Back to Journals » International Journal of General Medicine » Volume 16
The Role of Adipocyte Endoplasmic Reticulum Stress in Obese Adipose Tissue Dysfunction: A Review
Authors Xu S , Xi J, Wu T, Wang Z
Received 1 July 2023
Accepted for publication 19 September 2023
Published 27 September 2023 Volume 2023:16 Pages 4405—4418
DOI https://doi.org/10.2147/IJGM.S428482
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Shengjie Xu,1 Jiaqiu Xi,2 Tao Wu,1 Zhonglin Wang3
1The First Clinical College, Shandong University of Traditional Chinese Medicine, Jinan, 250000, People’s Republic of China; 2Shandong University of Traditional Chinese Medicine, Jinan, 250000, People’s Republic of China; 3Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, 250014, People’s Republic of China
Correspondence: Zhonglin Wang, Email [email protected]
Abstract: Adipose tissue dysfunction plays an important role in metabolic diseases associated with chronic inflammation, insulin resistance and lipid ectopic deposition in obese patients. In recent years, it has been found that under the stimulation of adipocyte endoplasmic reticulum stress (ERS), the over-activated ER unfolded protein response (UPR) exacerbates the inflammatory response of adipose tissue by interfering with the normal metabolism of adipose tissue, promotes the secretion of adipokines, and affects the browning and thermogenic pathways of adipose tissue, ultimately leading to the manifestation of metabolic syndrome such as ectopic lipid deposition and disorders of glucolipid metabolism in obese patients. This paper mainly summarizes the relationship between adipocyte ERS and obese adipose tissue dysfunction and provides an overview of the mechanisms by which ERS induces metabolic disorders such as catabolism, thermogenesis and inflammation in obese adipose tissue through the regulation of molecules and pathways such as NF-κB, ADPN, STAMP2, LPIN1, TRIP-Br2, NF-Y and SIRT2 and briefly describes the current mechanisms targeting adipocyte endoplasmic reticulum stress to improve obesity and provide ideas for intervention and treatment of obese adipose tissue dysfunction.
Keywords: endoplasmic reticulum stress, unfolded protein response, obesity, adipose tissue dysfunction
Introduction
Obesity is the excessive accumulation of adipose tissue resulting from chronic imbalances in energy intake and expenditure, often accompanied by low-grade inflammatory responses of the innate and adaptive immune systems.1 Obesity leads to impaired glucose tolerance and lipid metabolism disorders by affecting systemic metabolic homeostasis, and ultimately promotes the development of obesity-related metabolic diseases such as diabetes, atherosclerosis, and dyslipidemia.2,3 The alarming rise of the worldwide obesity epidemic has caused a great strain to our society, with nearly 108 million children (about 5% prevalence) and 604 million adults (about 12% prevalence) suffering from obesity worldwide.4 According to statistics, the annual medical cost of obesity prevention and treatment is about 2 trillion US dollars, making it one of the most expensive health issues in the country.5 Although some measures have been taken to prevent and control obesity, such as lifestyle changes, drugs, and surgery, the development of obesity and related metabolic diseases has not been effectively controlled.6 Therefore, it is very important to deeply explore the pathogenesis and potential targets of obesity. Recent studies have found that ERS was significantly increased in adipose tissue of obese mice and obese human subjects, highlighting the need for further research into the effects of obesity on human health.7
The endoplasmic reticulum (ER) is the center of protein folding, calcium homeostasis and lipid synthesis. It connects the cell membrane outside and the nuclear membrane inside, connects various structures in the cell as a whole, and has the role of transporting substances in the cell. This organelle is essential for the proper functioning of a cell, as it is responsible for the production and transport of its key components.8 Adipocytes are the main cells that make up adipose tissue and participate in the regulation of local and systemic inflammation, insulin sensitivity, and metabolism by secreting lipids, adipokines, and cytokines, making them integral to the body’s metabolism.9 In adipocytes, ER is directly involved in lipid droplet formation and maintenance of lipid homeostasis.10 ERS has been shown to be both a cause and a consequence of obesity.ERS can induce adipocyte inflammatory cascade and lipid metabolism disorder, and eventually lead to chronic inflammation, insulin resistance and ectopic fat deposition in obese patients.11,12
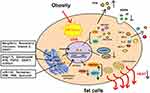
It can be seen from the above that reducing the effect of ERS on fat metabolism in obese patients is an important strategy to improve obesity metabolic syndrome (Figure 1). This review summarizes the effects and main mechanism of ERS on fat tissue decomposition, thermogenesis and inflammation in obesity, and briefly expounds the current related drugs targeting ERS to improve obesity, it is expected to provide new ideas for the treatment of adipose tissue dysfunction in obese patients.
