Back to Journals » International Journal of General Medicine » Volume 16
The Role of Combined Inflammatory Biomarkers in the Diagnosis of High- and Low-Virulence FRI Among High-Risk Lower Extremity Fractures
Authors Xu X, Wang H, Liu Y, Wang D , Diao S, Gao Y, Zhou J
Received 4 July 2023
Accepted for publication 1 August 2023
Published 8 August 2023 Volume 2023:16 Pages 3363—3371
DOI https://doi.org/10.2147/IJGM.S426608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Xiaopei Xu,* Hanzhou Wang,* Yang Liu, Dong Wang, Shuo Diao, Yuling Gao, Junlin Zhou
Department of Orthopedic Surgery, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junlin Zhou, Department of Orthopedic Surgery, Beijing Chaoyang Hospital, Capital Medical University, 8 Gongren Tiyuchang Nanlu, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel/Fax +86-1085231227, Email [email protected]
Objective: The aim of this study is to evaluate the diagnostic accuracy of infection-related biomarkers in high-risk lower limb injury patients with fracture-related infection (FRI) caused by high-/low-virulence microorganisms.
Methods: This study was a retrospective analysis of patients with high-risk lower extremity fractures (including tibial plateau, calcaneus, and Pilon fractures) who underwent open reduction internal fixation (ORIF) surgery from January 2017 to February 2022. Peripheral blood samples were collected within 24 hours of admission, and the following information was evaluated: gender, age, BMI, smoking, comorbidities, injury information, surgical details, values for serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), as well as neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR).
Results: A total of 576 patients receiving lower extremity fracture surgery were included in this study. Fifty-one patients (8.85%) were identified as FRI, and 28 (54.9%) of these 51 cases were further classified as high-virulence group. The median levels of CRP, ESR, NLR, and MLR were significantly higher in the FRI group than in the non-FRI group (p < 0.01). Similarly, the marginally significantly higher levels of CRP and NLR presented in the high-virulence group, compared to the low-virulence group (p < 0.1). The AUC areas of CRP, NLR, and CRP+NLR were 0.826, 0.650, and 0.873, respectively. We calculated the optimal cut-off points for CRP+NLR as diagnostic markers of high-virulent infection was 0.377.
Conclusion: This study showed the incidence of FRI in high-risk lower extremity fractures was 8.9%, and identified preoperative serum biomarkers, including CRP, ESR, NLR, and PLR, as useful tools for assisting in the diagnosis of infection. Additionally, the combination of CRP with NLR played a discriminating clinical role in postoperative infections caused by different virulence.
Level of Evidence: Clinical study.
Keywords: high-risk lower extremity fractures, fracture-related infection, high- / low-virulence, serum inflammatory biomarkers, receiver operating characteristic
Introduction
Postoperative infection after orthopedic surgery is a serious complication, which affects the therapeutic effect of clinical functions. Moreover, infection prolongs the hospitalization time and costs abundant economic consequences for patients.1 Especially, for some high-risk lower extremity fractures (including the tibial plateau, calcaneal, and Pilon fractures), the infection rate has reached 16% even if multi-stage and antibiotic treatments are performed.2 In order to standardize clinical research and improve the quality of published literature, the consensus definition of fracture-related infection (FRI) was achieved under an expert group consisting of scientific and medical organizations.3
Hence, early diagnosis and treatment of FRI among high-risk fractures are particularly important. At present, laboratorial inflammatory markers, such as white blood cell count (WBC), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), are economical, handy and widely available to present preoperative information. In addition, previous studies recommended preoperative neutrophil lymphocyte ratio (NLR), monocyte lymphocyte ratio (MLR) or platelet lymphocyte ratio (PLR) was associated with high probabilities of infections.4,5 However, these biomarkers seem to be short of sensitivity and identification when the microbiological result shows the existence of single low-virulence organisms from sinus drainage or operative proceed.6–8 In fact, the varied potential for pathogenicity microorganisms might cause different levels of host inflammatory response and clinical manifestation.
Until now, relevant clinical reports in orthopedic trauma remained blank. Therefore, our study aims to evaluate the diagnostic accuracy of infection-associated biomarkers for FRI caused by microorganisms with high-/low-virulence, among patients with high-risk lower limb injuries.
