhang Y, Wang M, Meng F, et al. A novel SRSF3 inhibitor, SFI003, exerts anticancer activity against colorectal cancer by modulating the SRSF3/DHCR24/ROS axis. Cell Death Discovery. 2022;8(1):238. doi:10.1038/s41420-022-01039-9
8. Sun J, Jin T, Su W, et al. The long non-coding RNA PFI protects against pulmonary fibrosis by interacting with splicing regulator SRSF1. Cell Death Differ. 2021;28(10):2916–2930. doi:10.1038/s41418-021-00792-1
9. Lv Y, Zhang W, Zhao J, et al. SRSF1 inhibits autophagy through regulating Bcl-x splicing and interacting with PIK3C3 in lung cancer. Signal Transduction Targeted Therapy. 2021;6(1):108. doi:10.1038/s41392-021-00495-6
10. Du JX, Luo YH, Zhang SJ, et al. Splicing factor SRSF1 promotes breast cancer progression via oncogenic splice switching of PTPMT1. J Exp Clin Cancer Res. 2021;40(1):171. doi:10.1186/s13046-021-01978-8
11. Lei S, Zhang B, Huang L, et al. SRSF1 promotes the inclusion of exon 3 of SRA1 and the invasion of hepatocellular carcinoma cells by interacting with exon 3 of SRA1pre-mRNA. Cell Death Discovery. 2021;7(1):117. doi:10.1038/s41420-021-00498-w
12. Ye Y, Yu F, Li Z, Xie Y, Yu X. RNA binding protein serine/arginine splicing factor 1 promotes the proliferation, migration and invasion of hepatocellular carcinoma by interacting with RecQ protein-like 4 mRNA. Bioengineered. 2021;12(1):6144–6154. doi:10.1080/21655979.2021.1972785
13. Komoll RM, Hu Q, Olarewaju O, et al. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol. 2021;74(1):122–134. doi:10.1016/j.jhep.2020.07.039
14. Han TS, Hur K, Cho HS, Ban HS. Epigenetic associations between lncRNA/circRNA and miRNA in hepatocellular carcinoma. Cancers. 2020;12(9):64.
15. Wang X, Yao Z, Fang L. miR-22-3p/PGC1β suppresses breast cancer cell tumorigenesis via PPARγ. PPAR Res. 2021;2021:6661828. doi:10.1155/2021/6661828
16. Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 2022;38(6):613–626. doi:10.1016/j.tig.2022.02.006
17. Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of MicroRNA expression in cancer. Int J Mol Sci. 2020;21(5):578.
18. Xie D, Li S, Wu T, Wang X, Fang L. MiR-181c suppresses triple-negative breast cancer tumorigenesis by targeting MAP4K4. Pathol Res Pract. 2022;230:153763. doi:10.1016/j.prp.2022.153763
19. Li N, Zhu L, Zhou H, et al. miRNA-1183-targeted regulation of Bcl-2 contributes to the pathogenesis of rheumatic heart disease. Biosci Rep. 2020;40(11). doi:10.1042/bsr20201573
20. Wu W, Xi W, Li H, Yang M, Yao X. Circular RNA circ‑ACACA regulates proliferation, migration and glycolysis in non-small-cell lung carcinoma via miR‑1183 and PI3K/PKB pathway. Int J Mol Med. 2020;45(6):1814–1824. doi:10.3892/ijmm.2020.4549
21. Ji C, Zhu L, Fang L. Hsa_circ_0000851 promotes PDK1/p-AKT-mediated cell proliferation and migration by regulating miR-1183 in triple-negative breast cancer. Cell Signal. 2022;101:110494. doi:10.1016/j.cellsig.2022.110494
22. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. J Physiol. 2020;598(18):3793–3801. doi:10.1113/jp280389
23. National Research Council Committee for the Update of the Guide for the C, Use of Laboratory A. The National Academies Collection: Reports Funded by National Institutes of Health. Guide for the Care and Use of Laboratory Animals. National Academies Press (US); 2011.
24. Lee SE, Alcedo KP, Kim HJ, Snider NT. Alternative splicing in hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2020;10(4):699–712. doi:10.1016/j.jcmgh.2020.04.018
25. Zhou HZ, Li F, Cheng ST, et al. DDX17-regulated alternative splicing that produced an oncogenic isoform of PXN-AS1 to promote HCC metastasis. Hepatology. 2022;75(4):847–865. doi:10.1002/hep.32195
26. Yu L, Kim J, Jiang L, et al. MTR4 drives liver tumorigenesis by promoting cancer metabolic switch through alternative splicing. Nat Commun. 2020;11(1):708. doi:10.1038/s41467-020-14437-3
27. Anczuków O, Akerman M, Cléry A, et al. SRSF1-regulated alternative splicing in breast cancer. Mol Cell. 2015;60(1):105–117. doi:10.1016/j.molcel.2015.09.005
28. Liu Y, Zhang YM, Ma FB, Pan SR, Liu BZ. Long noncoding RNA HOXA11-AS promotes gastric cancer cell proliferation and invasion via SRSF1 and functions as a biomarker in gastric cancer. World j Gastroenterol. 2019;25(22):2763–2775. doi:10.3748/wjg.v25.i22.2763
29. Das T, Lee EY, You HJ, Kim EE, Song EJ. USP15 and USP4 facilitate lung cancer cell proliferation by regulating the alternative splicing of SRSF1. Cell Death Discovery. 2022;8(1):24. doi:10.1038/s41420-022-00820-0
30. Sheng J, Zhao Q, Zhao J, et al. SRSF1 modulates PTPMT1 alternative splicing to regulate lung cancer cell radioresistance. EBioMedicine. 2018;38:113–126. doi:10.1016/j.ebiom.2018.11.007
31. Wang J, Liu T, Wang M, et al. SRSF1-dependent alternative splicing attenuates BIN1 expression in non-small cell lung cancer. J Cell Biochem. 2020;121(2):946–953. doi:10.1002/jcb.29366
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.