Back to Journals » Infection and Drug Resistance » Volume 17
The Role of AGGF1 in the Classification and Evaluating Prognosis of Adult Septic Patients: An Observational Study
Authors Ji W, Wan T, Zhang F, Guo S , Mei X
Received 2 November 2023
Accepted for publication 27 February 2024
Published 21 March 2024 Volume 2024:17 Pages 1153—1160
DOI https://doi.org/10.2147/IDR.S447922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Wenqing Ji,* Tiantian Wan,* Fang Zhang, Shubin Guo, Xue Mei
Department of Emergency Medicine, Beijing Key Laboratory of Cardiopulmonary Cerebral Resuscitation, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shubin Guo; Xue Mei, Department of Emergency Medicine, Beijing Key Laboratory of Cardiopulmonary Cerebral Resuscitation, Beijing Chao-Yang Hospital, Capital Medical University, 8 South Workers Stadium Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86-10-85231000, Email [email protected]; [email protected]
Purpose: Angiogenic factor with G patch and FHA domains 1 (AGGF1) is a crucial angiogenic factor that is involved in a variety of diseases and in the regulation of inflammatory responses. However, its role in sepsis is poorly understood. We have investigated the role of AGGF1 in the classification and prognostic evaluation of adult septic patients in a clinical context.
Patients and Methods: A total of 126 septic patients who visited the Emergency Department of Beijing Chao-Yang Hospital and 76 non-sepsis patients visiting the Physical Examination Center of Beijing Chao-Yang Hospital were enrolled. AGGF1 levels in plasma were detected by enzyme-linked immunosorbent assay. Spearman correlation analysis was used to determine correlations between plasma AGGF1 and Sequential Organ Failure Assessment (SOFA) score, Acute Pathology and Chronic Health Evaluation II (APACHE II) score, procalcitonin and lactate. We evaluated the classification significance of AGGF1 in sepsis using receiver operating characteristic (ROC) curves. We also assessed the predictive significance of AGGF1 for 28-day mortality in sepsis using ROC curves and Kaplan–Meier analyses.
Results: Plasma AGGF1 levels were higher in sepsis patients than in non-sepsis patients (P < 0.001). Among sepsis patients, plasma AGGF1 levels were higher in non-survivors than in survivors (P < 0.001). Increased plasma AGGF1 levels were positively correlated with SOFA score, APACHE II score, procalcitonin and lactate. Plasma AGGF1 levels could distinguish sepsis patients from non-sepsis patients (area under the curve [AUC] = 0.777). AGGF1 had a higher predictive value than SOFA score, APACHE II score, lactate, procalcitonin, and white blood cell count for 28-day mortality in patients with sepsis (AUC = 0.876). Furthermore, Kaplan–Meier analysis indicated that lower plasma AGGF1 levels were associated with lower 28-day mortality compared with higher plasma AGGF1 levels (log rank P < 0.001).
Conclusion: AGGF1 is useful for the classification and evaluating prognosis of adult septic patients.
Keywords: AGGF1, sepsis, classification, predictor
Introduction
Sepsis results from a dysregulated reaction to an infection and is associated with substantial morbidity and mortality.1,2 A study has revealed that 29.5% of patients can develop sepsis on admission or during ICU stay.3 Death occurs in more than 25–30% of sepsis patients and hospital mortality of patients with septic shock is about 40–60%.4 Traditional blood culture methods are low-sensitivity, and they can delay the initial management of sepsis.5 This is unfavorable for the early diagnosis and treatment of sepsis. Emerging biomarkers, including miRNA, cell-free DNA, and presepsin, are promising for improving the diagnosis and treatment of sepsis.5 However, the search for sepsis biomarkers with increased diagnostic and prognostic utility and is ongoing.5
Angiogenic factor with G patch and FHA domains 1 (AGGF1) was initially discovered as a susceptibility gene for the congenital vascular disease, Klippel–Trenaunay syndrome, and it encodes a potent angiogenic factor.6 The pathophysiological process of sepsis is heavily influenced by the inflammatory response.7 Accumulating evidence indicates AGGF1 to play diverse roles in various pathological processes, including inflammation, apoptosis, and autophagy.8–10 Exogenous AGGF1 treatment improved behavior by reducing neuroinflammation and blood–brain barrier disruption after subarachnoid hemorrhage-induced brain injury in rats.11 In addition, AGGF1 can enhance angiogenesis and reduce inflammatory responses, myocardial apoptosis, and myocardial cell death, resulting in a decreased infarct size after ischemia/reperfusion injury in mice.9 Despite the established role of AGGF1 in inflammation, its effect on sepsis remains insufficiently investigated.
