Back to Journals » Nature and Science of Sleep » Volume 14
The Role of Dietary Inflammatory Index on the Association Between Sleep Quality and Long-Term Cardiovascular Risk: A Mediation Analysis Based on NHANES (2005–2008)
Authors Wang L, Sun M, Guo Y, Yan S, Li X, Wang X, Hu W, Yang Y, Li J, Li B
Received 11 January 2022
Accepted for publication 10 March 2022
Published 18 March 2022 Volume 2022:14 Pages 483—492
DOI https://doi.org/10.2147/NSS.S357848
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Ling Wang, Mengzi Sun, Yinpei Guo, Shoumeng Yan, Xiaotong Li, Xuhan Wang, Wenyu Hu, Yixue Yang, Jing Li, Bo Li
Department of Epidemiology and Biostatistics, School of Public Health, Jilin University, Changchun, 130021, People’s Republic of China
Correspondence: Bo Li, Department of Epidemiology and Biostatistics, School of Public Health, Jilin University, 1163 Xinmin Avenue, Changchun, 130021, People’s Republic of China, Email [email protected]
Objective: People with poor sleep quality have higher risk of cardiovascular disease (CVD), and one potential mechanism of CVD is chronic inflammation. The aim of this study was to investigate the role of dietary inflammation in the relationship between sleep quality and CVD risk.
Methods: This study involved 5594 participants from the National Health and Nutrition Examination Survey (NHANES) in 2005– 2008. Sleep quality, dietary inflammation, and 10-year CVD risk were evaluated via the Pittsburgh Sleep Quality Index (PSQI), the Energy-adjusted Dietary Inflammatory Index (E-DII), and the Framingham Risk Score (FRS), respectively. We used generalized additive model (GAM) and mediation analysis to investigate the relationship among sleep quality, 10-year CVD risk, and E-DII.
Results: PSQI had a non-linear relationship with 10-year CVD risk (P < 0.001). Meanwhile, among the participants with poor sleep quality, PSQI was positively associated with increased 10-year CVD risk (P < 0.001) and E-DII (P < 0.001). Furthermore, the association between sleep quality and CVD risk was partially mediated by E-DII, and the mediated proportion was 14.6%, and the mediating effect of E-DII varied in different gender and age groups. However, in the subjects with good sleep quality, the association among PSQI, E-DII, and 10-year CVD risk was not existed.
Conclusion: Ten-year CVD risk could be reduced by controlling the intake of inflammatory food, especially for whom with sleep disorders. In general, the reduction of inflammatory diet could weaken the effect of sleep disorders on the CVD risk.
Keywords: inflammatory diet, sleep quality, cardiovascular disease, NHANES, mediation
Introduction
Cardiovascular disease (CVD) is one of the common causes of death among adults in the United States, which includes atherosclerosis, cerebrovascular disease, and peripheral artery disease. Based on the mortality data in 2017, about 17.8 million deaths were attributed to CVD globally. Currently, the number of deaths owing to CVD have exceeded that of cancer and chronic lung disease combined.1 Therefore, the main aim of “Targeted for Prevention by Million Hearts 2022” is to prevent the occurrence of 1 million heart attacks, strokes, and other cardiovascular incidents.2 Noteworthily, Framingham Risk Score (FRS), constructed by D’Agostino et al, which could quantify 10-year CVD risk and helps identify people of high risk of CVD.3
Nowadays, many adults suffer from sleep-related problems. Previous studies have indicated that 50 to 70 million US adults have chronic sleep and wake disorders.4 Poor sleep quality was proved to be associated with many negative health outcomes, including CVD.5,6 Sleep deprivation or sleep disorder may play a role in the development of cardiometabolic disease.7 It is confirmed that both short and long sleep duration have been related to coronary heart disease (CHD) morbidity or mortality and stroke.8 Meanwhile, current studies have shown that levels of various markers of inflammation were associated with cardiovascular risk elevated, and inflammation could mediate the relationship between sleep quality and sleep-related diseases.9
The levels of inflammation in the body might be greatly influenced by diet, previous studies have confirmed that a pro-inflammatory diet could increase the body’s inflammatory burden.10 The dietary inflammatory index (DII) was a literature-based tool developed by Shivappa, which designed to evaluate the potential inflammatory levels of dietary components and examine the association between food consumption and inflammation.11 A systematic review suggested that more consumption of healthy diet might be associated with better sleep quality and highlighted the potential role of inflammation in the relationship between diet and sleep quality.12 Moreover, some studies indicated that pro-inflammatory diet was associated with higher risk of cardiovascular clinical events.13 A meta-study showed that each 1-point increase in DII score was associated with an 8% increase in CVD risk and mortality, confirming that an anti-inflammatory diet plays an important role in preventing CVD.14 Besides, some literatures have shown that higher DII scores were related to poorer sleep quality.15,16 However, few studies have evaluated the relationship among DII, sleep quality, and the risk of CVD. Therefore, we aim to investigate whether DII plays a mediating role in the relationship between sleep quality and CVD risk.
