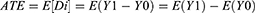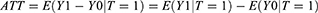Back to Journals » Infection and Drug Resistance » Volume 15
The Role of GeneXpert MTB/RIF in Reducing Treatment Delay Among Multidrug Resistance Tuberculosis Patients: A Propensity Score Matched Analysis
Authors Tamirat KS , Kebede FB , Baraki AG , Akalu TY
Received 2 November 2021
Accepted for publication 11 January 2022
Published 27 January 2022 Volume 2022:15 Pages 285—294
DOI https://doi.org/10.2147/IDR.S345619
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Koku Sisay Tamirat,1 Fentahun Bikale Kebede,2 Adhanom Gebreegziabher Baraki,1 Temesgen Yihunie Akalu1
1Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Ethiopia Public Health Institute, Addis Ababa, Ethiopia
Correspondence: Koku Sisay Tamirat, Email [email protected]
Background: GeneXpert MTB/RIF testing is a rapid molecular diagnostic test that is performed with an automated cartilage-based machine that makes treatment initiation prompt. This study aimed at evaluating the impact of GeneXpert in the reduction of treatment delay among multidrug-resistant tuberculosis (MDR-TB) patients in Amhara regional state of Ethiopia.
Methods: A facility-based retrospective follow-up study was conducted from January to February 2019, and a total of 465 MDR-TB patients were included in the study. Socio-demographic, clinical, and treatment-related characteristics were collected from patient’s chart retrospectively using data abstraction sheets. Binary logistic regression model was fitted to identify factors associated with treatment delay; adjusted odds ratio (AOR) with a 95% confidence interval (CI) was computed to assess the strength of association. A propensity score-matched (PSM) analysis was used to assess the impact of the GeneXpert MTB/RIF test on treatment delay through calculation of average treatment effect (ATE).
Results: The majority, 92.4%, of patients had the pulmonary form of TB, and 46.7% of patients were diagnosed by GeneXpert MTB/RIF. The presence of cavitation (AOR = 0.62, 95% CI: 0.39 0.96), extrapulmonary form of TB (AOR = 0.34, 95% CI: 0.14 0.81), and GeneXpert (AOR = 0.15, 95% CI: 0.10 0.24) were factors associated with treatment delay. The average treatment effect (ATE) of PSM analysis showed that GeneXpert MTB/RIF has significantly reduced treatment delay by 41% compared to matched control groups.
Conclusion: This study revealed that GeneXpert test has a strong association with the reduced treatment delays among MDR-TB patients. This underscores that rapid molecular tests could help improve the health system and lead to prompt initiation of MDR-TB treatment. Therefore, expansion and decentralization of GeneXpert tests to peripheral health facilities are highly recommended. In turn, the case detection and control of the disease will be hastened.
