Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
The Role of ElastPQ in Assessing Liver Stiffness for Non-Alcoholic Fatty Liver Disease in Patients Treated with Atypical Antipsychotic Drugs
Authors Sun L , Li N, Zhang L, Chen J
Received 3 March 2023
Accepted for publication 14 June 2023
Published 30 June 2023 Volume 2023:19 Pages 1491—1502
DOI https://doi.org/10.2147/NDT.S409210
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Linlin Sun,1 Nan Li,1 Ligang Zhang,2 Jingxu Chen2
1Department of Ultrasound, Peking University Huilonguan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, People’s Republic of China; 2Department of Psychiatry, Peking University Huilonguan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, People’s Republic of China
Correspondence: Jingxu Chen, Department of Psychiatry, Peking University Huilonguan Clinical Medical School, Beijing Huilongguan Hospital, Huilongguan Town, Changping District, Beijing, 100096, People’s Republic of China, Email [email protected]
Objective: To evaluate the role of elastography point quantification (ElastPQ) for the quantitative assessment of stiffness in the fatty liver disease in mental disorder patients and to provide a noninvasive detection method for non-alcoholic fatty liver (NAFLD) caused by atypical antipsychotics drugs (AAPDs).
Methods: A total number of 168 mental disorder patients treated with AAPDs and 58 healthy volunteers were enrolled in this study. All the subjects underwent ultrasound and ElastPQ tests. The basic data of the patients were analyzed.
Results: BMI, liver function, and the value of ElastPQ were considerably higher in the patient group than that in the healthy volunteers. The values of liver stiffness obtained by ElastPQ were increased gradually from 3.48(3.14– 3.81) kPa in the normal liver to 8.15(6.44– 9.88) in the severe fatty liver. The receiver operating characteristic (ROC) for the diagnosis of fatty liver with ElastPQ were 0.85, 0.79, 0.80, and 0.87 for the diagnosis of normal, mild, moderate, and severe steatosis, respectively, with a sensitive/specificity of 79%/76.4%, 85.7%/78.3%, 86.2%/73%, and 81.3%/82.1%, correspondingly. Moreover, ElastPQ in the olanzapine group was higher than those in the risperidone and aripiprazole groups (5.11(3.83– 5.61) kPa vs 4.35(3.63– 4.98) kPa, P < 0.05; 5.11(3.83– 5.61) kPa vs 4.79(4.18– 5.24) kPa, P < 0.05). After one-year treatment, the value of ElastPQ was 4.43(3.85– 5.22) kPa, but it was 5.81(5.09– 7.33) kPa in patients treated for more than three years. This value increased with treatment prolongation (P < 0.05).
Conclusion: ElastPQ is a real-time, quantitative method for assessing the stiffness of NAFLD. The liver stiffness value could be varied in the different stages of fatty liver. Olanzapine has a considerable influence on liver stiffness. The long-term use of AAPDs can increase the stiffness value of fatty liver.
Keywords: non-alcoholic fatty liver, ultrasound, elastography, antipsychotics drugs
Introduction
Atypical antipsychotics (AAPDs) such as olanzapine represent the mainstay for the treatment of schizophrenia and bipolar disorder.1 AAPDs are a diverse drug class grouped together based on their lack of extrapyramidal side effects caused by typical antipsychotics. However, the administration of AAPDs is associated with other effects, the most notable of which are weight gain and metabolic syndrome (MS). Clinically significant weight gain (> 7%),2 hyperglycemia, and hyperlipidemia often occur in more than 50% of patients receiving an atypical antipsychotic.3
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in the world.4 In China, the prevalence of NAFLD in adults was estimated to be 20.1%–29.2%, and has increased over time.5 NAFLD is closely associated with metabolic syndrome, obesity, type 2 diabetes, and dyslipidemia.6,7 As NAFLD is considered as independent hepatic manifestation of the metabolic syndrome,8 it is expected1 that NAFLD could be more common among people with mental disorders. For example, patients with schizophrenia had higher prevalence of chronic liver disease than the general population due to higher prevalence of metabolic syndrome, unhealthy lives, and antipsychotics use.9 NAFLD is divided into two major subtypes: non-alcoholic fatty liver (NAFL, also termed simple steatosis) and NASH (non-alcoholic steatohepatitis), which is the progressive form of NAFLD that can lead to cirrhosis, hepatocellular carcinoma (HCC) and liver-related mortality characterized by the presence of steatosis, ballooning degeneration, and lobular inflammation.10 Thus, the burden of NAFLD is tremendous and the prevention of the disease and the early detection of its complications are of crucial importance.