In the present study, we assessed the classification and prognostic utility of AGGF1 in sepsis in an effort to improve the diagnosis and treatment of the disease.
Materials and Methods
Ethics Statement
The present study was approved by the Ethics Committee of Beijing Chao-Yang Hospital (2016-ke-173) and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all subjects or their legal guardians.
Participants
In total, 126 patients treated for sepsis in the Emergency Department of Beijing Chao-Yang Hospital from September 2018 to June 2022 were included. The inclusion criteria were as follows: 1) meeting the diagnostic criteria for sepsis according to the Third International Consensus Definition of Sepsis and Septic shock (Sequential Organ Failure Assessment [SOFA] score ≥ 2 points).12 The SOFA score was based on evaluating the organ dysfunction consequent to the infection, which included the PaO2/FiO2 ratio, platelet count, bilirubin level, mean arterial pressure, or use of vasopressor drugs, creatinine level, or urine output, and Glasgow Coma Scale score; 2) age ≥18 years; 3) No medical treatment was performed before blood samples collection; 4) patients whose sample collection time was within 24 hours after admission to the Emergency Department of Beijing Chao-Yang Hospital and 5) willing to participate in this study. The exclusion criteria were as follows: 1) age < 18 years; 2) pregnancy or nursing; 3) imminent death; 4) recent receipt of immunosuppressive drug therapy; 5) presence of complicated tumors; and 6) presence of complicated autoimmune disease. In addition, 76 non-sepsis patients of matched age and sex who visited the Physical Examination Center of Beijing Chao-Yang Hospital were enrolled. The sample collection time was within 24 hours after admission to the Physical Examination Center of Beijing Chao-Yang Hospital.
Collection of Blood Samples and Laboratory Analysis
Peripheral venous blood (4 mL) was obtained from each patient with non-sepsis and sepsis. Venous blood was centrifuged for 15 minutes, and then the upper plasma was collected and stored at −80°C until use. Plasma AGGF1 levels were analyzed using an enzyme-linked immunosorbent assay (ELISA) kit, with the lowest amount less than or equal to 59 pg/mL (Cloud-clone Corp, Wuhan, China).
Data Collection
The clinical information of subjects, including age, sex, incidence of septic shock, SOFA score, Acute Pathology and Chronic Health Evaluation II (APACHE II) score, and results of crucial laboratory blood tests, such as procalcitonin (PCT), lactate, and white blood cell (WBC) count were collected within 24 hours after admission to the Emergency Department of Beijing Chao-Yang Hospital or within 24 hours after admission to the physical examination center of Beijing Chao-Yang Hospital. The endpoint of the study was 28-day mortality.
Statistical Analysis
SPSS version 22.0 (IBM, Armonk, NY, USA) or GraphPad Prism 9 (GraphPad, San Diego, CA, USA) was used for data analysis. Continuous variables are presented as the mean ± standard error of the mean or the median (interquartile range). Comparisons between two groups were performed using Student’s t-test or the nonparametric Mann‒Whitney U test. One-way analysis of variance or the nonparametric Kruskal–Wallis H test was used for multiple-group comparisons. Categorical variables were calculated as numbers (percentages) using the chi-squared test. The utility of AGGF1 for classifying sepsis and predicting prognosis was evaluated using receiver operating characteristic (ROC) curves. Kaplan–Meier survival curves and the Log rank test were used to predict 28-day mortality. P < 0.05 was considered statistically significant.
Results
Demographic Characteristics of the Included Subjects
This study included 126 patients with sepsis, among whom 36 (28.6%) were non-survivors. Values of the study’s variables are presented for each group in Table 1. The sex ratio did not differ between the groups (P > 0.05). Compared with non-sepsis, WBC counts were markedly higher in survivors with sepsis (P < 0.001). Meanwhile, age, WBC count, the incidence of septic shock, lactate level, PCT level, and SOFA and APACHE II scores were significantly higher in non-survivors than in survivors (all P < 0.05).
 |
Table 1 Baseline Characteristics of the Study Objects |
Plasma AGGF1 Levels were Increased in Patients with Sepsis
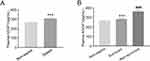
As presented in Figure 1, plasma AGGF1 levels were markedly higher in patients with sepsis than in non-sepsis patients (P < 0.001). Among patients with sepsis, plasma AGGF1 levels were markedly higher in non-survivors than in survivors (P < 0.001).