ERS and UPR
The ER is an important organelle responsible for the synthesis, folding, and maturation of secreted transmembrane proteins, as well as the site of Ca2+ storage and lipid biosynthesis.13 Ribosomes attached to the ER membrane release newly synthesized peptides into the lumen of the ER, and the protein chaperones and foldases on the ER further translate and fold these peptides correctly, release them to the Golgi complex for modification, and finally transport them to their final destination.14,15 However, the abnormal inflammatory response in the body, insufficient Ca2+ content, and redox reaction imbalance can all cause ER homeostasis imbalance,1–3 leads to the accumulation of immature proteins, which triggers the UPR in an attempt to reestablish ER homeostasis. ERS occurs when UPR stress induction fails to correct unfolded/misfolded proteins.16
There are three main stress-sensing pathways for signal transduction between UPR and cells: the inositol-requiring enzyme 1α (IRE1α) pathway and the protein kinase R-like ER kinase (PERK) pathway, the activating transcription factor 6 (ATF6) pathway.17 Under non-stress conditions, these three transducers bind the immunoglobulin heavy chain binding protein (Bip) at the ER membrane. When external stimuli or changes in ER homeostasis lead to excessive accumulation of misfolded or unfolded proteins, Bip combines with abnormal proteins amassed in the ER lumen, dissociates from IRE1α, PERK and ATF6 and activates their respective functions, thereby boosting ER protein folding ability and curbing intracellular protein synthesis to restore ER homeostasis.18,19 However, when the degree of ERS exceeds the regulatory range of UPR, it will lead to the imbalance of ER homeostasis, resulting in a variety of metabolic abnormalities related to ER function.20 Therefore, under the condition of obesity-induced ER homeostasis imbalance, the regulation of ERS by UPR signaling plays an important role in the metabolism of adipose tissue in obesity.
Obesity and ERS in Adipocytes
Adipocytes are one of the major cell types involved in the pathogenesis of obesity metabolic syndrome, the demand for protein folding in adipocytes under obese conditions exceeds the ability of the ER to mature these proteins, triggering ERS and activating the UPR in adipocytes.21 Menikdiwela22 found that the levels of ERS markers such as Bip and ATF4 in fat cells of obese mice were significantly increased. In a study published by Guenther et al23, the differential expression of subcutaneous fat proteins in healthy and obese subjects was compared by proteomic analysis, three of the 19 differentially upregulated proteins were found to be related to ERS, and Western blot experiments also showed the upregulation of several other UPR-related proteins, including calnexin, membrane-bound chaperone, etc. These results suggest that obesity triggers ERS in adipose tissue and leads to sustained activation of UPR.
Recent research has suggested that the microenvironmental imbalance in the body caused by excess nutrients during obesity may be an important cause of ERS in adipocytes.24 Excessive food intake stimulates lipogenesis to store excess energy. Adipocytes need to continuously store excess nutrients in the form of triglycerides (TG) in lipid droplets in the cytoplasm, which induces progressive hypertrophy of adipocytes, the ER is therefore continuously stimulated to synthesize more proteins for the formation of lipid droplets.25,26 At the same time, excess lipid metabolites produced by obese patients can also affect ER membrane flow and thickness through ER sensors IRE1α and PERK, thereby directly leading to ERS.27 Therefore, obesity alters the folding environment of ER proteins and exacerbates the accumulation of misfolded or unfolded proteins by stimulating adipocytes as well as disrupting adipocyte homeostasis, leading to ERS and activating UPR in adipocytes.28 Recent studies have shown that the phosphorylation of the ERS marker PERK in obese mice fed a high-fat diet (HFD) was significantly higher than that of mice fed a normal diet, accompanied by abnormal protein degradation and lipid accumulation, this further indicates that HFD-induced abnormal lipid metabolism may be related to the activation of PERK protein in the UPR signaling pathway.29 At the same time, by comparing the detection results of ERS markers in subcutaneous adipose tissue of obese patients before and after gastric bypass (GBP) surgery, it was found that the BiP and mRNA levels of X-box binding protein 1 (Xbp1) in adipose tissue of patients with weight loss after surgery were significantly lower than those of obese patients, accompanied by a decrease in the phosphorylation levels of PERK and c-Jun N-terminal kinase (JNK).30 These results suggest that obesity induces ERS in adipose tissue and activation of the UPR signaling pathway, and weight loss can significantly reduce ERS in adipose tissue and improve the imbalance of ER homeostasis in adipocytes.