Materials and Methods
Study Design
With institutional review board approval, the operation performed by the same expert team was retrospectively reviewed for all patients undergoing open reduction and internal fixation (ORIF) from January 2017 to February 2022.
Inclusion criteria for enrollment were as follows (1) Over 18 years old; (2) Imaging diagnosis of a tibial plateau/ calcaneus / Pilon fracture; (3) Conforming to the definition of high-risk lower extremity fractures, which involved with a delayed definitive treatment at >3 days after the injury/a multistage treatment due to a soft tissue problem;9,10 (4) follow-up more than a year. Exclusion criteria included (1) Open or pathologic fractures at the surgical site; (2) Incomplete medical records; (3) Evacuation from this study during follow-up (Figure 1).
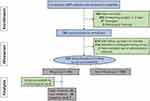 |
Figure 1 Flow diagram of the study design. |
Perioperative Treatments
Within 24 hours after admission, all peripheral venous blood from patients were collected and then transported for laboratorial testing within an hour. All blood samples underwent anticoagulated and processed in a blood analyze machine in our institution for the blood cell counts, differential counts of leukocytes, serum ESR and CRP. We also calculated the NLR, PLR and MLR, which was defined as the ratio of the neutrophil, platelet and monocyte count to lymphocyte count, retrospectively.
After admission, patients underwent anticoagulant therapy with 4100 u (0.4 mL) of nadroparin calcium per day until the operation and the subsequent treatment involving the same dosage after wound closure. A routine ultrasound examination of both lower limbs before days 10 were needed or earlier if there was any clinical suspicion of thrombotic event.
All patients received prophylactic use of 1.5 to 3 g of cefuroxime on the basis of weight within 2 hours preoperatively (clindamycin if allergic to penicillin). Surgical technique including temporary external fixations (if necessary) and ORIF was performed by a same professional team. Worth mentioning that all patients were instructed to follow the same principle on wound care and physical exercises.
The following clinical data for each patient was extracted from electronic records: gender, age, BMI, smoking comorbidities including hypertension, diabetes mellitus and coronary heart disease, injury site, injury type, injury severity score (ISS), time to surgery, ASA classification and surgical details (blood loss, operative time and drainage use).
Participants Allocation
During follow-ups, we referred the confirmatory FRI, which further described in the previous study by Goormans et al.11 FRI consisted of postoperative infections within one year that met at least 1 of the following conditions: (1) Clinical signs including the presence of a fistula, sinus, wound breakdown or purulent exudation, (2) Confirmed pathogens by culture results from at least two separate deep tissue/implant specimens during paracentesis or operations. Based on this, patients were separated into two groups: the FRI group and the non-FRI group.
For further analysis, each FRI case was taken at least two microbiological specimens from distinct suspected sites according to standardized guidelines.12 Of note, every sample was stored in a new sterile instrument each time and sent instantly with retrieved implants to the laboratory within 30 minutes after execution.
In all FRI cases, the pathogens were separated in high-virulence pathogens: Staphylococcus aureus (including methicillin-resistant Staphylococcus aureus), Pseudomonas, Enterococci, Streptococci and Enterobacteriaceae and low-virulence pathogens: Propionibacterium, coagulase-negative Staphylococci and Cut bacterium spp.
Polymicrobial infection combined with high- and low-virulence pathogens was assigned to the high-virulence group. If microbiological results presented negative, cases can be determined based on clinical features such as the type of surgical incision, antibiotics used before surgery, and clinical symptoms.
Statistical Analysis
This study was analyzed using statistics software SPSS (version 26.0, Chicago, IL). The Shapiro–Wilk test was used to evaluate the continuous variables of a normal distribution. Descriptive data were presented as mean ± standard deviation (SD) for normally distributed continuous variables or median and interquartile range (IQR) for non-normally distributed data, and frequency (percentage) for enumeration variables. The statistical differences for continuous data between or among two were compared using the independent sample t-test, while the Mann–Whitney U-test was used to analyze the numerical variables with non-normal distribution or unequal variance. The chi-squared test and Fisher’s exact test (if any variable less than 5) were used to analyze the enumeration data.
