Back to Journals » Infection and Drug Resistance » Volume 16
The Role of Intestinal Flora and Its Metabolites in Heart Failure
Received 20 September 2022
Accepted for publication 25 November 2022
Published 5 January 2023 Volume 2023:16 Pages 51—64
DOI https://doi.org/10.2147/IDR.S390582
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xueqing Guan, Zhijun Sun
Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Zhijun Sun, Department of Cardiology, Shengjing Hospital, No. 39 of Huaxiang Road, Tiexi District, Shenyang, 110021, People’s Republic of China, Tel +86 18940251218, Fax +86 18940251218, Email [email protected]
Abstract: Intestinal flora is a complex collection of microbial communities that participate in the physiological and pathological activities of the human body through various pathways. In recent years, numerous studies have reported that intestinal flora are involved in the occurrence and development of heart failure (HF) and its metabolic products could play an important role in this progression, suggesting a great value in the clinical treatment of this condition. This study reported the interaction between intestinal flora and HF, and with intestinal flora metabolites, such as short-chain fatty acids, trimethylamine N-oxide and bile acids and urotoxins, considered as the starting point, the mechanism of the roles in HF was summarized. Additionally, the current research status and the development prospects of applying flora and metabolites to the clinical therapeutic decision of HF were discussed.
Keywords: intestinal flora, heart failure, trimethylamine N-oxide, inflammation, probiotics, targeted therapy, fecal microbiota transplantation
Introduction
Heart failure is a complex group of clinical syndromes caused by abnormal changes in the structure and/or function of the heart due to multiple causes; it results in impaired ventricular systolic and/or diastolic function, which primarily manifests as dyspnea, fatigue, and fluid retention (pulmonary congestion, systemic circulation congestion, and peripheral edema).1 HF is characterized by a high incidence and a high mortality rate. Data from developed countries show that the prevalence of HF in the current population is 1.5% to 2.0%, and 5-year mortality rates for inpatients with HF are 10.4%, 22%, and 42.3%, respectively.2 Patients with HF often have a decreased cardiac output combined with peripheral circulation congestion, causing intestinal ischemia and edema. In this case, the function of the intestinal barrier is weakened. Increased permeability of the intestinal wall and dislocation of the flora occur since harmful metabolites can enter the bloodstream through the damaged intestinal mucosal barrier, producing more inflammatory stimuli and aggravating HF.3
Intestinal flora is a complex collection of microbial communities which closely connected with the human body through direct or indirect pathways. In one way, intestinal flora can be recognized by intestinal cells and thus act directly. Peptidoglycan, LPS, flagellin, and formyl peptides in bacterial structures, which are called micro-associated molecular patterns (MAMPs), can be selectively recognized by the pattern recognition receptors on the surface of intestinal mucosal cells, such as the host toll-like receptors and receptors containing nucleotide oligomerization domains.4 In addition, intestinal flora might also act as an endocrine organ, metabolizing and secreting active substances that indirectly affect other physiological functions of the host through various pathways.
The current research status of the influence of the metabolic secretion products of intestinal flora on HF and its clinical application were summarized in the present study to discuss the possibility of applying the products in the diagnosis and treatment of HF.
Intestinal Flora Alteration Induced by Heart Failure
Patients with HF, especially those with right heart involvement, often have a decreased cardiac output combined with peripheral circulation congestion, causing intestinal ischemia and edema. In this case, the function of the intestinal barrier is weakened. The earliest description of altered intestinal flora in patients with HF was published in 2015, which uncovered that patients with HF had an increased intestinal aggregation of harmful bacteria.5 Although limitations existed in the sample collection and data processing, it may still function as an important guide for subsequent investigations. The study revealed that the alpha diversity of the intestinal flora in patients with HF decreased correspondingly with increasing severity of cardiac insufficiency (the New York Heart Association [NYHA] classification), while inflammation and oxidative stress levels increased concurrently.3 In clinical practice, intestinal ischemia can be determined by decreases in intestinal mucosal pH6 and passive carrier-mediated d-xylose transport.7 Intestinal edema can manifest as a thickening of the intestinal wall. In addition, intestinal permeability can be determined by lactose mannitol and cellobiose tests.5,8
A decrease in the production of short-chain fatty acids (SCFAs) by related flora (eg, Lachnospiraceae) has been observed in patients with HF. Among them, the decrease in the abundance of related flora, such as Faecalibacterium prausnitzii, Eubacterium rectale, and Roseburia, with the production of butyrate was more prominent.9 The abundance of microbial genes related to the production of LPS and TMA N-oxide (TMAO) was increased. Pasini et al discovered that patients with stable chronic HF with reduced ejection fraction had increased intestinal pathogenic bacteria, including Candida, Campylobacter, Shigella, Salmonella, and Yersinia, compared with healthy controls5 (Figure 1).