According to World Federation for Ultrasound in Medicine and Biology (WFUMB), two methods are considered reference standards in diagnosing hepatic steatosis (HS): liver biopsy (LB) and magnetic resonance imaging-proton density fat fraction (MRI-PDFF).11 Depending on the percentage of hepatocytes with fatty infiltration, a pathohistological grading system for HS has been developed. It serves as a reference for testing the accuracy of the development of non-invasive methods:12 steatosis grade 0 (normal liver) refers to a percentage of fatty transformed hepatocytes (FTH) < 5%; steatosis grade 1 (mild fatty liver) refers to a percentage of FTH within 5%–33%; steatosis grade 2 (moderate fatty liver) refers to a percentage of FTH 33%–66%; steatosis grade 3 (severe fatty liver) refers to a percentage of FTH >66%. A clinically important entity is the transition to S ≥ 2, as this significantly increases the possibility of the presence of metabolic syndrome factors and liver fibrosis (LF) progression. Risk reduction related to S < 2 regression is clinically plausible and physiologically justified.13 Liver biopsy has been commonly performed in chronic liver disease to assess liver fibrosis, and it is required for the diagnosis of NASH. However, the invasiveness of the procedure entails a risk for the patient.14
Conventional ultrasound (US) has been the most routinely used method for the assessment of HS. Furthermore, some studies suggest that non-invasive methods in patients suspected of having NAFLD can be implemented to select patients who may benefit from liver biopsy. However, its disadvantages have been revealed over the years, including the high inter-observer variability, low sensitivity for detection of mild steatosis (liver fat content < 20%) and lower accuracy in patients with liver fibrosis.15 NAFLD degree is important to know to rule out significant fibrosis (F ≥ 2). Several studies about TE were reported in NAFLD and NASH. Wong et al compared TE with liver biopsy in 246 consecutive patients with NAFLD and revealed 91% sensitivity and 75% specificity for predicting severe fibrosis (F ≥ 3), using a cut-off value of 7.9 kPa.16
Point share wave elastography (pSWE) is a recently developed method for liver stiffness assessment, which is characterized by better stability of results and a higher successful rate than those of other elastography techniques.17 This novel approach is regarded as a standardized method for the diagnosis of the fibrosis stage in patients with chronic liver disease.18 A recent study revealed that pSWE is a viable imaging technique for non-invasive staging of liver fibrosis in patients with NAFLD, especially in those with advanced cirrhosis and fibrosis.19,20 ElastPQ is an ARFI-based pSWE technique, that has been confirmed to be accurate for the staging liver fibrosis in studies performed using liver biopsy as a reference standard.21–23 However, the liver stiffness obtained by ElastPQ for assessing different stage of steatosis has been rarely reported, especially its application for the differentiation of a normal liver from a fatty liver, and for the quantitative assessment of the progression from a mild fatty liver to a severe fatty liver. Moreover, to date, only few studies have been performed with ElastPQ in mental disorder patients with NAFLD.
Therefore, the purpose of this study was to apply ElastPQ to quantitatively analyze the variation of the stiffness values in fatty liver and to provide a noninvasive method for monitoring the NAFLD in patients during their AAPDs treatment.
Materials and Methods
Subjects
All subjects were Chinese and provided written inform consent before any study-related tests were performed. All procedures were reviewed and approved by the Ethics Committee of Beijing Huilongguan Hospital. This investigation was performed in accordance with the guidelines laid out in the Declaration of Helsinki as revised.
From March 2018 to May 2020, three hundred and eighty-seven patients were diagnosed with mental disorders and received standard AAPDs treatment, regular diet, and hospital care during the hospitalization. All patients were diagnosed based on the Diagnostic and Statistical Manual (DSM-IV).24 Diagnostic assessments were completed by two independent senior psychiatrists for each patient and confirmed using the Structured Clinical Interview for DSM-IV (SCID).25 Of them, a total number of 168 patients were enrolled in this study based on the inclusion and exclusion criteria. One hundred and fifteen of 168 patients were diagnosed with schizophrenia (77 with undifferentiated schizophrenia, 28 with paranoid schizophrenia, 6 with refractory schizophrenia, and 4 with residual schizophrenia), 12 patients were with mania, 14 patients were with major depression (MD) with psychotic symptoms, 22 patients were with bipolar disorder, 3 patients were with paranoia, and acute transient mental disorder was diagnosed in 2 cases. The inclusion criteria were as follows: (1) The patient was regularly treated with AAPDs as first-line treatment during hospitalization; (2) Aged from 18 to 80 years. The exclusion criteria in this study were as follows: (1) Liver chronic disease before hospitalization, including autoimmune hepatitis, viral hepatitis, and schistosomiasis; (2) Single or multiple intrahepatic solid tumors; (3) Alcoholic hepatitis caused by long-term alcohol drinking; (4) Other diseases, such as hemopathy (eg, acute leukemia), induced liver disease, or heart failure-induced hepatomegaly, and other drug-induced liver injury; (5) Poor compliance with abdominal ultrasound examination.