Keywords: drug-resistant tuberculosis, treatment delay, Ethiopia
Introduction
Drug-resistant tuberculosis (DR-TB) is a major public health problem worldwide. According to the World Health Organization (WHO) 2020 report, over half a million people had multidrug resistance tuberculosis (MDR-TB), and 156, 071 were enrolled in MDR-TB treatment.1 Drug-resistance testing coverage ranges from 46% to 83% among new and previously treated TB patients, respectively.1 Ethiopia is also one of the 30 TB high burden countries with an increasing number of MDR-TB and successful treatment outcomes reached above 70%, which is above the global target of 56%.2 However, only one in three MDR-TB patients were enrolled in treatment.1 In addition, delayed initiation of MDR-TB treatment leads to continued transmission of the disease, which could be responsible for the increased incidence of primary MDR-TB cases. The implementation of rapid diagnostic tests like GeneXpert and the expansion of MDR-TB treatment centers to peripheral health facilities were the interventions used to minimize gaps of low case detection and delayed treatment enrollment. The GeneXpert MTB/RIF testing is a rapid molecular diagnostic test that is performed with an automated cartilage-based GeneXpert machine (Cepheid, USA). The test function based on nucleic-acid amplification assay that detects Mycobacterium TB bacilli and Rifampicin resistance pattern from the patient’s sputum and other body fluids.3,4 The test minimizes median laboratory processing time to less than one day and makes MDR-TB treatment initiation prompt.5 The tests can be performed by trained midlevel healthcare professionals and enable the provision of the services at the point of care with minimal biosafety requirements. A study showed that GeneXpert test has contributed about 63.4 cases of same-day treatment initiation per 1000 screened individuals as compared to 40 cases of same-day treatment initiation with conventional culture and DST.4 A study conducted in South Africa showed that the median time from sputum collection to treatment initiation ranged from 18 days GeneXpert MTB/RIF to 64 days for culture and DST. Another study from Russia showed that the availability of the GeneXpert test for the initial diagnosis of drug-resistant tuberculosis had significantly reduced the time to initiation of second-line drugs.6 In addition, the GeneXpert test showed greater sensitivity compared to sputum smear microscopy and it has also a shorter laboratory processing time than other tests.7,8 The expansion of ambulatory MDR-TB treatment centers, conditional amendment of treatment guidelines, and introduction of short regimen are some of the interventions used to reduce treatment delay and related problems. Moreover, the implementation and scale-up of GeneXpert MTB/RIF tests in health facilities reduced the time to commencement of MDR-TB treatment. The National Tuberculosis Program (NTP) of Ethiopia has developed guidelines for a safe referral system of sputum samples to central laboratories through the postal office system.9 Apart with this previous studies determined the effect of socio-demographic, behavioral, and clinical related characteristics on the timely initiation of TB treatment.10 However, most of these previous studies are descriptive types determining the median time and making between-group comparisons without controlling potential confounders.11–14 There is a dearth of information about the impact of GeneXpert and its causal relationship with treatment delay in Ethiopia. Therefore, this study aimed to assess the impact of GeneXpert in reduction of treatment delay among MDR-TB in the study setting. The finding of this study could help clinicians, program planners, and policymakers assist evidence-based decision-making process. Given that all potential confounders are matched, an attempt is undertaken to determine to what degree the net difference in treatment delay observed among MDR-TB patients who underwent GeneXpert and other tests (LPA and conventional culture) might be ascribed to GeneXpert.
Method
Study Setting, Period, and Design
An institution-based retrospective follow-up study was conducted from September 2010 to December 2016 in the three selected public hospitals of the Amhara Regional State. A total of 465 DR-TB patients were enrolled in the study from three hospitals (University of Gondar Hospital, Boru Meda Primary Hospital, and Debre Markos Referral Hospitals). All MDR-TB patients who were diagnosed by GeneXpert MTB/RIF, line probe assay (LPA), and culture and drug susceptibility testing (DST) were the study population. Patients who have incomplete data, specifically time to initiation and undocumented methods of diagnosis were excluded from the study. A total of 465 drug-resistant TB patients who fulfilled the inclusion criteria took part in the study.
Data Collection Process
The data available on patient records were first examined, and the extraction checklist was prepared in English. Six data collectors and two supervisors (nurses and health officers) were recruited. A two-day training was given on research objectives and document review using the data extraction tool. Before the actual data collection process, records were identified by their medical registration numbers. The trained collectors reviewed and extracted data from patient charts and the MDR-TB registration logbook.
Variables of the Study
Treatment initiation delay for MDR-TB treatment was dichotomized as “1” if treatment delay was above 10 days and as “0” otherwise, whereas GeneXpert MTB/RIF test for the diagnosis of drug-resistant tuberculosis was categorized as “yes” or “no”. This variable was used as an independent variable in the logistic regression analysis and treatment variable in propensity score matching analysis for delayed treatment initiation among MDR-TB patients.