Then, we generated receiver operating characteristic curves to evaluate the clinical role of combined indicators. In order to create a more considerable and adequate model, we introduced the clinical factors which p value less than 0.1 among the high- and low-virulence comparison. The sensitivity, specificity, and area under the curve (AUC) were further analyzed the differential diagnostic value. In addition, the Youden index provided the optimal predictive cutoffs for the tested markers. A p value of <0.05 was considered statistically significant.
Results
Baseline Data
In general, a consecutive cohort of 1237 patients who underwent lower extremity fracture surgery in our trauma center were reviewed and finally 576 cases were enrolled in this study (Figure 1). According to the confirmative FRI definition, they were divided into two groups: the non-FRI group (n = 525) and the FRI group (n = 51). The endpoint of follow-up assessments was February 1st 2023. Overall, these patients had similar clinical data and operative details in gender, age, BMI, smoking, comorbidities, injury site, injury mode, ISS, time to surgery, ASA class, blood loss, operative time, and drainage use (p > 0.05, Table 1).
 |
Table 1 Demographics and Clinical Characteristics of FRI and Non-FRI Patients |
Biomarkers and Microbiology Characteristic
The data of five biomarkers were evaluated for normal distribution using the Shapiro–Wilk normality test. The results showed that none of the data followed a normal distribution (Table 2).
 |
Table 2 Distribution Tests Regarding Serological Levels of the Inflammatory Biomarkers |
In the comparison of serum inflammatory biomarker levels between the FRI and non-FRI groups, as shown in Table 3, the median levels of CRP, ESR, NLR, and MLR were significantly higher in the FRI group than in the non-FRI group (p < 0.01), while PLR did not differ significantly between the two groups (p > 0.05).
 |
Table 3 Comparison of Serum Inflammatory Biomarkers in the FRI/ Non-FRI |
Of all the included cases of infection, 23 cases (45.1%) were classified as the low-virulence group, and 28 cases (54.9%) were classified as the high-virulence group. The most common and correlated microorganisms causing the infections were Staphylococcus aureus (19 cases), followed by Coagulase-negative staphylococci (14 cases), Streptococci (5 cases), Propionibacterium acnes (4 cases) and Enterobacteriaceae (2 cases).
In the comparison of serum inflammatory biomarker levels between the high- and low-virulence groups, the median levels of CRP and NLR were significantly higher in the high-virulence group than in the low-virulence group (p < 0.1), while the other three biomarkers did not differ significantly between the two groups (p > 0.05, Table 4 and Figure 2).
 |
Table 4 Comparison of Serum Inflammatory Biomarkers in the High-/Low-virulence Groups |
The effectiveness of discrimination of the inflammatory biomarkers was analyzed, and the results showed that the AUC areas of CRP, NLR, and CRP+NLR were 0.826, 0.650, and 0.873, respectively, indicating that the combined indicators had the highest diagnostic value (Table 5 and Figure 3). Using the method described by Youden, we calculated the optimal cut-off points for CRP+NLR as diagnostic markers of high-virulent infection was 0.377.
 |
Table 5 The Diagnostic Value of Serum Biomarkers |
 |
Figure 3 ROC of the CRP, NLR and CRP+NLR for high- /low-virulence FRI diagnosis. |
Discussion
Fracture-related infection is a common and serious complication in trauma surgery.3 The infection rate of closed fracture after internal fixation is 1%, while the infection risk of severe open fracture is 15–55%.13 At present, with the visible improvement of the trauma emergency system worldwide, the effect of early treatment has made great progress. However, a previous literature reported that postoperative infection was closely connected with the use of implants during operation, and the related infection caused by implants led to the failure of internal fixation, which increased the percentage of revision operations.14 Immune rejection will present after implant fixation, and biofilm will generate around the steel plate. Unfortunately, systemic application of antibiotics cannot resist and destroy the biofilm. Therefore, early detection and evaluation are of great importance to improve the prognosis of patients with high-risk factors.