In addition, volunteers were recruited through an advertisement, who were enrolled into the control group, with an age within 18–60 years during the same period. Under the supervision of a research psychiatrist, trained researchers interviewed the participants in the control group. They did not have a personal history of mental disorder disease, as assessed by the researchers using SCID, and their first-degree relatives did not have any known history of mental illness. Each people in the control group were asked for a complete medical history including routine physical examination and laboratory testing to rule out other chronic liver diseases except NAFLD. The exclusion criteria for these volunteers were the same as it described above for enrolled inpatients. Finally, fifty-eight volunteers were enrolled in this study, and all of them were defined as healthy people. The subject flowchart in this study is shown in Figure 1.
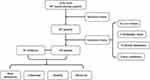 |
Figure 1 Subject flow chart of the present study. |
Clinical Measurements
The data of all subjects were collected and analyzed by the same doctor (Zhang Ligang) in this study. Gender, age, education, smoking status, height (cm), and weight (kg) were recorded. Then the body mass index (BMI) was calculated using the formula:  .
.
Medicine Treatment
All patients were treated with single or multiple AAPDs including olanzapine, risperidone, aripiprazole, clozapine, and quetiapine. The combinational treatment was defined as the treatment using more than two types of AAPDs or AAPDs combined with other antipsychotics. The treatment time was defined as the period during an AAPDs was first applied to the date that the value of ElastPQ was obtained. It ranged from 1 to 50 years with a median of 11.18(2–12) years. The treatment dose was (594.94 ± 237.50) mg·d-1 (equivalent dose of chlorpromazine).26
Laboratory Tests
After an overnight fast, samples of venous blood (5 mL) were taken from all the subjects between 6:30 and 7:00 in the morning. All the serum samples were tested in the Laboratory Department of Beijing Huilongguan Hospital. The alanine aminotransferase (ALT), aspartate aminotransferase (AST), total blood cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL) levels were tested in all subjects.
Diagnostic Ultrasonographic Fatty Liver Grading System
An iU-22 ultrasound system (Philips Medical Systems, Bothell, WA, USA) was used for the conventional ultrasound examination. The type of probe was C5-1 with a frequency of 3–5MHz. For the diagnoses of fatty liver, two senior abdominal radiologists (Linlin Sun and Nan Li, both with more than five years of liver ultrasonographic diagnostic experience), who were blinded to the patient information, made the final decision. The standard for the diagnoses of different of fatty liver degrees was based on the ultrasonographic grading system.27 The sonographic features employed for the diagnosis are presented in Figure 2.
 |
Figure 2 Schematic diagram of sonographic features for fatty liver diagnosis. |
Point Shear Wave Elastography
ElastPQ was performed immediately after the conventional ultrasound examination by an abdominal radiologist (Linlin Sun, with five-year experience in ultrasound elastography). The examiner was blinded to all clinical data during the measurements. Each patient was in the lying supine position with the right arm in maximum abduction. Then, the examinations proceeded through an intercostal approach to obtain the stiffness value of the right lobe of the liver, 1–2 cm under the liver capsule (18). Stiffness was expressed in kilopascals of young’s modulus. ElastPQ measurement box (1.5 × 0.5 cm2 in size) were positioned by the examiner in a specific region that was free of visible vessels or ducts with the real-time B-model. During the acquisition of real-time stiffness measurements, patients were instructed to hold breath. If the amount of non-shear wave motion surpassed the threshold, the calculation of liver stiffness was not performed and was present as “0 kPa”. It was regarded as invalid data when the obtained result less than 1 kPa. The failed measurement was defined as unable obtaining ten valid measurements after 15 attempts. Finally, the ElastPQ measurement mean value was calculated from 10 valid measurements in each of the subjects.
Statistical Analysis
Continuous variables are expressed as median (IQR) and categorical variables as numbers (percentages). The normality of data was tested by Kolmogorov–Smirnov (K–S test). The independent-sample t-test and the Mann–Whitney U-test were applied for normally and non-normally distributed data, respectively. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the diagnostic performance for detecting each grade of hepatic steatosis with ElastPQ, and the area under the ROC curve (AUROC) was calculated with 95% confidence intervals. Cutoff values of liver stiffness were determined to maximize the Youden index. Spearman’s test was implemented to evaluate the correlation between the ElastPQ value and age, BMI, ALT, AST, TC, TG, LDL, and treatment times. P < 0.05 was considered to be statistically significant. SPSS 27 and GraphPad Prism 8.0 statistical software was utilized for data analysis.