On the other hand, socio-demographic characteristics of patients like age group, residence, gender, TB treatment supporter, and clinical factors like the form of TB, complications,
- Functional status at admission: Classified based activity daily living (ADL) (eg, ambulating, feeding, dressing and keeping personal hygiene) as working, ambulatory, and bedridden.15
- Treatment supporter: A family member (FM) or any person who is willing to help and is accepted by the patient and answerable to the health services can also be a treatment supporter.16
- TB/HIV confection: A patient having HIV infection and TB disease at the same time.
- Cavitation: is an extensive necrosis involving the walls of airway in which semi-liquid necrotic material is discharged into the bronchial tree from where it is usually coughed up and may infect others.17
- Multidrug resistance (MDR-TB): resistance to at least both isoniazid and rifampicin or Rifampicin resistance (RR-TB) detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs.1
- Time to initiation of RR-TB treatment: The time interval between positive result and treatment initiation were covariates used to control potential confounders.10
Data Analysis
Initially, the filled checklists were checked for completeness by the principal investigator and three supervisors. The data were entered into Epi-data version 3.1 and exported to Stata version 14 statistical software for further analysis. Median with Inter Quartile Range (IQR) was used to estimate the time to initiation of MDR-TB treatment. The logistic regression model was fitted to identify factors associated with the treatment initiation delay.
The Propensity score matching (PSM) analysis was applied to compare treatment delay among MDR-TB patients diagnosed with GeneXpert MTB/RIF and other laboratory techniques. The PSM is a statistical method developed to estimate treatment effects (treatment effect in the case of this study is using GeneXpert) with non-experimental or observational data.18 The impact of the GeneXpert test on treatment delay was estimated using the endogenous regression model, which can be used to compare other laboratory tests. PSM was estimated in Stata version 14 using command psmatch2. The psmatch2 implements full Mahalanobis matching and a variety of propensity score matching methods to adjust differences between groups tested with GeneXpert and other tests. It was used to estimate the Average impact among tested with GeneXpert (ATT) and tested with other laboratory tests like LPA/culture (ATU), and the overall average treatment effect (ATE) (ATE is referring to the impact on treatment delay, but in this case the “treatment effect” is that of using GeneXpert). Using the PSM, the effect of a treatment on an individual can be evaluated by finding the difference in the outcome with and without treatment:
Where Di is the difference between outcomes of individual i with and without treatment and (Y1i, Y0i) shows the outcome of individual i with and without the treatment. Unfortunately, for each unit i, Y1i and Y0i are not observable at the same time because the same unit cannot simultaneously be in both the treatment and control groups. The unobserved outcome is called a “counterfactual outcome”.18,19 Alternatively, the Average Treatment Effect (ATE) can be estimated over the entire population as:
where E(Y1) is the expected value of Y for all the units in the treatment group, and E(Y0) is the expected value of Y for all the units in the control group. Moreover, the average treatment effect on the treated (ATT) is defined as follows:
Here, T = (0, 1) refers to the control and treatment conditions. The term E(Y0 | T = 1) is a counterfactual mean, which is not observable from the data. Finally, the standardized mean difference was calculated and was found to be 8.1%, which is below the 10% that reflects covariates between treated and control groups are balanced.
Results
Socio-Demographic Characteristics of the Samples
The median age of the patients was 28 years (IQR: 22, 38). Of 465 patients, 270 (58%) were males and 242 (52%) were rural residents. About 188 (40%) of study subjects were unemployed, and 195 (42%) had not attended formal education. Regarding behavioral characteristics, 40 (8.6%) patients chewed khat, 60 (12%) smoked cigarettes, and 88 (18.9%) had drunk alcohol (Table 1).