Correlation Analysis of AGGF1 and Sepsis Parameters
SOFA score, APACHE II score, PCT and lactate are crucial indicators of the extent of the inflammatory response and sepsis severity. Spearman correlation analysis indicated that plasma AGGF1 levels were positively correlated with SOFA (r = 0.622), APACHE II (r = 0.545), PCT (r = 0.508) and lactate (r = 0.391, Figure 2).
The Classification Utility of AGGF1 in Sepsis
As illustrated in Figure 3A, plasma AGGF1 levels distinguished patients with sepsis from non-sepsis patients with an optimal cutoff of 275.742 pg/mL (area under the curve [AUC] = 0.777). The sensitivity and specificity of AGGF1 were 72.20% and 80.30%, respectively. These results indicate that plasma AGGF1 is a promising biomarker for the classification of sepsis.
The Association of AGGF1 with 28-Day Mortality in Sepsis
Plasma AGGF1 levels distinguished survivors from non-survivors with an optimal cutoff of 329.929 pg/mL (AUC = 0.876, Figure 3B). The sensitivity and specificity of AGGF1 were 66.70% and 96.70%, respectively. None of the variables, SOFA score, APACHE II score, PCT, lactate and WBC count outperformed AGGF1 in 28-day mortality prediction (Table 2). This indicates that AGGF1 levels are predictive for sepsis prognosis.
 |
Table 2 ROC Curve Prediction of Septic Patient 28-Day Mortality |
Using the optimal cutoff of AGGF1, we then divided patients with sepsis into high and low AGGF1 groups. Twenty-eight-day mortality was significantly higher in the high AGGF1 group than in the low AGGF1 group (log-rank P < 0.001, Figure 4). Together, the above results illustrate that AGGF1 has potential as a parameter for predicting 28-day mortality in patients with sepsis.
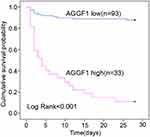 |
Figure 4 Kaplan-Meier survival curves to confirm the predictive utility of AGGF1 for 28-day mortality in patients with sepsis. |
Discussion
Sepsis is a pathological syndrome associated with a high in-hospital mortality rate.12 It is characterized by persistent maladjusted inflammation and immunosuppression resulting from disturbed immune homeostasis caused by invading pathogens.13 The early signs of sepsis are not specific;14 therefore, it is crucial to identify novel biomarkers for the early classification of sepsis to permit timely treatment to reduce the sepsis mortality rate. Our goal is to help classify septic patients earlier and manage them more promptly when they are septic or at risk of septic shock.12
Patients with sepsis were distinguished from non-sepsis patients using AGGF1 as a classification marker in this study. Increased plasma AGGF1 levels in sepsis were positively correlated with the extent of inflammatory response and disease severity. Furthermore, in patients with sepsis, high plasma AGGF1 levels predicted 28-day mortality. Therefore, our data demonstrated that AGGF1 is useful for the classification and predicting the prognosis of sepsis.
AGGF1 is expressed in vascular smooth muscle, endothelial, and myocardial cells.8 AGGF1 expression is significantly upregulated in myocardial ischemia/reperfusion injury, acute myocardial infarction, and subarachnoid hemorrhage-induced brain injury.8,9,11 AGGF1 plays a crucial role in the induction of angiogenesis, and increased AGGF1 expression is one of the causes of Klippel–Trenaunay syndrome.6,9,15–17 AGGF1 has been demonstrated in numerous studies to play a key regulatory role in a variety of conditions, such as inflammation, fibrosis, cancer, and cardiovascular diseases.18–21 We are particularly interested in the regulatory role of AGGF1 in the inflammatory response. Lipopolysaccharide, a major component of the outer membrane of gram-negative bacteria, induces dysregulated inflammatory responses and sepsis.22,23 AGGF1 is upregulated in a concentration-dependent manner after lipopolysaccharide stimulation in human dental pulp cells. However, inflammatory factor expression was increased by lipopolysaccharide stimulation in AGGF1-depleted cells through the NF-κB signaling pathway.21 Given these findings, we speculate that AGGF1 has an important involvement in sepsis.