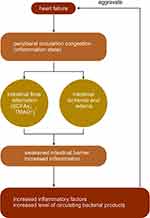 |
Figure 1 The interaction between heart failure and intestinal flora. Note: ↑ mean increase, ↓ mean decrease. |
Intestinal Flora in Human Body
A Close Relationship of Symbiosis
There are approximately 3.8×1013 microbial cells in each human body, far exceeding the number of human cells.10 Most of these microorganisms are present in the intestinal tract, especially the colon, and some are also distributed in the nasopharynx, mouth, lungs, skin, and cervix. These microbes are called “human microbiota” and consist of approximately 3.3 million microbial genes, which exceeds the number of human genes (approximately 25,000).11 Intestinal flora starts to colonize from birth and increases rapidly after two to three years of age; then, it maintains a dynamic balance under the influence of genes, the environment, and diet for most of a person’s life span. It tends to decrease again when an individual ages to a certain level or develops a serious disease.12 The intestinal microbiota varies from person to person and even between locations within the same individual, and some of the flora that perform major metabolic functions, which are known as core flora, are similar. The intestinal microbial community is composed of four main phyla: Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria.13 The flora in these four major groups contains most of the dominant flora, which plays an important role in supporting the physiological functions of the host and determines the physiopathological significance of the flora to the host. Of these phyla, Bacteroidetes and Firmicutes account for 90% of the total bacterial population in a healthy intestine.
The Functions of Intestinal Flora
Intestinal flora is involved in many metabolic processes in the body, including the absorption of indigestible fibers (eg, polysaccharides and resistant starches of plant cell walls) and energy utilization, the production of vitamins and amino acids, and the metabolism of drugs. In addition to acting directly with intestinal cells through substances such as LPS in the structure, intestinal flora might also act as an endocrine organ, metabolizing and secreting active substances that indirectly affect other physiological functions of the host through various pathways. The details of these actions including: a) promote gut mucus barrier, modulate the immune activation and against restrain inflammatory via SCFA pathways,14,15 b) participate in the formation of atherosclerotic plaques via TMA and TMAO pathways,16,17 c) act on HF through primary and secondary bile acid (BA) pathways,18,19 and d) regulate the mitochondrial function in cardiomyocytes and influence myocardial contractility via the urotoxin (eg, indole-3-propionic acid) pathways.20
Intestinal Flora Acts on Heart Failure Through Metabolic Secretory Pathways
Short-Chain Fatty Acids
Short-chain fatty acids are the end products of dietary fiber fermentation by gut microbiomes such as Bacteroides, Bifidobacterium and Faecalibacterium;21,22 they are produced primarily by the glycolytic metabolic pathway and the protein metabolic pathway and include various ammonias, amines, thiols, etc. Short-chain fatty acids, such as acetic acid, propionic acid, and butyric acid, produced by the catabolism of glucose are the main sources of energy for colonocytes (which maintain the intestinal mucosal barrier) and are closely associated with the promotion of intestinal immune function. It has been proved that SCFAs act not only as energy sources, but also as ligands and act in various pathways. SCFAs mainly combine with G protein-coupled receptors (GPCRs), including GPR41, GPR43 and GPR109a. They can also recognize with Olfr78, and inhibit the activation of histone deacetylase.23 Butyrate induces the proliferation of Forkhead box P3+ regulatory T cells and activates G protein-coupled receptors to inhibit the production of Th17 cells, thus producing anti-inflammatory effects.24 In addition, butyrate helps maintain the relative physiological hypoxia of the colonic epithelial mucosa by activating hypoxia-inducible factors, which are essential for maintaining the function of the intestinal barrier.25 In terms of immunity, butyric acid promotes the maturation of T cells on the surface of the intestinal mucosa, which is involved in chronic immune activation and is beneficial in suppressing ventricular remodeling (VR)26.
It was found that SCFAs appeared to promote post-infarction cardiac repair by inducing the infiltration of CX3CR1+ monocytes in the peri-infarct zone.27 Pluznick et al stated that SCFAs (acetic acid/propionic acid) inhibited myocardial fibrosis and suppressed VR. Short-chain fatty acids also play a role in other cardiovascular diseases; for example, SCFAs including acetic, propionate as well as butyrate are involved in the regulation of blood pressure23,28 (Table 1 and Figure 2).
 |
Table 1 Microbial Metabolites and Heart-Failure-Related Research Reference |
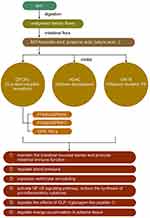 |
Figure 2 The synthesis of short-chain fatty acids and its effects. |
The abilities of intestinal regulation and anti-inflammatory of short-chain fatty acids have been demonstrated. However, its more direct protective effect on the heart in heart failure, such as anti-myocardial fibrosis, needs further experiments to support it. At present there are only animal trials of short-chain fatty acids can reduce myocardial fibrosis and lack enough supports from clinical trials.