 |
Table 1 Socio-Demographic and Behavioral Characteristics of Drug-Resistant Tuberculosis MDR-TB Patients in Amhara Region, Ethiopia (N = 465) |
Clinical Characteristics of DR-TB Patients
Out of 465 MDR-TB patients 430 (92.5%) of patients had the pulmonary form of TB, of which, 118 of 465 (25%) had TB/HIV co-infection. Regarding chronic comorbidities, out of 465 patients 42 (9%) had other chronic illnesses like hypertension, Diabetes Mellitus (DM), and congestive heart failure (CHF). Moreover, 406 (87.3%) of patients had tuberculosis treatment history. Out of 465 MDR-TB patients, 419 (90.1%), 434 (93.3%), and 313 (67.3%) had cough, fever, and chest pain symptoms at presentation, respectively. Out of 465 patients, 217 (46.7%), 167 (35.9%), and 81 (17.4%) diagnosed by GeneXpert, line probe assay (LPA), and conventional culture and DST methods, respectively. About 200 (43%) of patients had comorbid conditions like COPD, bronchiectasis, and fibrotic changes (Table 2).
 |
Table 2 Clinical Characteristics of Drug-Resistant Tuberculosis MDR-TB Patients in Amhara Region, Ethiopia (N = 465) |
Treatment Delay Among MDRTB Patients
The median time to initiate DR-TB treatment was 10 days with an IQR of 2 to 44.5 days. Of all, 12.5% of patients were initiated MDR-TB treatment on the date of diagnosis. Likewise, the median time waited to initiate DR-TB treatment among patients diagnosed with GeneXpert MTB/RIF, LPA, and culture and DST were 5, 30, and 40 days, respectively. In the binary logistic regression analysis, the presence of cavitation, the extrapulmonary form of tuberculosis, and patients diagnosed with GeneXpert MTB/RIF were significantly associated with MDR-TB treatment delays. The odds of DR-TB treatment delay among patients who had cavitation was decreased by 38% (AOR = 0.62, 95% CI: 0.39, 0.96), compared to patients without cavitation. Similarly, patients with extrapulmonary form of tuberculosis had a 66% (AOR = 0.34, 95% CI: 0.14, 0.81), reduction in MDR-TB treatment delay compared to patients with pulmonary form of TB. Moreover, the odds of MDR-TB treatment delay among patients diagnosed by GeneXpert MTB/RIF was 85% (AOR = 0.15, 95% CI: 0.10, 0.24), lower compared to patients diagnosed by other methods (Table 3).
 |
Table 3 Binary Logistic Regression Model to Identify Factors Associated with Treatment Delay Among DR-TB Patients in Amhara Region, Ethiopia (N = 465) |
The Impact of GeneXpert MTB/RIF Test on Treatment Delay Among MDR-TB Patients
The result of matching estimates from a propensity score analysis showed that ATT and ATE had a significant impact on the reduction of treatment initiation among MDR-TB patients. Thus, the ATT value of 0.301 and 0.732 in treated and control groups showed that MDR-TB patients who were diagnosed by other methods like LPA, culture, and DST had a 70% higher probability of delayed treatment initiation compared to patients who were diagnosed by GeneXpert. Meanwhile, the ATE value showed that the difference between treated and control patients was −0.41. This indicates that the GeneXpert MTB/RIF test has significantly reduced the probability of treatment delay by 41% compared to matched control groups (Table 4).
 |
Table 4 Matching Estimates of GeneXpert MTB/RIF Test with Other Diagnostic Tests on Treatment Delay Among MDR-TB Patients (N = 456) |
Furthermore, Table 5 shows the mean values of each variable before and after matching in both GeneXpert tested and other laboratory tests (LPA and culture and DST) groups. A bias before and after matching was calculated for each variable, and the change in this bias has also been reported. In addition, Table 5 shows the percentage bias reduction for all matched variables after matching. Moreover, the difference between the matched pairs was evaluated using the t-test and the last column shows the significance level of the t-test. It was found that almost all covariates show that the mean difference was not significant after matching, that is, covariates were sufficiently balanced. There were significant differences between individuals in unmatched cases for around all covariates, which became insignificant after matching. As we can see from the table, the percentage of bias reduction for covariates varies from −418.3% in complications to 88.7% point in the covariate of the form of TB. The detail is presented in the table (Table 5).