Results
Patient Characteristics and Examination Sets Subjected to Analysis
A total number of 168 mental disorder patients who were treated with AAPDs and 58 volunteers were included in this study. The baseline characteristics of the total number of 226 involved subjects are summarized in Table 1. The value of ElastPQ in the treatment group was 5.02(3.82–5.57) kPa, which was significantly higher than that in the control group, which was 4.01(3.58–5.47) kPa (P < 0.001).
 |
Table 1 Characteristics of the Study Population |
Diagnostic Value of ElastPQ for Fatty Liver Evaluation
In the present study 73 subjects (45 patients and 28 control subjects) had normal liver as detected by conventional ultrasound examination, and the value of stiffness obtained by ElastPQ was 3.48(3.14–3.81) kPa (patients: 3.39(3.07–3.67) kPa vs control: 3.25(3.31–4.01) kPa, P = 0.288). The values of ElastPQ in 76 mild fatty livers (53 patients and 14 controls), 70 moderate fatty livers (57 patients and 13 controls), and 16 severe fatty livers (13 patients and 3 controls) were 4.45(4.14–4.81) kPa (patients: 4.35(4.21–4.62) kPa vs controls: 4.33(3.82–4.58) kPa, P = 0.620), 5.82(5.13–6.11) kPa (patients: 5.53(5.82–6.78) kPa vs controls: 5.37(4.91–5.78) kPa, P = 0.413), and 8.15(6.44–9.88) kPa (patients: 8.50(7.08–10.93) kPa vs control: 6.6(6.1–8.1) kPa, P = 0.028), respectively. The result was showed in the Table 2. The ElastPQ value gradually increased with the rise in the fatty liver severity degree (P < 0.001) (Figure 3).
 |
Table 2 The Stiffness Value Using ElsatPQ in Different Stages of Fatty Liver |
The receiver operating characteristic curves (ROC) for ElastPQ for the diagnosis of the different fatty liver grade are illustrated in Figure 4. The area under the curve (AUC), cut-off value, sensitivity, and specificity value of ElastPQ for the diagnosis of different fatty liver grade are listed in Table 3. ElastPQ had good performance for the diagnosis of different stages of fatty liver disease. Moreover, no failed measurement occurred in this study.
 |
Table 3 The AUC, Cut-off Value, Sensitivity, and Specificity for the Diagnosis of Fatty Liver Grade |
 |
Figure 4 The receiver operating characteristic curves for ElastPQ values used for the detection and differentiation of fatty liver grades. |
Correlation of Liver Stiffness with BMI, Liver Function Test Results, and Age
We performed correlation analyses to identify the association of liver stiffness with BMI, liver function (ALT, AST, TC, TG, and LDL), and age. The results showed a good positive correlation between liver stiffness and BMI (R=0.513, P < 0.001), ALT (R=0.488, P < 0.001), AST (R=0.265, P < 0.001), TC (R=0.179, P =0.020), TG (R=0.458. P < 0.001), LDL (R=0.226, P = 0.003), and treatment time (R=0.226, P=0.028), but no obvious correlation with age (P = 0.659) in the patients. On the contrast, the value of liver stiffness has good positive correlation with BMI (R=0.325, P=0.014), ALT (R=0.379, P=0.004), and AST (R=0.449, P=0.001 in the control group.
Comparison of ElastPQ in Different Types of AAPDs and Different Therapy Times
As can be seen from the results presented in Table 4, ninety-two of the 112(82.14%) patients treated with olanzapine were diagnosed with fatty liver by ultrasound, whereas the liver in the remaining subjects was normal. The rate of fatty liver disease in the olanzapine group was higher (P < 0.05) than those in any of the groups in which other types of AAPDs were used. In addition, the mean value of ElastPQ in patients who received olanzapine was significantly higher than those in the risperidone and aripiprazole groups (5.11(3.83–5.61) kPa vs 4.35(3.63–4.98) kPa, P = 0.038; 5.11(3.83–5.61) kPa vs 4.79(4.18–5.24) kPa, P = 0.044). However, there was no obvious different in ElastPQ between olanzapine and combination groups (P > 0.05).
 |
Table 4 The Result of ElastPQ and Fatty Liver in Different Types of AAPs |
To further evaluate the variations in ElastPQ values after different treatment durations, we separated the patients into three groups based on the differences in their treatment time: less than one year, more than one year but less than three years, and more than three years. The results are presented in Table 5. The ElastPQ value after one-year treatment was 4.43(3.85–5.22) kPa, but it was 5.02(3.82–5.57) kPa in patients treated for more than three years. The value of ElastPQ increased with treatment prolongation (P = 0.041).
 |
Table 5 The Variation of ElastPQ in Different Treatment Time |
Discussion
In the present study, the patients treated in our hospital had more severe metabolic syndrome manifestations than the volunteers. The distributions of NAFLD from mild fatty liver to severe fatty liver were 31.55%, 33.93%, and 7.74%, respectively. More importantly, the liver stiffness value obtained with ElastPQ was higher than that in the volunteers, with a value gradually elevated from 3.48(3.14–3.81) kPa to 8.15(6.44–9.88) kPa during the period of changing the liver tissue from normal to steatosis. Stiffness presented a good diagnostic value for the identification and differentiation of different fatty liver stages. The distribution of fatty liver as well as the liver stiffness value in the mental disorder patients treated with olanzapine was different from those of patients treated with other AAPDs. In addition, the AAPDs treatment time influenced the liver stiffness value, presenting a positive correlation.