Trimethylamine N-Oxide
Trimethylamine N-oxide is primarily derived during the process of digestion and the decomposition of food. During digestion process food can be broken down into choline and L-carnitine, which are later fermented by intestinal microbiota such as Firmicutes and Proteobacteria into TMA.29 TMA is then absorbed into the blood and transported to the liver, where it is catalyzed by flavin-containing monooxygenases and consequently metabolised to TMAO.
It has been verified that TMAO may be directly involved in the generation of coronary atherosclerotic plaques.30,31 In HF, TMAO was determined to be associated with an increased 5-year mortality rate in patients with HF, a poor prognosis in acute HF, and a higher NYHA classification.32,33 Trimethylamine N-oxide is correlated with b-type natriuretic peptide and the echocardiographic indicators of diastolic function (eg, E/Ea and left atrial volume index), while patients with high TMAO levels had worse left ventricular diastolic insufficiencies and clinical prognoses than those with low TMAO levels. However, TMAO is not correlated with systolic function, such as left ventricular ejection fraction.34,35 The observation results of choline-fed mice showed a direct effect of elevated TMAO levels on the activation of the myocardial pro-fibrotic pathway.36
Trimethylamine N-oxide also plays a role in kidney injury in addition to promoting myocardial fibrosis and participating in VR. In a cardiac study including 1434 participants without chronic kidney disease (CKD) at the baseline, it was revealed that the levels of choline and TMAO were independent predictors of future CKD.35 In animal experiments, mice fed with choline or TMAO had increased renal fibrosis and elevated markers of kidney injury, such as cysteine inhibitor-c.36 In one study, the development of CKD and cardiac hypertrophy was delayed in mice fed with iodomethylcholine, an inhibitor of TMA production.37 These results further supported the close relationship between TMAO and CKD (Table 1 and Figure 3).
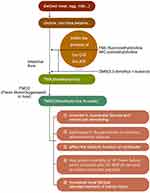 |
Figure 3 The synthesis of trimethylamine N-oxide and its effects. |
Given that patients with chronic heart failure are often combined with renal dysfunction while TMAO is associated with kidney injury, it may lead to a decline in the prognosis ability of TMAO in patients with HF and CKD. In addition, interindividual dietary differences and ethnic differences would also make it more difficult to set a standard for TMAO and its application in clinical practice needs more in-depth research.
Bile Acids
The physiological function of BA is to promote the absorption of dietary fat, fat-soluble molecules, and cholesterol and gut bacteria such as Bacteroides, Clostridium, and Lactobacillus could affect bile salt hydrolase gene expression.38 However, recent studies have found that BA not only has physiological significance but also plays a role in diseases such as HF.39 Farnesoid X receptors are highly expressed in the liver and ileum and negatively regulate BA synthesis by regulating different transcriptional networks.40,41 Tauro-β-muricholic acid is an abundant primary BA produced by intestinal flora, which upregulates the size and composition of the BA library and acts as a farnesoid X receptor antagonist. The ratio of the secondary to primary BAs in the blood was increased in patients with chronic HF, and a univariate analysis revealed that this ratio might be correlated with a decrease in overall survival.39,42 Multiple metabonomic studies also showed that the mechanism of HF was closely related to the metabolic disorder of bile acids.43,44 Some bile acids, such as ursodeoxycholic acid found to be significantly reduced in HF, have been proved to be beneficial to improving the peripheral blood flow of patients with HF and protecting the heart from reperfusion injury.45 The discovery of bile acid receptors has greatly expanded the understanding of the role of bile acids in HF, such as farnesoid X receptor (called FXR) and G-protein coupled bile acid receptor 1 (called TGR5). Farnesoid X receptors are highly expressed in the liver and ileum and negatively regulate BA synthesis by regulating different transcriptional networks.40,41 FXR activation can improve bile acid ratio and inhibit NF-kb, thereby reducing inflammation and improving myocardial function.46 Bile acids, especially TGR5 agonists, can induce protective changes in cardiac cells and improve the myocardial response of mice to physiological, positive muscle force and hemodynamic stress.47 These results suggested that BA metabolism might be regulated by intestinal flora, thus slowing the progression of HF (Table 1 and Figure 4).
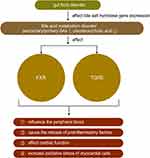 |
Figure 4 The role of bile acids in heart failure. Note: ↑ mean increase, ↓ mean decrease. |
Urotoxins
Tryptophan is derived from dietary protein and is metabolized by intestinal microorganisms to produce a variety of indole derivatives.48 Indole may be involved in a variety of normal physiological activities as a signaling molecule, such as affecting intestinal colony distribution, regulating gene expression in intestinal epithelial cells that affect barrier function, regulating glucagon-like peptide-1 secretion, and regulating anti-inflammation and antioxidation. However, some tryptophan metabolites, such as indole sulfate (IS), may have negative effects on the organism. Indole sulfate is metabolized from indole in the liver under the action of cytochrome P450 enzymes. In patients with renal insufficiency, urinary excretion is reduced, and IS accumulates in the body as a urotoxin, inducing tubulointerstitial fibrosis, glomerulosclerosis, and oxidative stress in the endothelial cells.49–51 In cardiovascular disease, IS may activate p38 mitogen-activated protein kinase, p42/44 mitogen-activated protein kinase, and nuclear factor-kB pathways, thereby stimulating the myocardial fibroblasts and collagen synthesis and resulting in adverse cardiac remodeling. In vitro studies also confirmed the direct effect of IS on the induction of cardiomyocytes hypertrophy and collagen synthesis in cardiac fibroblasts.49,52,53 A study in 2021 showed that the microbial tryptophan derivative indole-3-propionic acid could be identified as a regulator of mitochondrial function in cardiomyocytes. Acute treatment induced an enhanced maximal mitochondrial respiration, while chronic exposure resulted in mitochondrial dysfunction in cardiomyocytes in isolated perfused hearts of mice. The latter effect could also be observed in human hepatic and endothelial cells20 (Table 1 and Figure 5).