 |
Table 5 Covariate Balance Check and Absolute Bias Reduction of Treatment Delay |
Discussion
Timely initiation of drug-resistant tuberculosis treatment is one of the strategies used to reduce the transmission of infection and unfavorable treatment outcomes. This study showed that the median number of days from health seeking to the initiation of TB treatment was 10 days with significant variability among techniques used to diagnose the disease. Thus, those patients diagnosed with GeneXpert MTB/RIF test had been waited for a median of 5 days to initiate MDR-TB treatment, which was lower than 30 and 40 days for LPA, culture and DST test, respectively. Thus, molecular test like GeneXpert MTB/RIF has significantly improved the time to diagnosis of MDR-TB which in turn significantly reduced treatment delay. This finding was supported by previous studies conducted in Ethiopia, South Africa, and Russia.3,6,7,20
Moreover, this study also revealed that patients who had the extrapulmonary form of TB and had cavitation were associated with lower odds of treatment delay compared to their counterparts. This finding was supported by another study.21 Thus, patients with an extrapulmonary form of TB may have less severe disease and be able to initiate their treatment in an ambulatory model of care. However, extrapulmonary TB disease has diagnostic challenges and sometimes patients start MDR-TB treatment through the decision of the PMDT panel team with no laboratory test results. Meanwhile, drug-resistant tuberculosis treated in a hospitalized model of care created a long waiting list for admission and commencement of treatment.22 Patients having findings of cavitation on the lungs from the radiological investigation were associated with lower odds of treatment delay as compared to those who had no such findings. This could be because patients with cavitation are more symptomatic, which may lead to early health-seeking. In addition, patients with cavitation findings may expectorate sputum samples with a high bacillary load that enables to detect the disease easily.17
This study also revealed that GeneXpert had an impact on the shortening of treatment initiation time after diagnosis of the disease.4,7,23 The average treatment effect on treated showed that GeneXpert MTB/RIF has significantly reduced the delayed initiation of MDR-TB treatment. Therefore, since the test could be operated by midlevel professionals at peripheral health facilities, it could be used to reduce patients travel, and sputum sample transportation to a central reference laboratory. Therefore, by reducing treatment delay we can reduce the cost of expenditure, halt the transmission of the disease and ultimately improve patient outcomes. However, the median time to initiate MDR-TB treatment in this study is unacceptably long. Therefore, to make the waiting time shorter where GeneXpert is not available, interventions such as the implementation of an electronic short message to notify laboratory results and prompt initiation of treatment shall be considered.24 This study has implications for patients, clinicians, policymakers, and other stakeholders to strengthen programmatic management of drug-resistant tuberculosis.
Strength and Limitation
This study is the first to assess the impact of GeneXpert in Ethiopia using propensity score matching (PSM) that enables measuring the average treatment effect of exposure after controlling the potential confounders. As a result, the effect of a single exposure from the observational study was assessed. However, due to the retrospective nature of the data, we missed important variables such as the patient’s economic condition and health facility-related characteristics that might have an association with treatment delay. Due to the lack of a standard cut-off point, the median point was used to classify treatment delay status; this may lead to under- or over-estimation of the treatment effect.
Conclusion
This study showed that the median time to start DR-TB treatment was ten days, with significant variation among laboratory tests. Meanwhile, the use of GeneXpert test has a strong association with reduced treatment delay among multidrug-resistant tuberculosis patients. This study finding underscores that rapid molecular tests have an indispensable role in improving the health system and leading to prompt initiation of MDR-TB treatment. Consequently, further interventions on expansion and decentralization of the GeneXpert test may hasten case detection, treatment initiation, and ultimately control of the disease.