Fisher et al reported that a higher NLR on admission was related with higher fracture presence, postoperative myocardial events and infection rate.15 In addition, Tekin et al observed that preoperative NLR and MLR were independent risk factors in mortality within a year after surgery.16 Our study showed significant higher serum biomarkers (CRP, ESR, MLR and NLR) among the confirmative FRI patients, which was consistent with previous studies.4,14,15
Given the strong suggestion investigating the laboratory evidence of microorganisms, Metsemakers et al highlighted that positive bacterial cultures in more than two different locations were recognized as the gold standard for the confirmative FRI.17 Wang et al reported that infection diagnosis was established when at least 5 specimens were collected around the implant for microbiological examination, and at least 2 specimens thereof were cultured with the same bacteria.18 Holinka et al took at least 3 peri prosthesis soft tissue samples from the inflammation area during the operation, as well as joint fluid extraction for identification of pus, gram staining and bacteriological culture.19 In this study, we performed the collection of microbiological specimens at least two sites from distinct suspected area following the aseptic principle and sent it to the lab within 30 minutes, which was conformed to the consensus of the international expert group.3
A multicenter study reported that infections after high-energy lower extremity trauma could up to 23%.20 Related factors for post-traumatic infection consisted of obesity, open fractures, tobacco use, alcoholism, inadequate debridement of the fracture site, and malnutrition.21 Prevention of infection for certain high-risk injury pattern, including tibial plateau, Pilon, and calcaneus fractures, is quite vital. We referred previous descriptions of “high-risk fractures”, which involved with fractures requiring planned surgical delay or staged fixation due to soft-tissue concerns.22,23 Hence, we investigated the prediction value of serum biomarkers in regard of FRI details among high-risk lower extremity fractures, which was not mentioned in other literatures.
Laboratory inflammatory tests are generally low-cost and widely available. However, to our knowledge, there is limited research on the predictive role of preoperative inflammatory biomarkers in high-risk lower extremity fracture-related infections, particularly in relation to microbial type or virulence. In this study, we found that the levels of CRP and NLR were influenced by the virulence of the microorganisms causing FRI. Similarly, in studies on periprosthetic joint infections (PJI) in the hip, knee, and shoulder, CRP has been considered a diagnostic tool for both high-virulent and low-virulent microbial PJI.8,24 This may be attributed to the fact that high-virulent microorganisms induce acute planktonic infections that trigger a robust inflammatory response characterized by the release of cytokines and elevation of CRP levels, whereas low-virulent microorganisms rapidly adhere to implant surfaces and form biofilms, enabling them to evade the host immune system and leading to reduced inflammation.25 Several studies have reported that NLR has the potential to serve as a more effective serum parameter than CRP and ESR for the early detection of PJI.26,27
In further performance evaluation, the AUC of CRP, NLR, and CRP+NLR were 0.826, 0.65, and 0.873, respectively, indicating good accuracy of CRP+NLR in detecting high-/low-virulence FRI. Additionally, we calculated the optimal cutoff values for CRP, NLR, and CRP+NLR using the Youden index, which were 18.77 mg/l−1, 4.39, and 0.377, respectively, providing the best balance of sensitivity and specificity for a given continuous biomarker in a single measurement. Furthermore, we found that CRP+NLR had high sensitivity (89.5%) and specificity (91.5%), indicating good diagnostic efficacy of this combined index. The single serum CRP showed moderate sensitivity (89%) but lower specificity (61%), consistent with reported sensitivity and specificity in the literature ranging from 60% to 83% and 34% to 86%, respectively.18,28,29 The single inflammatory marker NLR also demonstrated low specificity (35%), indicating poor performance of these diagnostic tests.
The findings of our study have important clinical implications. Preoperative assessment of inflammatory biomarkers such as CRP+NLR could be used as a simple and inexpensive tool to identify patients at high risk of developing postoperative infections caused by high-virulence bacteria. Early identification of these patients could lead to more aggressive prophylactic measures, such as targeted antibiotic therapy or closer postoperative monitoring, which could potentially reduce the incidence of postoperative infections and improve patient outcomes.
Limitations
This study has several limitations. First, the retrospective design and collection of data from single center may have limited its academic value. Inversely, the operating room and ward environment were always maintained the same, preventing many biases in this study. Second, finally, some patients might receive subsequent treatments for FRI at another trauma center within a year postoperatively, of which we were unaware. Lastly, while we used the same FRI definition and perioperative guideline, clinical misdiagnosis should still exist, which included some situations when a patient was infected and did not meet the criteria and vice versa.