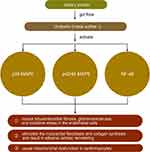 |
Figure 5 The role of urotoxins in heart failure. Note: ↑ mean increase. |
Based on the effect of IS on cardiac fibrosis and the characteristics of its transrenal metabolism in the above studies, the inhibition of its production or accelerate its clearance to reduce its accumulation in body is essential for patients with heart failure and renal insufficiency in improving their prognosis.
The Clinical Value of Intestinal Flora in Heart Failure (Figure 6)
Potential Predictive Markers
The high mortality rate and poor prognosis of heart failure are still problems to be solved. As for the inner reason, the current lack of understanding of the pathogenesis contributes to the predicament to the same extant as the frequent multi-systemic involvement. In clinical practice, assessing HF disease development requires more abundant, more objective and more accurate indicators. Studies have found that in patients with acute heart failure, TMAO has a significant correlation with renal function (BUN, eGFR) and cannot be used as an independent predictor. However, after combining the clinical algorithm and NT-BNP it can predict mortality during hospital stay and mortality within 1 year.32 In a trial containing a sample of 720 patients, CHF patients had significantly higher TMAO levels and a 3.4-fold increased risk of death when compared with age-, sex-matched non-HF patients. Higher TMAO levels can still predict a 5-year increased risk of mortality even after adjustment for traditional risk factors and cardiorenal index.33 Furthermore, a multicenter study involving 11 European countries showed that patients with lower TMAO levels at baseline or follow-up had higher survival rates, and persistently elevated TMAO levels before and after treatment were associated with higher mortality.32
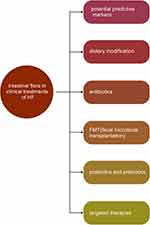 |
Figure 6 The clinical value of intestinal flora in heart failure. |
However, the production of TMAO can be significantly influenced by diet and the level of TMAO in plasma varies in different regions and ethnic groups.54 Thus, there are still no specific criteria for the level of elevated TMAO, and further clinical trials are needed to evaluate the effectiveness of combining TMAO and other indicators to predict heart failure.
Feasible Strategies of Treatment
Dietary Modification
Several studies have shown that the consumption of fiber-rich foods is associated with healthier intestinal microbiota and lower mortality rates. A systematic review and meta-analysis of randomized controlled trials showed that the Mediterranean diet reduced the incidence of HF by 70%.55 In addition, since TMAO is derived primarily from dietary choline and carnitine, reducing the intake of red meat can also reduce the production of TMAO in the body.56 In addition, an ingredient called DMB, which can be found in virgin olive oil can inhibit the production of TMA.57 However, due to the complexity of food sources, more precise control of variables is necessary to obtain more definitive results in the investigation of the dietary effects on HF.
Antibiotics
It has been suggested that the oral administration of polymyxin B, which can selectively kill gram-negative bacteria, could reduce the production of pro-inflammatory factors by monocytes in patients with HF, which in turn could improve flow-mediated dilation and improve vascular endothelial function.58 However, the results of multiple trials have shown that due to the poor selectivity of antibiotics, although pathogenic flora was removed, the beneficial flora was significantly weakened. Therefore, since the function of the intestinal flora was seriously weakened, an increased risk of cardiovascular disease, diabetes mellitus, and other diseases was revealed, and the long-term disadvantages outweighed the advantages.
Fecal Microbiota Transplantation
The primary function of fecal microbiota transplantation (FMT) is to deliver the intestinal flora from healthy individuals to the intestine of a patient to regulate the balance of their intestinal flora, usually in an endoscopic route. In current clinical circumstances, FMT is often used in the treatment of severe infections or inflammatory bowel diseases (IBD). In heart failure treatments, FMT is adopted as a potential auxiliary therapy. There are several experiments on FMT and its effects on cardiovascular diseases for now. In an experiment on atherosclerosis pathogenesis, fecal microbiota transplanted from WT mice could improve gut microbiome composition and reduce the atherosclerosis level of atherosclerosis-prone CTRP9-KO mice.59 Studies also showed that FMT could alleviate inflammatory infiltration, and thus reduce myocardial injury.60 However, FMT also carries risk, and in a clinical trial, one of the two patients who accepted a FMT treatment died due to improper donor screening.61 The procedure and operation of FMT still need further standardization and optimization, including screening for suitable donors, developing gentler non-invasive delivery methods, and extracting more specific ingredients for more individualized therapeutic strategies.