The Effect of ERS on the Function of Obese Adipose Tissue
ERS-induced adipose tissue dysfunction plays an important role in the pathology of metabolic disorders in the context of obesity overnutrition.31 Under continuous ERS stimulation caused by obesity, the overactivated UPR signaling pathway interferes with the normal metabolism of adipose tissue, aggravates the inflammatory response of adipose tissue, promotes the secretion of FFA and adipokines in adipose tissue, and affect the normal browning and thermogenesis pathways of adipose tissue.32 Eventually lead to metabolic syndrome such as ectopic fat deposition and glucose and lipid metabolism disorders in obese patients.
ERS and Adipose Tissue Inflammation
Obesity is now recognized as a chronic inflammatory state, and ERS may be a major cause and/or consequence of inflammatory activation in obese adipose tissue. An increase in the ERS marker CCAAT/enhancer-binding protein homolog (CHOP) /ATF4 was found in adipose tissue of obese mice, accompanied by the upregulation of gene expression of inflammatory cytokines including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1), this suggests that obesity induces ERS and activates the UPR signaling pathway in adipocytes, leading to abnormal inflammatory responses in adipose tissue.33 Suzuki et al34 found that the ERS protein CHOP was up-regulated in the adipocytes of HFD-fed obese mice, and the up-regulated CHOP stimulated adipocytes to produce T helper 2 cell (Th2) cytokines by mediating ERS, thereby changing the microenvironment of white adipocytes, eventually triggering the recruitment of pro-inflammatory M1 macrophages and the production of excessive pro-inflammatory cytokines, aggravating the inflammatory response of adipose tissue; In contrast, downregulation of Th2 cytokine expression was evident by inhibiting ERS in cultured adipocytes. This suggests that under obesity, ERS can cause abnormal inflammatory microenvironment of adipose tissue and lead to adipose tissue inflammation by promoting the secretion of various inflammatory cytokines by adipocytes and mediating the infiltration of macrophages into white adipose tissue. In addition, Alicka et al35 noted that adipose tissue inflammation can in turn activate the UPR through all three branches (PERK, IRE1α, and AT6), and that prolonged and sustained inflammatory stimulation induces ERS.
ERS and Abnormal Fat Breakdown
Abnormal lipid breakdown and ectopic lipid deposition caused by obesity are high risk factors for fatty liver and cardiovascular disease in patients.16 In a study on human fat, it was found that the degree of lipolysis in the subcutaneous fat tissue of the legs of obese patients was significantly higher than that of normal body subjects,36 this suggests that obesity will promote abnormal fat decomposition by affecting certain factors. In recent years, more and more studies have found that ERS plays an important role in the abnormal decomposition of obesity lipids. Recent studies in ERS mice induced with tunicamycin have demonstrated that free fatty acid (FFA) and glycerol in circulating serum increased by 49% and 30%, respectively, compared with normal groups. This was accompanied by a decrease in adipose tissue mass and lipid droplets. The formation of a large number of lipid droplets in the liver cells of ERS mice was further observed by transmission electron microscopy, and obvious FFA deposition was also found in the gastrocnemius muscle and heart of mice.37 This suggests that ERS accelerates the lipolysis of adipose tissue and is a key factor causing ectopic lipid deposition. Torre et al38 also observed an abnormal increase in lipolysis in the subcutaneous fat of HFD-induced obese ERS mice; Lipid synthesis was also found to be inversely correlated with ERS in adipocytes of obese mice, and increased phosphorylated expression of hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) was observed in adipocytes. In contrast, treatment with ERS inhibitors significantly reduced the expression of lipolytic enzymes and attenuated lipolysis. These experimental results suggest that ERS under obese conditions may be one of the underlying mechanisms that induce abnormal lipolysis of adipose tissue, leading to increased circulating FFA and lipid deposition to other organs.