The current study had several limitations. First, ideal blood tests should be done in the early phase of sepsis. Although we collected blood samples within 24 hours of admission to hospital, it was not always in the early stages of sepsis. Second, this paper only classifies sepsis according to the Third International Consensus Definition of Sepsis and Septic shock, and does not study the severity of patients in the sepsis group based on changes in the SOFA score. Third, it was a single-center study with a relatively small sample size. A multiple-center study with sufficient sample size is needed for definitive conclusions. Fourth, the source and molecular mechanism of elevated plasma AGGF1 levels is unknown. Future studies should assess AGGF1 levels in the heart, vascular tissues, brain and other organs during the course of sepsis. In addition, the molecular mechanism of increased AGGF1 levels in organs also needs to be investigated; therefore, we will use quantitative real–time polymerase chain reaction, western blot, ELISA, immunohistochemistry and other methods to verify the above content in mice.
For the diagnosis and prognosis of sepsis, no clear clinical presentation or laboratory indicator has been established as the gold standard. To diagnose sepsis, traditional detection methods, such as blood culture, are inefficient and time-consuming.24 Several recent studies found that sepsis can be diagnosed early using a variety of laboratory indicators, such as PCT, lactate, interleukin-6, and C-reactive protein, which have different sensitivities and specificities.25–27 However, none of them can be used for the accurate diagnosis of sepsis.25–27 For example, PCT and C-reactive protein levels are also increased in non-septic diseases.28 In the prognostic evaluation of sepsis, the use of SOFA and APACHE II scores to assess the prognosis of sepsis is limited because of their poor specificity.29 It remains challenging to identify a new biomarker for classification and predicting the prognosis of sepsis with high specificity and sensitivity. In our study, we assessed the classification and prognostic significance of AGGF1 in patients with sepsis. We found that AGGF1 could distinguish patients with sepsis from non-sepsis patients, indicating that it is a promising biomarker for the classification of sepsis. Furthermore, after correlation analysis, significant positive correlations between plasma AGGF1 and APACHE II score, SOFA score, PCT and lactate were detected, indicating that increased plasma AGGF1 levels were positively correlated with both the inflammatory response and severity of sepsis. In addition, ROC curves illustrated that AGGF1 can distinguish survivors from non-survivors, indicating that AGGF1 can be used to evaluate the prognosis of patients with sepsis. To further examine the significance of AGGF1 as a predictor of sepsis-related mortality after 28 days, we divided patients with sepsis into high and low AGGF1 groups according to the optimal cutoff of plasma AGGF1 levels in survivors and non-survivors. Kaplan–Meier survival curves indicated that high AGGF1 levels were associated with a higher mortality rate than low AGGF1 levels. This study illustrates that AGGF1 has the classification and prognostic significance in sepsis.
Conclusion
In summary, AGGF1 has high classification value for adult sepsis patients and non-sepsis patients, and it may be predictive of 28-day mortality.
Data Sharing Statement
Data will be made available on request.
Ethical Approval and Consent to Participate
This study was approved by the Ethics Committee of Beijing Chao-Yang Hospital (2016-ke-173) and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all subjects or their legal guardians.
Acknowledgments
This work was supported by Capital Clinical Special Application Research Project (Z171100001017057) and Sailing Plan Key Medical Speciality (ZYLX202132).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van der Poll T, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54(11):2450–2464. doi:10.1016/j.immuni.2021.10.012
2. Sevransky JE, Rothman RE, Hager DN, et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients With Sepsis: the VICTAS Randomized Clinical Trial. JAMA. 2021;325(8):742–750. doi:10.1001/jama.2020.24505
3. Vincent JL, Marshall JC, Namendys-Silva SA, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–386. doi:10.1016/S2213-2600(14)70061-X
4. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/S0140-6736(18)30696-2
5. Sandquist M, Wong HR. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev Clin Immunol. 2014;10(10):1349–1356. doi:10.1586/1744666X.2014.949675