Probiotics and Prebiotics
Probiotic therapy is the direct administration of certain beneficial flora, such as Bifidobacteria, to promote the balance of intestinal flora. Prebiotics, such as dietary fiber, have certain specific ingredients that can produce a therapeutic effect. The consumption of probiotics or prebiotics can improve the distribution of intestinal flora and reduce the production of related harmful metabolites and inflammatory factors. The results of animal experiments suggested that certain lactic acid bacteria could have cardioprotective effects. Rats were given a supplement containing Lactobacillus plantarum 299v, followed by a coronary artery ligation and observation. Reduced myocardial infarct size and improved left ventricular function were observed in rats fed with probiotics.62 Another study found that the addition of L. rhamnosus GR-1 to a rat model with myocardial ischemia resulted in similar cardioprotective effects.63 In terms of the investigation on prebiotics, a recent randomized controlled trial showed that the addition of inulin or inulin-propionate ester to the diet in experimental animals increased the delivery of SCFAs in the colon.64 Although current scientific evidence does not yet support the application of probiotics or prebiotics in the treatment of patients with HF, the production of SCFAs via supplementation with inulin or other prebiotics provides a strategy for the treatment of cardiovascular disease.
Targeted Therapies
There is a broad prospect of taking metabolic products as targeted drug in treatments of cardiovascular diseases. Several inhibitors of enzymes have been tested in trials. Fluoromethylcholine (FMC) and iodomethylcholine (IMC) are inhibitors of cutC/D, a kind of enzyme participants the produce of TMA. They can permanently inactivate cutC/D without affecting the viability of the commensal bacteria. In animal models, FMC and IMC significantly reduced systemic TMAO levels and reversed TMAO-induced platelet hyperactivity as well as thrombosis.65 Inhibitors of FMOs in the liver can reduce TMAO generation, but can also cause TMA accumulation and lead to complications such as acute hepatitis.66 Short-chain fatty acids entering the systemic circulation were shown to have the ability to regulate cardiovascular disease risk factors, including lowering blood pressure and regulating blood glucose and lipid homeostasis. Oral charcoal adsorbent (AST-120) has been used clinically to remove uremic toxins, such as indoxyl sulfate, in patients with advanced renal failure. Moreover, existing animal trials have shown that AST-120 can prevent the progression of LV hypertrophy and myocardial fibrosis.67 At present, most of the studies on the role of metabolites are animal experiments, but it is foreseeable that the application of microbiota metabolic products in the treatment of heart failure is of great value.
Conclusions and Future Perspectives
Heart failure affects intestinal microbiota community structure, leading to the disfunction of gut microbiota. On the other hand, microbiota dysbiosis and the accumulation of some microbiota-derived metabolites aggravate heart failure progression in multiple aspects. Intestinal flora is similar to an endocrine organ and is involved in many metabolic pathways of the body, both directly and indirectly, that affect the functions of the host. There are emerging evidences that intestinal flora is closely connected with diabetes mellitus, obesity, hypertension and other chronic diseases.
There is quite a lot of clinical data to prove that intestinal microbiota experiences a dysbiosis in patients with heart failure compared to healthy cohorts, even in HFpEF patients.68–70 Further studies also uncovered the fact that diets could effect cardiovascular risk factors such as the blood lipid by means of intestinal microbiome.26 In laboratory investigations with rats, we found that the effects of microbiota-derived metabolites on inflammation factors, fibrosis substances and other aspects correlated with heart failure.3,71,72 However, the mass insufficiency in the studies for now should not be ignored. On one hand, the superficial analysis of the diversity changes in microbiota is far from enough and specific function of bacterium ought to be explored. Only in that way could we make more precise evaluation on the effects of microbiota and its metabolites. On the other hand, more clinical trials are needed to transmit the achievements in animal studies into practical treatments. Meanwhile, the current clinical outcomes should also be verified with larger scales of experiments. What is more, the effect of medicine applied in current heart failure treatments is an essential part too. For example, the SGLT-2 inhibitors, which are introduced into the clinical treatments in heart failure in recent years seems to share similar accesses with gut microbiota when they come into effect.23,73 Therefore, the study on correlations between drugs and microbiota is of great significance. However, it is undeniable that the regulation of the microflora and its metabolites will have an extremely important role in the long-term treatment of heart failure.