NAFLD is closely associated with MS, including central obesity, dyslipidemia, hypertension, hyperglycemia, and persistent abnormalities detected in liver function tests.28 In general, NAFLD is a common denominator for a broad spectrum of damage to the liver, which can be due to hepatocyte injury, inflammatory processes, and fibrosis.29 There is growing evidence linking the incidence of NAFLD with psychiatric illnesses such as schizophrenia, bipolar disorder, and depression mechanistically via the administration of genetic, metabolic, inflammatory, and psychiatric medications.30 In fact, patients prescribed with antipsychotic medications, regardless of their diagnoses, have a higher incidence of NAFLD than the normal population. The mechanistic pharmacology of antipsychotic-associated NAFLD is beginning to emerge. Furthermore, much more evidence has gradually revealed an association of the risk of MetS and AAPDs tacking including diabetes, obesity, and hyperlipidemia.31,32 In this study, we attempted to reduce the influence of other risk factors on NAFLD, including the administration of non-AAPD drugs and those of the environment. All the patients were treated with regular AAPDs during the hospital stay; unified healthy diet and hospital care were implemented for each patient. More importantly, smoking was forbidden during the hospital stay. Our results indicated that AAPDs might encourage the development of NAFLD in patients. The prevalence rates of fatty liver and DM in the treatment group were both higher than those in the control group. These findings were consistent with other results revealing that AAPDs might contribute to weight gain and liver dysfunction.33,34
Recently, the utilization of elastography techniques for the quantitative analysis of the liver fiber stage has received extensive attention.20,35 Ultrasound elastography examines the degree of deformation (stiffness) of an organ or lesion. Thus, such alterations are well manifested in the presence of hardening, fibrosis, or cirrhosis of the liver. Liver stiffness measurements using TE have shown good diagnostic performance for staging liver fibrosis and detecting cirrhosis.36 Moreover, studies on the quantitative evaluation of hepatic steatosis using controlled attenuation parameter (CAP), which can quantify the degree of ultrasound (US) beam attenuation by the tissue, have also been conducted for several years and have yielded promising results.37–40 Since steatosis and fibrosis assessment is required in NAFLD patients, a multiparametric approach using either TE with CAP is needed for a comprehensive evaluation.37,41 However, few studies have revealed the value of liver stiffness in fatty liver staging. In our study, only one parametric test was applied to assess the stage of the liver tissue. We quantitatively evaluated the stiffness value of the liver with ElastPQ and established that 3.48(3.14–3.81) kPa was the median stiffness value of a normal liver. This result was consistent with the ones of other investigations that involved healthy people with normal liver stiffness and reported similar values of 3.6 kPa42 and 3.8 kPa.43 In addition, these results also showed that the liver stiffness value in a fatty liver was significantly higher than that in a normal liver, and its increase was associated with the raised level of liver steatosis. Furthermore, our results showed that ElastPQ has a good diagnostic ability and high sensitivity and specificity for different fatty liver degrees. Furthermore, the median stiffness value in the severe fatty liver was 8.15(6.44–9.88) kPa, which was similar to the value of liver fibrosis. Additionally, the cut-off value for diagnosing a severe fatty liver was 6.4 kPa with an AUC of 0.87 and sensibility and specificity of 81.3% and 82.1, respectively. In previous research, Lee et al revealed that the values of liver stiffness for fibrosis stages from F1 to F4 were 6.9(6.0–8.6) kPa, 8.5(6.8–10.7) kPa, 9.7(6.8–10.5) kPa, and 13.0(7.4–16.6) kPa, respectively.44 The dissimilarities in the results might have been due to the differences between the stiffness in liver steatosis and liver fibrosis.45 More importantly, we found that the stiffness value of liver tissue was higher in the liver steatosis than that in normal liver tissue. With a progressively increasing stiffness value, a severe fatty liver tissue might change and progress to liver fibrosis. This result was consisted with the pathological changing. Thus, we consider that ElastPQ provides good diagnostic accuracy in stage-significant fatty liver and good ability for dynamic monitoring of the progression of liver steatosis to liver fibrosis.