Conclusion
In summary, the infection rate of FRI among high-risk lower extremity fractures was 8.9% and preoperative serum biomarkers in assisted diagnosis of infection were identified, consisting of CRP, ESR, NLR and PLR. In addition, we observed that combination of CRP and NLR proved its discriminating clinical role in different virulence. Special emphasis shall be given that our findings should be achieved in the context of limitations, and the prospective and multicenter studies will be carried out in the future.
Abbreviations
FRI, fracture-related infection; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Ethics Approval
This study was approved by the Ethics Committee of Beijing Chaoyang Hospital. The Ethics Committee of Beijing Chaoyang Hospital waived the requirement for written informed consent because the study was retrospective, it did not have any adverse effect on patients’ health, and it reported anonymized patient data. The authors announce that all methods were performed in accordance with the relevant guidelines and regulations.
Acknowledgments
Xiaopei Xu and Hanzhou Wang are co-first authors for this study. We would like to acknowledge the help provided by residents and other staff members of the of orthopedic surgery in conducting this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The correspondence author Junlin Zhou discloses receipt of the following financial support for the research, authorship, and publication of this article: National Natural Science Foundation of China (82272469) and Beijing Key Clinical Specialty Project.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Iliaens J, Onsea J, Hoekstra H, Nijs S, Peetermans WE, Metsemakers WJ. Fracture-related infection in long bone fractures: a comprehensive analysis of the economic impact and influence on quality of life. Injury. 2021;52(11):3344–3349.
2. Stall A, Paryavi E, Gupta R, Zadnik M, Hui E, O’Toole RV. Perioperative supplemental oxygen to reduce surgical site infection after open fixation of high-risk fractures: a randomized controlled pilot trial. J Trauma Acute Care Surg. 2013;75(4):657–663. doi:10.1097/TA.0b013e3182a1fe83
3. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49(3):505–510. doi:10.1016/j.injury.2017.08.040
4. Xu H, Xie JW, Liu L, Wang D, Huang ZY, Zhou ZK. Combination of CRP with NLR is a sensitive tool for screening fixation-related infection in patients undergoing conversion total Hip arthroplasty after failed internal fixation for femoral neck fracture. Bone Joint J. 2021;103-B(9):1534–1540. doi:10.1302/0301-620X.103B.BJJ-2021-0105.R1
5. Marom O, Paz I, Segal D, et al. Proximal femur fractures in the elderly-a novel modality to predict mortality: the neutrophil-to-lymphocyte ratio. J Clin Med. 2023;12(2). doi:10.3390/jcm12020456
6. Stone WZ, Gray CF, Parvataneni HK, et al. Clinical evaluation of Synovial Alpha defensin and Synovial C-reactive protein in the diagnosis of periprosthetic joint infection. J Bone Joint Surg Am. 2018;100(14):1184–1190. doi:10.2106/JBJS.17.00556
7. Akgün D, Bürger J, Pumberger M, Putzier M. C-reactive protein misdiagnoses delayed postoperative spinal implant infections in patients with low-virulent microorganisms. Eur Spine J. 2019;28(12):2990–2995. doi:10.1007/s00586-019-05889-3
8. Akgün D, Wiethölter M, Siegert P, et al. The role of serum C-reactive protein in the diagnosis of periprosthetic shoulder infection. Arch Orthop Trauma Surg. 2022;142(8):1715–1721. doi:10.1007/s00402-021-03779-2
9. Paryavi E, Stall A, Gupta R, et al. Predictive model for surgical site infection risk after surgery for high-energy lower-extremity fractures: development of the risk of infection in orthopedic trauma surgery score. J Trauma Acute Care Surg. 2013;74(6):1521–1527. doi:10.1097/TA.0b013e318292158d
10. Major Extremity Trauma Research Consortium. Effect of supplemental perioperative oxygen on SSI among adults with lower-extremity fractures at increased risk for infection: a randomized clinical trial. J Bone Joint Surg Am. 2022;104(14):1236–1243. doi:10.2106/JBJS.21.01317