Abbreviation
HF, heart failure; LPS, lipopolysaccharide; CRP, C-reactive protein; TNF-α, tumour necrosis factor alpha; VR, ventricular remodeling; pH, potential of hydrogen; TMA, trimethylamine; TMAO, trimethylamine N-oxide; MAMPs, microassociated molecular patterns; prr, pattern recognition receptors; SCFAs, short chain fatty acids; E/Ea, the ratio of peak velocity of early tricuspid inflow wave (E) to peak velocity of early diastolic wave of the lateral tricuspid annulus (Ea); CKD, chronic kidney disease; IMC, iodomethylcholine; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; GLP-1, glucagon-like peptide 1; IS, Indoxyl sulfate; 16sRNA, 16S ribosomal DNA identification; RCT, randomized clinical trial; FMT, fecal microbiota transplantation; BA, bile acids; DMB, 3,3-dimethyl-1-butanol; IBD, inflammatory bowel diseases; SGLT-2 inhibitors, sodium-glucose cotransporter-2 inhibitors; HFpEF, heart failure with preserved ejection fraction.
Data Sharing Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical A, Chinese Heart Failure Association of Chinese Medical Doctor A and Editorial Board of Chinese Journal of C. 中国心力衰竭诊断和治疗指南 [Chinese guidelines for the diagnosis and treatment of heart failure 2018]. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46(46):760–789. Chinese. doi:10.3760/cma.j.issn.0253-3758.2018.10.004
2. Maggioni AP, Dahlström U, Filippatos G, et al. EURObservational research programme: regional differences and 1-year follow-up results of the heart failure pilot survey (ESC-HF pilot). Eur J Heart Fail. 2013;15:808–817. doi:10.1093/eurjhf/hft050
3. Yuzefpolskaya M, Bohn B, Nasiri M, et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J Heart Lung Transpl. 2020;39(880–890):880–890. doi:10.1016/j.healun.2020.02.004
4. Maddox J. Immunology made accessible. Nature. 1984;310:183. doi:10.1038/310183a0
5. Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220–227. doi:10.1016/j.jchf.2015.10.009
6. Krack A, Richartz BM, Gastmann A, et al. Studies on intragastric PCO2 at rest and during exercise as a marker of intestinal perfusion in patients with chronic heart failure. Eur J Heart Fail. 2004;6:403–407. doi:10.1016/j.ejheart.2004.03.002
7. Sandek A, Bjarnason I, Volk H-D, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–85. doi:10.1016/j.ijcard.2010.12.016
8. Sandek A, Bauditz J, Swidsinski A, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi:10.1016/j.jacc.2007.07.016
9. Cui X, Ye L, Li J, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8:635. doi:10.1038/s41598-017-18756-2
10. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi:10.1371/journal.pbio.1002533
11. Willyard C. New human gene tally reignites debate. Nature. 2018;558:354–355. doi:10.1038/d41586-018-05462-w
12. Tran TTT, Cousin FJ, Lynch DB, et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome. 2019;7(1):39. doi:10.1186/s40168-019-0654-1
13. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi:10.1038/nature08821
14. Vijay A, Astbury S, Le Roy C, Spector TD, Valdes AM. The prebiotic effects of omega-3 fatty acid supplementation: a six-week randomised intervention trial. Gut Microb. 2021;13:1. doi:10.1080/19490976.2020.1863133
15. Desai MS, Seekatz AM, Koropatkin NM, et al. Gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1. doi:10.1016/j.cell.2016.10.043
16. Vallance HD, Koochin A, Branov J, Rosen-Heath A, Bosdet T, Wang Z. Marked elevation in plasma trimethylamine-N-oxide (TMAO) in patients with mitochondrial disorders treated with oral l-carnitine. Mol Genet Metab Rep. 2018;15:130–133. doi:10.1016/j.ymgmr.2018.04.005
17. Koeth RA, Lam-Galvez BR, Kirsop J, et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2019;129:373–387. doi:10.1172/JCI94601
18. Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi:10.1016/j.cmet.2009.08.001
19. Downes M, Verdecia MA, Roecker AJ, et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell. 2003;11:1079–1092. doi:10.1016/S1097-2765(03)00104-7
20. Gesper M, Nonnast ABH, Kumowski N, Stoehr R, Schuett K. Gut-derived metabolite indole-3-propionic acid modulates mitochondrial function in cardiomyocytes and alters cardiac function. Front Med. 2021;8:648259. doi:10.3389/fmed.2021.648259
21. Baxter NT, Lesniak NA, Sinani H. The glucoamylase inhibitor acarbose has a diet-dependent and reversible effect on the murine gut microbiome. mSphere. 2019;4:1.