Abbreviations
AOR, adjusted odds ratio; ATE, average treatment effect; ATT, average treatment effect among treated; ATU, average treatment on untreated; CI, confidence interval; COPD, chronic obstructive pulmonary disease; DR-TB, drug-resistant tuberculosis; DST, drug sensitivity test; LPA, line probe assay; MDR-TB, multidrug-resistant tuberculosis; MTB/RIF, Mycobacterium tuberculosis bacilli/rifampicin; NTP, National Tuberculosis Program; PMDT, programmatic management drug-resistant tuberculosis; PSM, propensity score matching.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author with a reasonable request.
Ethics Statement
Ethical approval was secured from the Institutional Review Board of the University of Gondar, College of Medicine and Health Sciences with an approval number of (Ref/no H/R/T/T1/638/09). In addition, permission letters are also from the regional health bureau and other respected organizations, and hospitals. The study was conducted in accordance to the Declaration of Helsinki. To maintain confidentiality, we collect data using codes rather than participant names. The study was based on a secondary source and collected retrospectively the ethics committee of the University of Gondar waived the requirements for patient’s consents.
Acknowledgments
We would like to thank our data collectors and the supervisor for their invaluable effort; without them, this study would not have come to be completed. Our deep gratitude also goes to clinical directors, nurses, and administrators of the hospitals.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global tuberculosis report 2020: executive summary; 2020.
2. Kibret KT, Moges Y, Memiah P, Biadgilign S. Treatment outcomes for multidrug-resistant tuberculosis under DOTS-Plus: a systematic review and meta-analysis of published studies. Infect Dis Poverty. 2017;6(1):1–8. doi:10.1186/s40249-016-0214-x
3. Iruedo J, O’Mahony D, Mabunda S, Wright G, Cawe B. The effect of the Xpert MTB/RIF test on the time to MDR-TB treatment initiation in a rural setting: a cohort study in South Africa’s Eastern Cape Province. BMC Infect Dis. 2017;17(1):1–9. doi:10.1186/s12879-017-2200-8
4. Pooran A, Theron G, Zijenah L, et al. Point of care Xpert MTB/RIF versus smear microscopy for tuberculosis diagnosis in Southern African primary care clinics: a multicentre economic evaluation. Lancet Glob Health. 2019;7(6):e798–e807. doi:10.1016/S2214-109X(19)30164-0
5. Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLoS One. 2012;7(5):e36966. doi:10.1371/journal.pone.0036966
6. Ershova JV, Volchenkov GV, Somova TR, et al. Impact of GeneXpert MTB/RIF® on treatment initiation and outcomes of RIF-resistant and RIF-susceptible TB patients in Vladimir TB dispensary, Russia. BMC Infect Dis. 2020;20(1):1–9. doi:10.1186/s12879-020-05243-9
7. Schmidt B, Geldenhuys H, Tameris M, et al. Impact of Xpert MTB/RIF rollout on management of tuberculosis in a South African community. S Afr Med J. 2017;107(12):1078–1081. doi:10.7196/SAMJ.2017.v107i12.12502
8. Castro A, Moreira AR, Oliveira J, et al. Clinical impact and cost analysis of the use of either the Xpert MTB Rif test or sputum smear microscopy in the diagnosis of pulmonary tuberculosis in Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2018;51(5):631–637. doi:10.1590/0037-8682-0082-2018
9. Shiferaw MB, Yismaw G, Getachew H. Specimen rejections among referred specimens through referral network to the Amhara Public Health Institute for laboratory testing, Bahir Dar, Ethiopia. BMC Res Notes. 2018;11(1):1–6. doi:10.1186/s13104-018-3891-7