To date, the risk factors influencing liver stiffness have been evaluated by several studies.46,47 But results are still in controversial. Such as Li et al reported that their findings indicated that NAFLD was significantly associated with the risk of hypertension in males than in females.48 However, their findings also need to be confirmed in future prospective studies. A recent investigation revealed that liver fibrosis was associated with multiple cardiovascular risk factors, including obesity, metabolic syndrome, diabetes, hypertension, and high-density lipoprotein cholesterol. Moreover, several studies revealed that the abnormal laboratory finding could indicate NAFLD.49,50 Consisting with the previous studies, the value of liver stiffness obtained by ElastPQ has a good positive correlation with MetS risk factors, such as BMI, ALT, AST, TC, TG, LDL, and treatment time in the patient prescribed with AAPDs. However, the liver stiffness value has positive correlation with the BMI, ALT, and AST. Base on the results, we believe that the ElastPQ could reflect the change of liver function which was similar to the laboratory test. Compared with psychotic patient, TC, TG, and LDL were not correlated with liver stiffness. The reason mainly due to the patient’s habits and AAPDs applying for a long time.51
In an earlier cross-sectional study, MetS occurred in 46.7% of patients with schizophrenia treated with AAPDs,51 whereas the morbidity of MetS change between 14% and 69% in the general population. Moreover, the incidence reached 20% after one-year AAPDs administration.52 Although NAFLD has been recognized as a part of MetS, scarce research has been focused on the correlation between NAFLD and AAPDs use in schizophrenia patients. According to recent case-control research, a higher incidence of liver disease was present in patients with schizophrenia.53 In our study, we evaluated the role ElastPQ in tests for fatty liver disease in schizophrenia patients prescribed with AAPDs, according to other acknowledged information that has previously been published.54 The results of this study revealed that olanzapine has the greatest effect on fatty liver compared those of other drugs. Meanwhile, the value of liver stiffness in the olanzapine group was higher than those in the groups treated with other AAPD drugs. Furthermore, the course of treatment influenced the liver stiffness value; a long treatment time led to a higher stiffness value. The chronic use of AAPDs may produce hepatic damage due to its toxic-free radical metabolic intermediates extensively metabolized by the liver.51
In spite of the promising results, several limitations exist in our study that need to be acknowledged. First, the absence of biopsy results was due to ethical and equipment limitations. Therefore, we applied the most common examination, grayscale ultrasound, to diagnose the different stages of fatty liver. Moreover, the progression of liver steatosis to liver fibrosis could not be completely detected by ElastPQ due to the absence of liver biopsy samples. Thus, in this study, we focused on the early effects of AAPSs on liver steatosis or liver stiffness. Second, the sample of subjects enrolled in this study was small, and olanzapine was the main type of antipsychotic medication for the enrolled patients, whereas a small number of patients received other medications. Different ElastPQ result s could have been obtained in the other treatment groups with an increase in the sample size in a further study. Third, comparative analysis was performed on a relatively small number of volunteers, because only 58 healthy participants matched the inclusion criteria in this study.
Conclusion
ElastPQ is a real-time, convenient, and quantitative detection method for assessing NAFLD liver stiffness. The fatty liver stiffness value was varied in different stages of liver steatosis. Olanzapine has a significant influence on fatty liver development as well as on liver stiffness values. The long-term use of AAPDs can increase the stiffness value of a fatty liver.
Disclosure
The authors report no conflicts of interest in this work.
References
1. De Oliveira IR, Juruena MF. Treatment of psychosis: 30 years of progress. J Clin Pharm Ther. 2006;31:523–534. doi:10.1111/j.1365-2710.2006.00784.x
2. Ahmer S, Khan RA, Iqbal SP. Association between antipsychotics and weight gain among psychiatric outpatients in Pakistan: a retrospective cohort study. Ann Gen Psychiatry. 2008;7(1):12. doi:10.1186/1744-859X-7-12
3. Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi:10.1001/jama.2009.1549
4. Diehl AM, Day C, Longo DL. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377(21):2063–2072. doi:10.1056/NEJMra1503519
5. Li Z, Xue J, Chen P, Chen L, Yan S, Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol. 2014;29(1):42–51. doi:10.1111/jgh.12428
6. Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation. 2019;103:22–27. doi:10.1097/TP.0000000000002484
7. Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28(Suppl 4):64–70. doi:10.1111/jgh.12271
8. Huang X, Liu X, Yu Y. Depression and chronic liver diseases: are there shared underlying mechanisms? Front Mol Neurosci. 2017;10:134. doi:10.3389/fnmol.2017.00134
9. Hsu JH, Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of chronic liver disease in patients with schizophrenia: a population-based cohort study. Psychosomatics. 2014;55:163–171. doi:10.1016/j.psym.2013.06.001
10. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10(11):686–690. doi:10.1038/nrgastro.2013.171
11. Ferraioli G, Berzigotti A, Barr RG, et al. Quantification of liver fat content with ultrasound: a WFUMB position paper. Ultrasound Med Biol. 2021;47(10):2803–2820. doi:10.1016/j.ultrasmedbio.2021.06.002
12. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi:10.1002/hep.20701
13. Berzigotti A. Getting closer to a point-of-care diagnostic assessment in patients with chronic liver disease: controlled attenuation parameter for steatosis. J Hepatol. 2014;60(5):910–912. doi:10.1016/j.jhep.2014.01.017
14. Gunn NT, Shiffman ML. The use of liver biopsy in nonalcoholic fatty liver disease: when to biopsy and in whom. Clin Liver Dis. 2018;22(1):109–119. doi:10.1016/j.cld.2017.08.006
15. Dietrich CF, Shi L, Lowe A, et al. Conventional ultrasound for diagnosis of hepatic steatosis is better than believed. Z Gastroenterol. 2022;60(08):1235–1248. doi:10.1055/a-1491-1771
16. Wong GL. Update of liver fibrosis and steatosis with transient elastography (Fibroscan). Gastroenterol Rep. 2013;1:19–26. doi:10.1093/gastro/got007
17. Jiang W, Huang S, Teng H, et al. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: a meta-analysis. BMJ Open. 2018;8(8):e021787. doi:10.1136/bmjopen-2018-021787
18. Argalia G, Tarantino G, Ventura C, et al. Shear wave elastography and transient elastography in HCV patients after direct-acting antivirals. Radiol Med. 2021;126(6):894–899. doi:10.1007/s11547-020-01326-4
19. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology. 2012;55(6):2005–2023. doi:10.1002/hep.25762
20. Leong WL, Lai LL, Nik Mustapha NR, et al. Comparing point shear wave elastography (ElastPQ) and transient elastography for diagnosis of fibrosis stage in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35(1):135–141. doi:10.1111/jgh.14782
21. Conti F, Serra C, Vukotic R, et al. Accuracy of elastography point quantification and steatosis influence on assessing liver fibrosis in patients with chronic hepatitis C. Liver Int. 2017;37(2):187–195. doi:10.1111/liv.13197
22. Ferraioli G, Tinelli C, Lissandrin R, et al. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787–4796. doi:10.3748/wjg.v20.i16.4787
23. Ma JJ, Ding H, Mao F, Sun HC, Xu C, Wang WP. Assessment of liver fibrosis with elastography point quantification technique in chronic hepatitis B virus patients: a comparison with liver pathological results. J Gastroenterol Hepatol. 2014;29:814–819. doi:10.1111/jgh.12479
24. Hasin D, Hatzenbuehler ML, Keyes K, Ogburn E. Substance use disorders: Diagnostic and Statistical Manual of mental disorders, fourth edition (DSM-IV) and International Classification of Diseases, tenth edition (ICD-10). Addiction. 2006;101(Suppl 1):59–75. doi:10.1111/j.1360-0443.2006.01584.x
25. Kundakci T, Sar V, Kiziltan E, Yargic IL, Tutkun H. Reliability and validity of the Turkish version of the Structured Clinical Interview for DSM-IV dissociative disorders (SCID-D): a preliminary study. J Trauma Dissociation. 2014;15:24–34. doi:10.1080/15299732.2013.821434
26. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi:10.4088/JCP.v64n0607
27. Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis. 2007;11:37–54, viii. doi:10.1016/j.cld.2007.02.014
28. Majumdar S, Thakur MB, Ran A. Comparative evaluation of serum copeptin in obesity with non alcoholic fatty liver disease. J Assoc Physicians India. 2023;71:1.