Materials and Methods
Study Design and Participants
The data of our study was from the National Health and Nutrition Examination Survey (NHANES), which was a stratified multistage research program designed to assess the health status of the US non-institutionalized civilian population, conducted by the Centers for Disease Control and Prevention (CDC). Based on the 2005 to 2008 NHANES databases, this survey utilized a complex probability sampling design, and collected information by standardized interviews, physical examinations.17 All surveys were reviewed and approved by the CDC and Prevention National Center for Health Statistics Research (NCHS) Ethics Review Board, and all included participants have provided written informed consent.
A total of 20,497 participants were included in 2005–2008 of NHANES. We selected subjects who met the following inclusion criteria: participants aged 30 years and above, who provided information on sleep quality and CVD risk, and completed the dietary interview about the total nutrient intakes. Meanwhile, participants with CVD (congestive heart failure, coronary heart disease, angina pectoris, heart attack and stroke), extreme diet data (total energy intakes of <500 or >5000 kcal/day for females and <500 or >8000 kcal/day for males) and absence of covariates were excluded.
Cardiovascular Disease Risk
CVD risk was estimated via the Framingham risk score (FRS), which was predicted 10-year general CVD risk and risk of individual CVD events.3 This algorithm was used to assess 10-year CVD risk based on gender, age, diabetes status (diabetes was defined by self-report of a physician diagnosis), smoking status (smoking was defined as current smokers, and non-smoking was defined as people who never smoke and former smokers), treated (taking prescribed medicine for hypertension) and untreated hypertension, systolic blood pressure (SBP), total cholesterol (TC), and high-density lipoprotein (HDL) cholesterol. We used the total FRS points based on the above process and expressed as percent of FRS (FRS%). Specially, FRS% were divided into three risk levels: low (<10%), moderate (10–19%), and high (≥20%).
Assessment of Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) was developed to assess the quality and patterns of sleeping in adolescents or adults.18,19 In NHANES 2005–2008, eight self-reported items were performed to evaluate PSQI. Based on analyzing the score of PSQI in sleep latency, sleep disturbance and daytime dysfunction, the sleep quality could be divided into “poor” from “good”. Sleep latency was measured using the following two items: (a) “How long does it usually take you to fall asleep at bedtime (minutes)?”, and (b) “In the past month, how often did you have trouble falling asleep?”. Sleep disturbances were measured using the following two items: (a) “In the past month, how often did you wake up during the night and have trouble getting back to sleep?” and (b) “In the past month, how often did you wake up early in the morning and was unable to get back to sleep?”. Meanwhile, daytime dysfunction was measured using the following two items: (a) “In the past month, how often did you feel unrested during the day, no matter how many hours of sleep you had?” and (b) “In the past month, how often did you feel excessively or overly sleepy during the day?”. Under normal situation, the scores on time to fall asleep was categorized as follows: ≤15 minutes (score of 0), 16–30 minutes (score of 1), 31–60 minutes (score of 2), and ≥60 minutes (score of 3); And possible answers to the other questions were as follows: never-0 time a month (score of 0), rarely-1 time a month (score of 1), sometimes-2 to 4 times a month (score of 2), often-5 to 15 times a month (score of 3), and almost always-16 to 30 times a month (score of 4). A total score of PSQI (range: 0–23) was calculated via adding scores of each part (latency, disturbances, and daytime dysfunction). A score of greater than 5, defined as poor sleep quality, and a higher score indicated poorer sleep quality.