6. Tian XL, Kadaba R, You SA, et al. Identification of an angiogenic factor that when mutated causes susceptibility to Klippel-Trenaunay syndrome. Nature. 2004;427(6975):640–645. doi:10.1038/nature02320
7. Zhang Z, Yan C, Miao J, Pu K, Ma H, Wang Q. Muscle-Derived Mitochondrial Transplantation Reduces Inflammation, Enhances Bacterial Clearance, and Improves Survival in Sepsis. Shock. 2021;56(1):108–118. doi:10.1097/SHK.0000000000001681
8. Lu Q, Yao Y, Hu Z, et al. Angiogenic Factor AGGF1 Activates Autophagy with an Essential Role in Therapeutic Angiogenesis for Heart Disease. PLoS Biol. 2016;14(8):e1002529. doi:10.1371/journal.pbio.1002529
9. Liu Y, Yang H, Song L, et al. AGGF1 protects from myocardial ischemia/reperfusion injury by regulating myocardial apoptosis and angiogenesis. Apoptosis. 2021;26(11–12):657–659. doi:10.1007/s10495-021-01695-9
10. Hu FY, Wu C, Li Y, et al. AGGF1 is a novel anti-inflammatory factor associated with TNF-alpha-induced endothelial activation. Cell Signal. 2013;25(8):1645–1653. doi:10.1016/j.cellsig.2013.04.007
11. Zhu Q, Enkhjargal B, Huang L, et al. Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-kappaB pathway after subarachnoid hemorrhage in rats. J Neuroinflammation. 2018;15(1):178. doi:10.1186/s12974-018-1211-8
12. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
13. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi:10.1038/nri.2017.36
14. Arriaga-Pizano LA, Gonzalez-Olvera MA, Ferat-Osorio AE, et al. Accurate diagnosis of sepsis using a neural network: pilot study using routine clinical variables. Comput Methods Programs Biomed. 2021;210:106366. doi:10.1016/j.cmpb.2021.106366
15. Fan C, Ouyang P, Timur AA, et al. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function. J Biol Chem. 2009;284(35):23331–23343. doi:10.1074/jbc.M109.036079
16. Lu Q, Yao Y, Yao Y, et al. Angiogenic factor AGGF1 promotes therapeutic angiogenesis in a mouse limb ischemia model. PLoS One. 2012;7(10):e46998. doi:10.1371/journal.pone.0046998
17. Hu Y, Li L, Seidelmann SB, et al. Identification of association of common AGGF1 variants with susceptibility for Klippel-Trenaunay syndrome using the structure association program. Ann Hum Genet. 2008;72(5):636–643. doi:10.1111/j.1469-1809.2008.00458.x
18. Xu W, Zeng S, Li M, Fan Z, Zhou B. Aggf1 attenuates hepatic inflammation and activation of hepatic stellate cells by repressing Ccl2 transcription. J Biomed Res. 2017;31(5):428–436. doi:10.7555/JBR.30.20160046
19. Ding L, Lu S, Zhou Y, et al. The 3’ Untranslated Region Protects the Heart from Angiotensin II-Induced Cardiac Dysfunction via AGGF1 Expression. Mol Ther. 2020;28(4):1119–1132. doi:10.1016/j.ymthe.2020.02.002
20. Wang W, Zhu G, Lai S, et al. Angiogenic Factor with G Patch and FHA Domains 1 (AGGF1) Acts as Diagnostic Biomarker and Adverse Prognostic Factor of Hepatocellular Carcinoma (HCC): evidence from Bioinformatic Analysis. Med Sci Monit. 2020;26:e919896. doi:10.12659/MSM.919896
21. Shen S, Shang L, Liu H, Liang Q, Liang W, Ge S. AGGF1 inhibits the expression of inflammatory mediators and promotes angiogenesis in dental pulp cells. Clin Oral Investig. 2021;25(2):581–592. doi:10.1007/s00784-020-03498-9
22. Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83:99–128. doi:10.1146/annurev-biochem-060713-035600
23. Bryant CE, Spring DR, Gangloff M, Gay NJ. The molecular basis of the host response to lipopolysaccharide. Nat Rev Microbiol. 2010;8(1):8–14. doi:10.1038/nrmicro2266
24. Wang J, Wang J, Wei B. The Diagnostic Value of Fe3+ and Inflammation Indicators in the Death of Sepsis Patients: a Retrospective Study of 428 Patients. Ther Clin Risk Manag. 2021;17:55–63. doi:10.2147/TCRM.S291242
25. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–435. doi:10.1016/S1473-3099(12)70323-7
26. Celik IH, Demirel FG, Uras N, et al. What are the cut-off levels for IL-6 and CRP in neonatal sepsis? J Clin Lab Anal. 2010;24(6):407–412. doi:10.1002/jcla.20420
27. Faix JD. Biomarkers of sepsis. Crit Rev Clin Lab Sci. 2013;50(1):23–36. doi:10.3109/10408363.2013.764490
28. Guo S, Mao X, Liang M. The moderate predictive value of serial serum CRP and PCT levels for the prognosis of hospitalized community-acquired pneumonia. Respir Res. 2018;19(1):193. doi:10.1186/s12931-018-0877-x
29. Zhao D, Li S, Cui J, Wang L, Ma X, Li Y. Plasma miR-125a and miR-125b in sepsis: correlation with disease risk, inflammation, severity, and prognosis. J Clin Lab Anal. 2020;34(2):e23036. doi:10.1002/jcla.23036
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.