22. Di Iorio BR, Rocchetti MT, De Angelis M, Cosola C, Marzocco S, Di Micco L. Nutritional therapy modulates intestinal microbiota and reduces serum levels of total and free indoxyl sulfate and p-cresyl sulfate in chronic kidney disease (medika study). J Clin Med. 2019;8:1. doi:10.3390/jcm8091424
23. Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110(11):4410–4415. doi:10.1073/pnas.1215927110
24. Carrillo-Salinas FJ, Anastasiou M, Ngwenyama N, et al. Gut dysbiosis induced by cardiac pressure overload enhances adverse cardiac remodeling in a T cell-dependent manner. Gut Microb. 2020;12:1–20. doi:10.1080/19490976.2020.1823801
25. Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial hif augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi:10.1016/j.chom.2015.03.005
26. Vijay A, Astbury S, Panayiotis L, Marques FZ, Spector TD. Dietary interventions reduce traditional and novel cardiovascular risk markers by altering the gut microbiome and their metabolites. Front Cardiovasc Med. 2021;8:691564. doi:10.3389/fcvm.2021.691564
27. Tang TWH, Chen H-C, Chen C-Y, et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139:647–659. doi:10.1161/CIRCULATIONAHA.118.035235
28. Poll BG, Xu J, Jun S, et al. Acetate, a short-chain fatty acid, acutely lowers heart rate and cardiac contractility along with blood pressure. J Pharmacol Exp Ther. 2021;377:39–50. doi:10.1124/jpet.120.000187
29. Gupta N, Buffa JA, Roberts AB, et al. Targeted inhibition of gut microbial trimethylamine N-oxide production reduces renal tubulointerstitial fibrosis and functional impairment in a murine model of chronic kidney disease. Arterioscler Thromb Vasc Biol. 2020;40:1239–1255. doi:10.1161/ATVBAHA.120.314139
30. Koren O, Spor A, Felin J, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4592–4598. doi:10.1073/pnas.1011383107
31. Yu H, Li L, Deng Y, et al. The relationship between the number of stenotic coronary arteries and the gut microbiome in coronary heart disease patients. Front Cell Infect Microbiol. 2022;12:903828. doi:10.3389/fcimb.2022.903828
32. Suzuki T, Yazaki Y, Voors AA, et al. Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure: results from BIOSTAT-CHF. Eur J Heart Fail. 2019;21:877–886. doi:10.1002/ejhf.1338
33. Tang WHW, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–1914. doi:10.1016/j.jacc.2014.02.617
34. Trøseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. doi:10.1111/joim.12328
35. Rhee EP, Clish CB, Ghorbani A, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–1338. doi:10.1681/ASN.2012101006
36. Tang WHW, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi:10.1161/CIRCRESAHA.116.305360
37. Zhang W, Miikeda A, Zuckerman J, et al. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Sci Rep. 2021;11:518. doi:10.1038/s41598-020-80063-0
38. Wan Y, Yuan J, Li J, et al. Unconjugated and secondary bile acid profiles in response to higher-fat, lower-carbohydrate diet and associated with related gut microbiota: a 6-month randomized controlled-feeding trial. Clin Nutr. 2020;39:395–404. doi:10.1016/j.clnu.2019.02.037
39. Mayerhofer CCK, Ueland T, Broch K, et al. Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail. 2017;23:666–671. doi:10.1016/j.cardfail.2017.06.007
40. Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci USA. 2004;101:3668–3673. doi:10.1073/pnas.0400046101
41. Xu M, Shen Y, Cen M, et al. Modulation of the gut microbiota-farnesoid X receptor axis improves deoxycholic acid-induced intestinal inflammation in mice. J Crohns Colitis. 2021;15:1197–1210. doi:10.1093/ecco-jcc/jjab003
42. Ryan PM, Stanton C, Caplice NM. Bile acids at the cross-roads of gut microbiome-host cardiometabolic interactions. Diabetol Metab Syndr. 2017;9:102. doi:10.1186/s13098-017-0299-9
43. Xu J, Li X, Zhang F, et al. Integrated UPLC-Q/TOF-MS technique and MALDI-MS to study of the efficacy of yixinshu capsules against heart failure in a rat model. Front Pharmacol. 2019;6(10):1474. doi:10.3389/fphar.2019.01474
44. Liu S, Pi Z, Liu Z, Song F, Liu S. Fecal metabolomics based on mass spectrometry to investigate the mechanism of qishen granules against isoproterenol-induced chronic heart failure in rats. J Sep Sci. 2020;43(23):4305–4313. doi:10.1002/jssc.202000622
45. von Haehling S, Schefold JC, Jankowska EA, et al. Ursodeoxycholic acid in patients with chronic heart failure: a double-blind, randomized, placebo-controlled, crossover trial. J Am Coll Cardiol. 2012;59(6):585–592. doi:10.1016/j.jacc.2011.10.880
46. Purcell NH, Tang G, Yu C, Mercurio F, DiDonato JA, Lin A. Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci USA. 2001;98(12):6668–6673. doi:10.1073/pnas.111155798