Energy-Adjusted Dietary Inflammatory Index (E-DII)
All dietary data for NHANES 2005–2008 participants were obtained based on the average of two 24-hour dietary recall interviews. The calculation of DII was based on the standardized z-scores.11 Each participant’s dietary data was linked to the global database which provided a robust estimate of a mean intake and a standard deviation for each of the 45 food parameters. These parameters then were used to derive the participant’s exposure relative to the standard global mean as a z-score, derived by subtracting the mean of the energy-adjusted regionally representative database from each food parameter and dividing this value by the parameter’s standard deviation. To minimize the effect of “right skewing”, these z-scores were converted to the centered proportion score as multiplied by 2 and subtracting 1. To acquire the food parameter-specific DII score, the final value was multiplied by its respective overall food parameter score. All these food parameter-specific E-DII scores were then summed to create the overall E-DII score for each subject in the study. Importantly, even if the number of nutrients applied for the calculation of E-DII is less than 30, the E-DII score is still available.11 In this study, based on the 45 possible food items in DII list, 26 food parameters including protein, carbohydrate, alcohol, vitamins B12/B6, β-carotene, caffeine, cholesterol, total fat, fiber, folic acid, Fe, Mg, Zn, Se, MUFA, niacin, n-3 fatty acids, n-6 fatty acids, PUFA, riboflavin, saturated fat, thiamin, vitamins A/C/E, were finally applied to compute the E-DII.
Sociodemographic and Health-Related Variables
The following data were extracted from the NHANES database: Sociodemographic variables including age, gender, ethnicity (ie, Mexican American, Non-Hispanics White, Non-Hispanics Black, other Hispanics and other races) and marital status; Body mass index (kg/m2) and waist circumference (cm); Smoking status was divided as never (smoked less than 100 cigarettes in life and not currently smoking), former smoker (smoked at least 100 cigarettes in life and not currently smoking), current smoker (smoked at least 100 cigarettes in life and currently smoking);20 Physically active classified into “yes” or “no” based on those question “Did you do moderate activities for at least 10 minutes over past 30 days?” and some moderate activities such as brisk walking, bicycling for pleasure, golfing, dancing, and some moderate work.21
Statistical Analysis
To obtain population-representative characteristics, weighted means, and standard errors (SEs) were calculated to describe continuous variables. Meanwhile, weighted proportions and SEs were used to describe the categorical variables. Kruskal-Wallis test and chi-square test were performed for the comparison, respectively. Generalized additive model (GAM) is an extension of the generalized linear model (GLM), which allows the evaluation for the non-linear relationship of the outcome and the predictors.22 Two variables represented a linear relationship when the estimated degree of freedom (EDF) in GAM was equal to one. GAM was used it to explore the associations between PSQI, E-DII, and 10-year CVD risk. All statistical analyses were performed via R 4.0.5 statistical software and the package “gam”,23 and “survey”.24
The mediation analyses were carried out using the Mplus 8.3. To investigate whether the E-DII scores mediated the relationship between sleep quality and 10-year CVD risk, three pathways (a, b and c) were used to assess the mediation (Figure 1). Total effect evaluated the relationship between PSQI (exposure) and 10-year CVD risk (outcome). Path a was used to assess the relationship between PSQI and E-DII (mediator). Path b was conducted to evaluate the relationship between E-DII (mediator) and 10-year CVD risk (outcome). The influence of E-DII on the link between PSQI and 10-year CVD risk were assessed through path c (direct effect).
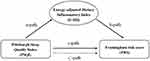 |
Figure 1 Path diagram of the mediation analysis models. |
The proportion of the mediated effect was calculated using the following formula: (mediated effect/total effect) × 100%. Bootstrapping was used for significance testing for mediation analysis. Significance level was set at α= 0.05, all tests were two-sided.