11. Govaert GAM, Hobbelink MGG, Reininga IHF, Bosch P, Ijpma FFA. The accuracy of diagnostic imaging techniques in patients with a suspected fracture-related infection (ifi) trial: study protocol for a prospective multicenter cohort study. BMJ Open. 2019;9(9):e027772. doi:10.1136/bmjopen-2018-027772
12. Hellebrekers P, Rentenaar RJ, McNally MA, et al. Getting it right first time: the importance of a structured tissue sampling protocol for diagnosing fracture-related infections. Injury. 2019;50(10):1649–1655. doi:10.1016/j.injury.2019.05.014
13. Morgenstern M, Kühl R, Eckardt H, et al. Diagnostic challenges and future perspectives in fracture-related infection. Injury. 2018;49(Suppl 1):S83–S90. doi:10.1016/S0020-1383(18)30310-3
14. Parvizi J, Tan TL, Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309–1314.e2. doi:10.1016/j.arth.2018.02.078
15. Fisher A, Srikusalanukul W, Fisher L, Smith P. The neutrophil to lymphocyte ratio on admission and short-term outcomes in orthogeriatric patients. Int J Med Sci. 2016;13(8):588–602. doi:10.7150/ijms.15445
16. Sigmund IK, Dudareva M, Watts D, Morgenstern M, Athanasou NA, McNally MA. Limited diagnostic value of serum inflammatory biomarkers in the diagnosis of fracture-related infections. Bone Joint J. 2020;102-B(7):904–911. doi:10.1302/0301-620X.102B7.BJJ-2019-1739.R1
17. Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49(3):511–522. doi:10.1016/j.injury.2016.09.019
18. Wang S, Yin P, Quan C, et al. Evaluating the use of serum inflammatory markers for preoperative diagnosis of infection in patients with nonunions. Biomed Res Int. 2017;2017:9146317. doi:10.1155/2017/9146317
19. Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29(4):617–622. doi:10.1002/jor.21286
20. Harris AM, Althausen PL, Kellam J, Bosse MJ, Castillo R. Complications following limb-threatening lower extremity trauma. J Orthop Trauma. 2009;23(1):1–6. doi:10.1097/BOT.0b013e31818e43dd
21. Meza BC, Talwar D, Flynn JM. Measures to reduce end-of-case wound contamination: the impact of intra-wound vancomycin powder and betadine irrigation on surgical site infections in posterior spinal fusion. Spine Deform. 2020;8(1):45–50. doi:10.1007/s43390-020-00033-4
22. Qadir R, Costales T, Coale M, et al. Vancomycin powder use in fractures at high risk of surgical site infection. J Orthop Trauma. 2021;35(1):23–28. doi:10.1097/BOT.0000000000001863
23. O’Toole RV, Joshi M, Carlini AR, et al. Effect of intrawound vancomycin powder in operatively treated high-risk tibia fractures: a randomized clinical trial. JAMA Surg. 2021;156(5):e207259. doi:10.1001/jamasurg.2020.7259
24. Unter Ecker N, Suero EM, Gehrke T, et al. Serum C-reactive protein relationship in high- versus low-virulence pathogens in the diagnosis of periprosthetic joint infection. J Med Microbiol. 2019;68(6):910–917. doi:10.1099/jmm.0.000958
25. Høiby N, Ciofu O, Johansen HK, et al. The clinical impact of bacterial biofilms. Int J Oral Sci. 2011;3(2):55–65. doi:10.4248/IJOS11026
26. Yombi JC, Schwab PE, Thienpont E. Neutrophil-to-lymphocyte ratio (NLR) distribution shows a better kinetic pattern than C-reactive protein distribution for the follow-up of early inflammation after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3287–3292. doi:10.1007/s00167-015-3921-0
27. Yu BZ, Fu J, Chai W, Hao LB, Chen JY. Neutrophil to lymphocyte ratio as a predictor for diagnosis of early Periprosthetic joint infection. BMC Musculoskelet Disord. 2020;21(1):706. doi:10.1186/s12891-020-03704-5
28. Bosch P, van den Kieboom J, Plate J, et al. Limited predictive value of serum inflammatory markers for diagnosing fracture-related infections: results of a large retrospective multicenter cohort study. J Bone Jt Infect. 2018;3(3):130–137. doi:10.7150/jbji.26492
29. Omar M, Suero EM, Liodakis E, et al. Diagnostic performance of swab PCR as an alternative to tissue culture methods for diagnosing infections associated with fracture fixation devices. Injury. 2016;47(7):1421–1426. doi:10.1016/j.injury.2016.04.038
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