47. Eblimit Z, Thevananther S, Karpen SJ, Taegtmeyer H, Moore DD, Adorini L. TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice. Cardiovasc Ther. 2018;36(5):e12462. doi:10.1111/1755-5922.12462
48. Aronov PA, Luo FJG, Plummer NS, Quan Z, Holmes S. Colonic contribution to uremic solutes. J Am Soc Nephrol. 2011;22:1769–1776. doi:10.1681/ASN.2010121220
49. Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31(14):1771–1779. doi:10.1093/eurheartj/ehp574
50. Yisireyili M, Shimizu H, Saito S, Enomoto A, Nishijima F, Niwa T. Indoxyl sulfate promotes cardiac fibrosis with enhanced oxidative stress in hypertensive rats. Life Sci. 2013;92(24–26):1180–1185. doi:10.1016/j.lfs.2013.05.008
51. Camacho O, Rosales MC, Shafi T, Fullman J, Plummer NS. Effect of a sustained difference in hemodialytic clearance on the plasma levels of p-cresol sulfate and indoxyl sulfate. Nephrol Dial Transplant. 2016;31:1335–1341. doi:10.1093/ndt/gfw100
52. Yang K, Xu X, Nie L, et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol Lett. 2015;234:110–119. doi:10.1016/j.toxlet.2015.01.021
53. Yang K, Wang C, Nie L, et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol. 2015;26:2434–2446. doi:10.1681/ASN.2014060543
54. Yazaki Y, Salzano A, Nelson CP, et al. Geographical location affects the levels and association of trimethylamine N-oxide with heart failure mortality in BIOSTAT-CHF: a post-hoc analysis. Eur J Heart Fail. 2019;21:1291–1294. doi:10.1002/ejhf.1550
55. Liyanage T, Ninomiya T, Wang A, et al. Effects of the Mediterranean diet on cardiovascular outcomes-a systematic review and meta-analysis. PLoS One. 2016;11:e0159252. doi:10.1371/journal.pone.0159252
56. Wang Z, Bergeron N, Levison BS, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–594. doi:10.1093/eurheartj/ehy799
57. Wang G, Kong B, Shuai W, Fu H, Jiang X, Huang H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J Nutr Biochem. 2020;78:108341. doi:10.1016/j.jnutbio.2020.108341
58. Conraads VM, Jorens PG, De Clerck LS, et al. Selective intestinal decontamination in advanced chronic heart failure: a pilot trial. Eur J Heart Fail. 2004;6:483–491. doi:10.1016/j.ejheart.2003.12.004
59. Kim ES, Yoon BH, Lee SM, et al. Fecal microbiota transplantation ameliorates atherosclerosis in mice with C1q/TNF-related protein 9 genetic deficiency. Exp Mol Med. 2022;54:103–114. doi:10.1038/s12276-022-00728-w
60. Hu X-F, Zhang W-Y, Wen Q, et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res. 2019;139:412–421.
61. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381:2043–2050. doi:10.1056/NEJMoa1910437
62. Lam V, Su J, Koprowski S, et al. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727–1735. doi:10.1096/fj.11-197921
63. Gan XT, Ettinger G, Huang CX, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–499.
64. Johnson LP, Walton GE, Psichas A, Frost GS, Gibson G, Barraclough T. Prebiotics modulate the effects of antibiotics on gut microbial diversity and functioning in vitro. Nutrients. 2015;7:4480–4497. doi:10.3390/nu7064480
65. Roberts AB, Gu X, Buffa JA, et al. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–1417. doi:10.1038/s41591-018-0128-1
66. Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi:10.1016/j.cell.2015.11.055
67. Asanuma H, Chung H, Ito S, et al. AST-120, an adsorbent of uremic toxins, improves the pathophysiology of heart failure in conscious dogs. Cardiovasc Drugs Ther. 2019;33:277–286. doi:10.1007/s10557-019-06875-z
68. Beale AL, O’Donnell JA, Nakai ME, et al. The gut microbiome of heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e020654. doi:10.1161/JAHA.120.020654
69. Li L, Zhong S-J, Hu S-Y, Cheng B, Qiu H, Hu Z-X. Changes of gut microbiome composition and metabolites associated with hypertensive heart failure rats. BMC Microbiol. 2021;21:141. doi:10.1186/s12866-021-02202-5
70. Kummen M, Mayerhofer C, Vestad B, et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol. 2018;71:1184–1186. doi:10.1016/j.jacc.2017.12.057
71. Deng F, Zhang L-Q, Wu H, et al. Propionate alleviates myocardial ischemia-reperfusion injury aggravated by Angiotensin II dependent on caveolin-1/ACE2 axis through GPR41. Int J Biol Sci. 2022;18:858–872. doi:10.7150/ijbs.67724
72. Tayyeb JZ, Popeijus HE, Mensink RP, Konings MCJM, Mokhtar FBA, Plat J. Short-chain fatty acids (except hexanoic acid) lower NF-kB transactivation, which rescues inflammation-induced decreased apolipoprotein A-I transcription in HepG2 cells. Int J Mol Sci. 2020;22:21. doi:10.3390/ijms22010021
73. Oh TJ, Sul WJ, Oh HN, Lee Y-K, Lim HL, Choi SH. Butyrate attenuated fat gain through gut microbiota modulation in db/db mice following dapagliflozin treatment. Sci Rep. 2019;9:20300. doi:10.1038/s41598-019-56684-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