Results
General Characteristics
According to the specified inclusion and exclusion criteria, 5594 subjects were selected from a total of 20,497 survey participants in the analysis (Figure 2).
 |
Figure 2 Flow chart for study participants selection. Abbreviations: PSQI, Pittsburgh Sleep Quality Index; E-DII, Energy-adjusted Dietary Inflammatory Index; FRS, Framingham Risk Score. |
Based on 10-year CVD risk levels, the general characteristics of study participants weighted to the US population are depicted in Table 1. Statistically significant differences were observed among different CVD risk in age, gender, race, BMI, WC, energy intake, smoking status, physically active, E-DII and PSQI scores of subjects (P<0.05). Furthermore, there were also were statistically significant differences among sleep quality and different 10-year CVD risk group participants (P=0.001).
 |
Table 1 Characteristics of Subjects from the National Health and Nutrition Examination Survey (2005–2008) |
The FRS was regarded as a response in model 1 of GAM, and a smoothing spline function of PSQI and E-DII was viewed as predictor with adjustments (Figure 3A and B). Statistically significant non-linear associations were found in PSQI with FRS (P<0.001). The FRS was lowest when the PSQI score was 6, and the risk of CVD was increased with the score of PSQI for subjects with poor sleep quality (PSQI>5). Furthermore, a smooth curve was shown between PSQI and E-DII after regulating other variables, and a significant effective degree of freedom (EDF) (P<0.001) indicating a non-linear association (U-shape) between PSQI and E-DII (Figure 3C).
Mediation Analysis of E-DII
Based on the above analysis, we divided the subjects into two subgroups: good sleep quality (PSQI≤5) and poor sleep quality (PSQI>5). And subsequent mediation analysis was followed the steps shown in Figure 1. All mediation analyses were performed based on adjusting sociodemographic variables (age, gender and race), health behaviors (physical activity, smoking status), BMI, WC and energy intake.
As shown in Table 2, in the subjects with poor sleep quality, PSQI was positively associated with 10-year CVD risk (P<0.001) and E-DII (P<0.001). Meanwhile, E-DII was also positively associated with 10-year CVD risk (P<0.001). It was estimated that 14.6% of the total association between PSQI with 10-year CVD risk was mediated by E-DII. However, in the subjects with good sleep quality, the relationship in Figure 1 was not existed.
 |
Table 2 Mediation Effect of the E-DII on the Association Between PSQI and Cardiovascular Disease Risk |
Considering the effects of gender and age, we conducted a subgroup analysis of subjects with poor sleep quality in Table 3. In males, while the direct effects of PSQI on 10-year CVD risk were not significant (P=0.067), the total effects and indirect effects of PSQI on 10-year CVD risk were significant (total effects P=0.022; indirect effects P=0.015). E-DII completely mediated the effect of PSQI on 10-year CVD risk. In Females, E-DII mediated 14.3% of the total association between PSQI and 10-year CVD risk, similar to previous results. Noteworthy, in the subgroup analysis of age groups, the association between PSQI and 10-year CVD risk was partially mediated by E-DII in subjects younger than 60 years, but not in subjects ≥60 years.
 |
Table 3 Mediation Effect of the E-DII on the Association Between PSQI and Cardiovascular Disease Risk by Gender and Age Group Stratification in Subjects with Poor Sleep Quality |
Discussion
In the present study, we evaluated the associations between sleep quality and 10-year CVD risk, and the mediating role of E-DII in this relationship. The main findings of our study are as following: Firstly, PSQI had a non-linear relationship with 10-year CVD risk, E-DII had a linear association with 10-year CVD risk, and there was a U-shaped association between PSQI and E-DII. Secondly, we stratified the subjects based on sleep quality for further analysis. In the subjects with poor sleep quality, PSQI was associated with an increased risk of CVD, and also positively related to E-DII. Thirdly, the association between sleep quality and 10-year CVD risk was mediated by E-DII, and the proportion of mediation was 14.6%. Fourthly, the mediating effect of E-DII varied in different gender and age groups.