10. Tefera KT, Mesfin N, Reta MM, Sisay MM, Tamirat KS, Akalu TY. Treatment delay and associated factors among adults with drug resistant tuberculosis at treatment initiating centers in the Amhara regional state, Ethiopia. BMC Infect Dis. 2019;19(1):1–8. doi:10.1186/s12879-019-4112-2
11. Getnet F, Demissie M, Worku A, et al. Determinants of patient delay in diagnosis of pulmonary tuberculosis in Somali Pastoralist Setting of Ethiopia: a matched case-control study. Int J Environ Res Public Health. 2019;16(18):3391. doi:10.3390/ijerph16183391
12. Mnyambwa NP, Lekule I, Ngadaya ES, et al. Assessment of GeneXpert GxAlert platform for multi-drug resistant tuberculosis diagnosis and patients’ linkage to care in Tanzania. BMC Res Notes. 2018;11(1):1–6. doi:10.1186/s13104-018-3235-7
13. Naidoo P, Du Toit E, Dunbar R, et al. A comparison of multidrug-resistant tuberculosis treatment commencement times in MDRTBPlus line probe assay and Xpert® MTB/RIF-based algorithms in a routine operational setting in Cape Town. PLoS One. 2014;9(7):e103328. doi:10.1371/journal.pone.0103328
14. Oga-Omenka C, Tseja-Akinrin A, Sen P, et al. Factors influencing diagnosis and treatment initiation for multidrug-resistant/rifampicin-resistant tuberculosis in six sub-Saharan African countries: a mixed-methods systematic review. BMJ Glob Health. 2020;5(7):e002280. doi:10.1136/bmjgh-2019-002280
15. Verstraten CC, Metzelthin SF, Schoonhoven L, Schuurmans MJ, de Man‐van Ginkel JM. Optimizing patients’ functional status during daily nursing care interventions: a systematic review. Res Nurs Health. 2020;43(5):478–488. doi:10.1002/nur.22063
16. Izudi J, Tamwesigire IK, Bajunirwe F. Treatment supporters and level of health facility influence completion of sputum smear monitoring among tuberculosis patients in rural Uganda: a mixed-methods study. Int J Infect Dis. 2020;91:149–155. doi:10.1016/j.ijid.2019.12.003
17. Zhang L, Pang Y, Yu X, et al. Risk factors for pulmonary cavitation in tuberculosis patients from China. Emerg Microbes Infect. 2016;5(1):1–11.
18. Grilli L, Rampichini C. Propensity scores for the estimation of average treatment effects in observational studies. Training Sessions Causal Inference Bristol. 2011;46(3)28–29.
19. Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314(15):1637–1638. doi:10.1001/jama.2015.13480
20. Jacobson KR, Barnard M, Kleinman MB, et al. Implications of failure to routinely diagnose resistance to second-line drugs in patients with rifampicin-resistant tuberculosis on Xpert MTB/RIF: a multisite observational study. Clin Infect Dis. 2017;64(11):1502–1508. doi:10.1093/cid/cix128
21. Mozaffari A, Kianifar R. Factors affecting delay in diagnosis and treatment of pulmonary and extra pulmonary tuberculosis in Qom Province during the 2011–2017. J Med Chem Sci. 2021;4(2):100–106.
22. Bieh KL, Weigel R, Smith H. Hospitalized care for MDR-TB in Port Harcourt, Nigeria: a qualitative study. BMC Infect Dis. 2017;17(1):1–9. doi:10.1186/s12879-016-2114-x
23. Lessells RJ, Cooke GS, McGrath N, Nicol MP, Newell M-L, Godfrey-Faussett P. Impact of point-of-care Xpert MTB/RIF on tuberculosis treatment initiation. A cluster-randomized trial. Am J Respir Crit Care Med. 2017;196(7):901–910. doi:10.1164/rccm.201702-0278OC
24. Babirye D, Shete PB, Farr K, et al. Feasibility of a short message service (SMS) intervention to deliver tuberculosis testing results in peri-urban and rural Uganda. J Clin Tuberc Other Mycobact Dis. 2019;16:100110. doi:10.1016/j.jctube.2019.100110
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.