29. Liang W, Menke AL, Driessen A, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One. 2014;9:e115922. doi:10.1371/journal.pone.0115922
30. Gangopadhyay A, Ibrahim R, Theberge K, May M, Houseknecht KL. Non-alcoholic fatty liver disease (NAFLD) and mental illness: mechanisms linking mood, metabolism and medicines. Front Neurosci. 2022;16:1042442. doi:10.3389/fnins.2022.1042442
31. Beauchemin M, Geguchadze R, Guntur AR, et al. Exploring mechanisms of increased cardiovascular disease risk with antipsychotic medications: risperidone alters the cardiac proteomic signature in mice. Pharmacol Res. 2020;152:104589. doi:10.1016/j.phrs.2019.104589
32. Bicker J, Alves G, Falcao A, Fortuna A. Timing in drug absorption and disposition: the past, present, and future of chronopharmacokinetics. Br J Pharmacol. 2020;177(10):2215–2239. doi:10.1111/bph.15017
33. Kunst RF, Langlais AL, Barlow D, Houseknecht KL, Motyl KJ. Housing temperature influences atypical antipsychotic drug-induced bone loss in female C57BL / 6J mice. JBMR Plus. 2021;5(10):e10541. doi:10.1002/jbm4.10541
34. Carnagarin R, Tan K, Adams L, et al. Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD)-a condition associated with heightened sympathetic activation. Int J Mol Sci. 2021;22. doi:10.3390/ijms23010022
35. Ferraioli G, De Silvestri A, Reiberger T, et al. Adherence to quality criteria improves concordance between transient elastography and ElastPQ for liver stiffness assessment-A multicenter retrospective study. Dig Liver Dis. 2018;50(10):1056–1061. doi:10.1016/j.dld.2018.03.033
36. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–1183. doi:10.1001/jama.2020.2298
37. Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730. doi:10.1053/j.gastro.2019.01.042
38. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66(5):1022–1030. doi:10.1016/j.jhep.2016.12.022
39. Hong YM, Yoon KT, Cho M, et al. Clinical usefulness of controlled attenuation parameter to screen hepatic steatosis for potential donor of living donor liver transplant. Eur J Gastroenterol Hepatol. 2017;29(7):805–810. doi:10.1097/MEG.0000000000000876
40. de Ledinghen V, Hiriart JB, Vergniol J, Merrouche W, Bedossa P, Paradis V. Controlled Attenuation Parameter (CAP) with the XL probe of the fibroscan((R)): a comparative study with the M probe and liver biopsy. Dig Dis Sci. 2017;62:2569–2577. doi:10.1007/s10620-017-4638-3
41. Lee DH, Cho EJ, Bae JS, et al. Accuracy of two-dimensional shear wave elastography and attenuation imaging for evaluation of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2021;19(4):797–805 e797. doi:10.1016/j.cgh.2020.05.034
42. Ling W, Lu Q, Quan J, Ma L, Luo Y. Assessment of impact factors on shear wave based liver stiffness measurement. Eur J Radiol. 2013;82(2):335–341. doi:10.1016/j.ejrad.2012.10.004
43. Sporea I, Bota S, Gradinaru-Tascau O, Sirli R, Popescu A. Comparative study between two point shear wave elastographic techniques: Acoustic Radiation Force Impulse (ARFI) elastography and ElastPQ. Med Ultrason. 2014;16:309–314. doi:10.11152/mu.201.3.2066.164.isp1
44. Lee JE, Shin KS, Cho JS, et al. Non-invasive assessment of liver fibrosis with ElastPQ: comparison with transient elastography and serologic fibrosis marker tests, and correlation with liver pathology results. Ultrasound Med Biol. 2017;43:2515–2521. doi:10.1016/j.ultrasmedbio.2017.07.008
45. da Silva LCM, de Oliveira JT, Tochetto S, de Oliveira C, Sigrist R, Chammas MC. Ultrasound elastography in patients with fatty liver disease. Radiol Bras. 2020;53:47–55. doi:10.1590/0100-3984.2019.0028
46. Sirli R, Sporea I, Bota S, Jurchis A. Factors influencing reliability of liver stiffness measurements using transient elastography (M-probe)-monocentric experience. Eur J Radiol. 2013;82:e313–e316. doi:10.1016/j.ejrad.2013.03.002
47. Julian MT, Ballesta S, Pera G, et al. Abdominal obesity and dsyglycemia are risk factors for liver fibrosis progression in NAFLD subjects: a population-based study. Front Endocrinol. 2022;13:1051958. doi:10.3389/fendo.2022.1051958
48. Li XD, Qiu BH, Su FC, Sun SX. Gender impacts on the correlations between nonalcoholic fatty liver disease and hypertension in a Chinese population aged 45–60 y. Clin Exp Hypertens. 2016;38:639–643. doi:10.1080/10641963.2016.1182181
49. Zhao Y, Qiu C, Dong Y, et al. Technical acoustic measurements combined with clinical parameters for the differential diagnosis of nonalcoholic steatohepatitis. Diagnostics. 2023;13(9):1547. doi:10.3390/diagnostics13091547
50. Kong L, Yang Y, Li H, Shan Y, Wang X, Shan X. Prevalence of nonalcoholic fatty liver disease and the related risk factors among healthy adults: a cross-sectional study in Chongqing, China. Front Public Health. 2023;11:1127489. doi:10.3389/fpubh.2023.1127489
51. Platanic Arizanovic L, Nikolic-Kokic A, Brkljacic J, et al. Effects of several atypical antipsychotics closapine, sertindole or ziprasidone on hepatic antioxidant enzymes: possible role in drug-induced liver dysfunction. J Toxicol Environ Health A. 2021;84(4):173–182. doi:10.1080/15287394.2020.1844827
52. Bou Khalil R. Atypical antipsychotic drugs, schizophrenia, and metabolic syndrome in non-Euro-American societies. Clin Neuropharmacol. 2012;35:141–147. doi:10.1097/WNF.0b013e31824d5288
53. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med. 2006;21:1133–1137. doi:10.1111/j.1525-1497.2006.00563.x
54. Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010:CD006654. doi:10.1002/14651858.CD006654.pub2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