Currently, in addition to sleep duration, sleep disorders and insomnia have also been reported to be associated with CVD risk.6 Meanwhile, studies have indicated that multiple sleep parameters need to be assessed comprehensively when evaluating the association between sleep and health outcomes.7 In our study, the PSQI was calculated based on 8 self-reported items, which reflected the overall sleep quality of the included subjects. At the outset, association between PSQI and 10-year CVD risk was found in all subjects. Specially, we found a non-linear relationship between PSQI and CVD. Subsequently, a positive correlation between them was found in subjects with poor sleep quality. But in subjects with good sleep quality, the above relationship was not existed.
Previous research has indicated that disturbances of the physiologic processes during sleeping could increase the risk of CHD.25 Sleep disturbances makes the organism unable to reduce the sympathetic stimulation to the cardiovascular system at night, thereby preventing the body from rest and restoring energy.26 And we speculate that this series of physiological changes may not exist in people with good sleep quality. In addition, sleep disorders can also promote inflammation in the cardiovascular vessels, and inflammation not only promotes the blood vascular injury but also the occurrence of atherosclerosis, which was the main cause of CVD.27–29
Diet has been confirmed to regulate inflammation in the body.30 Current epidemiological evidence has confirmed that the consumption of sugary beverages, refined grains, red meat, margarine and other foods were associated with increasing levels of inflammation markers. Correspondingly, anti-inflammatory diets could reduce the levels of inflammatory markers.31 And DII, as a tool to quantify dietary inflammation, has been shown to be associated with a variety of diseases, especially for CVD.32,33 Diet could influence the activation of the immune system through the gut microbiota. Specially, as a component of the gut microbiota, previous studies have indicated that lipopolysaccharide (LPS) was a major inducer of the inflammatory responses.34,35 Interestingly, LPS does not have negative effects on the body under normal circumstances.36 However, some factors such as the high-fat diets can promote the transfer of LPS into the circulatory system, and induces an immune response and ultimately leads to a subclinical inflammatory status.37 Furthermore, inflammation can affect the cardiovascular system through oxidative stress and foam cell formation.38,39 We hypothesized that controlling inflammation in the body through an anti-inflammatory diet could reduce cardiovascular inflammation caused by sleep disturbances, thereby reducing the risk of CVD.
Sleep disorders were generally more prevalent in women and older adults.40 A study found an association between sleep disturbances and the risk of acute coronary events in women but not men.41 Interestingly, in our subgroup analysis, we had similar findings that there was no direct correlation between sleep quality and CVD risk in men, but the complete mediating role of E-DII in sleep quality and CVD risk may providing a new insight for the prevention of CVD in men. Furthermore, in our subgroup analysis, we found that sleep quality in older adult ≥60 years was not associated with CVD risk. We had similar findings in a study that, after controlling for variables, sleep problems in older adults were not associated with the risk of myocardial infarction events.42 This finding indicated that sleep may not be the focus of CVD prevention in older adults.
A recent study that examined the burden of CVD in US states found that the dietary risk was primary risk factor for most CVD.43 According to the survey of NHANES from 2005 to 2010, healthy diet was the most difficult to achieved in all 7 heart-healthy behaviors. Only 22% of adults had a healthy diet.44 Our study found that DII significantly mediated the relationship between sleep quality and 10-year CVD risk. And these results remind us that improving dietary health and controlling dietary inflammation effectively are beneficial to prevent CVD, especially for those with sleep disorders. Besides, our study speculates that sleep quality, DII, and CVD risk lack correlations in subjects with good sleep quality.
Some limitations were existed in our study. Firstly, data about sleep quality and diet in this study were self-reported, which might be subjected to recall bias. Secondly, our results require a larger sample size to verify. Finally, the cross-sectional design of NHANES limited the extrapolation of causality in the study. Therefore, prospective studies and interventional trials were needed to enlighten the mediation effect of inflammatory dietary burden on the relationship between sleep quality and CVD risk.
Conclusions
PSQI had a non-linear relationship with 10-year CVD risk. For subjects with poor sleep quality, PSQI was positively associated with increased risk of CVD and E-DII. Specially, 14.6% of the association between sleep quality and 10-year CVD risk was mediated by E-DII, and the mediating effect of E-DII varied in different gender and age groups. Further research is essential to confirm these results in prospective design and explore the mechanism of the associations.
Data Share Statement
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at https://wwwn.cdc.gov/nchs/nhanes/.
Ethics Approval and Consent to Participate
The NCHS Research Ethics Review Committee approved the NHANES survey protocol (https://www.cdc.gov/nchs/nhanes/irba98.htm), and all participants of the study provided informed written consent.
Consent for Publication
All authors have seen and approved the final version of the manuscript being submitted. This work was an original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in per part.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No.81973129).
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi:10.1161/CIR.0000000000000757
2. Ritchey MD, Wall HK, Owens PL, et al. Vital signs: state-level variation in nonfatal and fatal cardiovascular events targeted for prevention by million hearts 2022. Morb Mortal Wkly Rep. 2018;67(35):974–982. doi:10.15585/mmwr.mm6735a3
3. D’Agostino RB
4. Centers for Disease Control and Prevention. Perceived insufficient rest or sleep among adults - United States 2008. Morb Mortal Wkly Rep. 2009;58(42):1175–1179.
5. Yan LJ, Xie YJ. Associations between sleep quality and 10-year cardiovascular disease risk among female nurses in Hong Kong: a cross-sectional study. J Cardiovasc Nurs. 2021. doi:10.1097/JCN.0000000000000857
6. Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. doi:10.1016/j.beem.2010.07.001
7. Brindle RC, Yu L, Buysse DJ, et al. Empirical derivation of cutoff values for the sleep health metric and its relationship to cardiometabolic morbidity: results from the Midlife in the United States (MIDUS) study. Sleep. 2019;42(9). doi:10.1093/sleep/zsz116
8. Cappuccio FP, Cooper D, D’Elia L, et al. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. doi:10.1093/eurheartj/ehr007
9. Meng LL, Tang Y-Z, Ni C-L, et al. Impact of inflammatory markers on the relationship between sleep quality and incident cardiovascular events in type 2 diabetes. J Diabetes Complications. 2015;29(7):882–886. doi:10.1016/j.jdiacomp.2015.06.011
10. Johansson-Persson A, Ulmius M, Cloetens L, et al. A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur J Nutr. 2014;53(1):39–48. doi:10.1007/s00394-013-0496-8
11. Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi:10.1017/S1368980013002115
12. Godos J, Grosso G, Castellano S, et al. Association between diet and sleep quality: a systematic review. Sleep Med Rev. 2021;57:101430. doi:10.1016/j.smrv.2021.101430
13. Garcia-Arellano A, Ramallal R, Ruiz-Canela M, et al. Dietary inflammatory index and incidence of cardiovascular disease in the PREDIMED study. Nutrients. 2015;7(6):4124–4138. doi:10.3390/nu7064124
14. Shivappa N, Godos J, Hébert J, et al. Dietary inflammatory index and cardiovascular risk and mortality-A meta-analysis. Nutrients. 2018;10(2):200. doi:10.3390/nu10020200
15. Bazyar H, Zare Javid A, Bavi Behbahani H, et al. The association between dietary inflammatory index with sleep quality and obesity amongst Iranian female students: a cross-sectional study. Int J Clin Pract. 2021;75(5):e14061. doi:10.1111/ijcp.14061
16. Godos J, Ferri R, Caraci F, et al. Dietary inflammatory index and sleep quality in Southern Italian adults. Nutrients. 2019;11(6). 1324.
17. US Census Bureau. Current Population Survey (CPS) - definitions and explanations; 2008.
18. Vezina-Im LA, Nicklas TA, Baranowski T. Associations among sleep, body mass index, waist circumference, and risk of type 2 diabetes among U.S. childbearing-age women: national health and nutrition examination survey. J Womens Health. 2018;27(11):1400–1407. doi:10.1089/jwh.2017.6534
19. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
20. Strozyk D, Gress TM, Breitling LP. Smoking and bone mineral density: comprehensive analyses of the third National Health and Nutrition Examination Survey (NHANES III). Arch Osteoporos. 2018;13(1):16. doi:10.1007/s11657-018-0426-8
21. Wei Y, Zhu J, Nguyen A. Urinary concentrations of dichlorophenol pesticides and obesity among adult participants in the U.S. National Health and Nutrition Examination Survey (NHANES) 2005–2008. Int J Hyg Environ Health. 2014;217(2–3):294–299. doi:10.1016/j.ijheh.2013.07.003
22. Hastie T, Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res. 1995;4(3):187–196. doi:10.1177/096228029500400302
23. Hastie T. Gam: generalized additive models. R package version 1.20; 2020.
24. Lumley T. survey: analysis of complex survey samples. J Stat Softw. 2014;9. 1–9
25. Chandola T, Ferrie JE, Perski A, et al. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33(6):739–744. doi:10.1093/sleep/33.6.739
26. Burgess HJ, Trinder J, Kim Y, et al. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol. 1997;273(4):H1761–H1768. doi:10.1152/ajpheart.1997.273.4.H1761
27. Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22(6):960–968. doi:10.1016/j.bbi.2008.01.011
28. Ross R, Epstein FH. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi:10.1056/NEJM199901143400207
29. Páramo JA, Rodríguez JA, Orbe J. [Atherosclerosis in inflammatory diseases]. Med Clin. 2007;128(19):749–756. Italian. doi:10.1157/13106130
30. Salas-Salvadó J, Garcia-Arellano A, Estruch R, et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. 2008;62(5):651–659. doi:10.1038/sj.ejcn.1602762
31. O’Neil A, Shivappa N, Jacka FN, et al. Pro-inflammatory dietary intake as a risk factor for CVD in men: a 5-year longitudinal study. Br J Nutr. 2015;114(12):2074–2082. doi:10.1017/S0007114515003815
32. Ramallal R, Toledo E, Martínez JA, et al. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: the SUN cohort. Obesity. 2017;25(6):997–1005. doi:10.1002/oby.21833
33. Tyrovolas S, Koyanagi A, Kotsakis GA, et al. Dietary inflammatory potential is linked to cardiovascular disease risk burden in the US adult population. Int J Cardiol. 2017;240:409–413. doi:10.1016/j.ijcard.2017.04.104
34. Asadi Z, Yaghooti‐Khorasani M, Ghazizadeh H, et al. Association between dietary inflammatory index and risk of cardiovascular disease in the Mashhad stroke and heart atherosclerotic disorder study population. IUBMB Life. 2020;72(4):706–715. doi:10.1002/iub.2172
35. Moreira AP, Texeira TFS, Ferreira AB, et al. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108(5):801–809. doi:10.1017/S0007114512001213
36. Bayston KF, Cohen J. Bacterial endotoxin and current concepts in the diagnosis and treatment of endotoxaemia. J Med Microbiol. 1990;31(2):73–83. doi:10.1099/00222615-31-2-73
37. Laugerette F, Vors C, Géloën A, et al. Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem. 2011;22(1):53–59. doi:10.1016/j.jnutbio.2009.11.011
38. Crowley SD. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid Redox Signal. 2014;20(1):102–120. doi:10.1089/ars.2013.5258
39. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34(3):509–515. doi:10.1161/ATVBAHA.113.300156
40. Vgontzas AN, Kales A. Sleep and its disorders. Annu Rev Med. 1999;50:387–400. doi:10.1146/annurev.med.50.1.387
41. Meisinger C, Heier M, Löwel H, et al. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30(9):1121–1127. doi:10.1093/sleep/30.9.1121
42. Schwartz SW, Cornoni-Huntley J, Cole SR, et al. Are sleep complaints an independent risk factor for myocardial infarction? Ann Epidemiol. 1998;8(6):384–392. doi:10.1016/S1047-2797(97)00238-X
43. Roth GA, Johnson CO, Abate KH, et al. The burden of cardiovascular diseases among US states, 1990–2016. JAMA Cardiol. 2018;3(5):375–389. doi:10.1001/jamacardio.2018.0385
44. Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307(12):1273–1283. doi:10.1001/jama.2012.339
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