ERS and Defects in Fat Thermogenesis
Impaired adaptive thermogenesis in beige and brown fat plays an important role in obesity etiology,39 recent studies have found that ERS may be a key factor leading to impaired adaptive thermogenesis in obese individuals. Yuliana et al40 found that the expression of uncoupling protein 1 (UCP-1), which is involved in thermoregulation, was severely suppressed in the inguinal white fat of ERS mice compared with normal mice, at the same time, it was found that the mRNA level of peroxisome proliferator-activated receptor γ (PPARγ), which is involved in the regulation of Ucp1 expression, decreased significantly; on the contrary, after inhibiting ERS in adipose tissue, the expressions of PPARγ and Ucp1 proteins were significantly up-regulated. This indicates that ERS is a key factor affecting the energy balance of adipose tissue, and ERS inhibits the browning of white fat by down-regulating the expression of PPARγ in adipocytes, thereby reducing the thermogenic capacity of adipocytes. In the HFD-induced obesity mouse model, the key protein IRE1α in the adipose tissue ERS and UPR signaling pathways was also found to be up-regulated, this was accompanied by a downregulation of the rate-limiting enzyme tyrosine hydroxylase (TH) in catecholamine biosynthesis associated with activation of brown and beige fat thermogenesis and a reduction in UCP1 protein expression; In contrast, the expressions of UCP1 and TH were significantly increased in the brown adipose tissue of IRE1α knockout mice fed the same HFD.41 This suggests that ERS-mediated IRE1α signaling under obesity contributes to obesity-adaptive thermogenesis defects by inhibiting white fat browning and brown fat activity.
Mechanism of ERS Leading to Adipose Tissue Dysfunction in Obesity
Nuclear Factor Kappa-B (NF-κB)
NF-κB is an important signaling pathway involved in the response of cells to external stimuli, and plays a key role in cellular inflammatory responses, immune responses, etc.42 In recent years, it has been found that NF-κB is also an important transcription factor in the process of obesity ERS-induced adipose tissue inflammation.43 To explore whether NF-κB is involved in ERS-induced production of pro-inflammatory factors in adipose tissue, Chen et al44 used HFD to induce obese mice and found upregulation of ERS markers including glucose-regulated protein 78 (GRP78) and CHOP expression levels in their adipose tissue, and accompanied by the increase of resistin and TNF-α expression levels and the infiltration of macrophages and lymphocytes, the mRNA expressions of resistin and TNF-α in adipose tissue were significantly down-regulated after adding NF-κB inhibitors, this suggests that ERS is activated in the adipose tissue of HFD-induced obese mice and mediates downstream adipose tissue inflammatory responses through the NF-κB pathway. In addition, in vitro experiments in which 3T3-L1 adipocytes were preloaded with palmitate to generate artificially hypertrophied mature adipocytes also revealed that nuclear translocation of the NF-κB P65 subunit in adipocytes was significantly reduced by inhibiting the activated PERK and IRE1-related UPR pathways within the ER of hypertrophied mature adipocytes, thereby decreasing the expression and secretion of the NF-κB-dependent inflammatory cytokines MCP-1 and IL-6.45 It is suggested that reducing the NF-κB-dependent pathway by inhibiting the overactivated UPR pathway in adipocytes is a key measure to regulate abnormal inflammatory responses in adipocytes.
Adiponectin (ADPN)
ADPN is a protective adipokine produced only by adipocytes and plays multiple roles in anti-inflammation, regulation of energy homeostasis, and insulin sensitivity.46 Recent studies have found evidence that ERS inhibits ADPN processing and secretion in human adipocytes and contributes to obesity-associated metabolic disturbances.47,48 After induction of ERS in human adipose stem cells, it was found that the expression of TNF-α mRNA in adipocytes increased and led to the inflammation of adipose stem cells, while the mRNA and protein expressions of ADPN were significantly down-regulated.49 In contrast, treatment of HFD-induced obese mice with the ERS inhibitor tauroursodeoxycholic acid (TUDCA) found that serum levels of ADPN were significantly elevated and pro-inflammatory cytokines such as TNF-α and IL-6 were reduced in adipose tissue.50 This suggests that ERS-induced adipose tissue inflammation may be related to the decrease of ADPN level. Torre et al38 found that the reduction of ADPN synthesis was positively correlated with the increase of UPR markers in the visceral adipose tissue of obesity-induced ERS mice, and found that visceral fat lipolysis and circulating FFA were significantly increased in obese ERS mice compared with normal mice; After exposing subcutaneous primary adipocytes to drugs such as tunicamycin to induce ERS in vitro, maladaptive UPR also appeared in adipocytes, accompanied by decreased synthesis of ADPN and increased lipolysis. This suggests that ERS under obesity may affect lipolysis by inhibiting the secretion of ADPN and increase the release of FFA.
Six Transmembrane Protein of Prostate (STAMP2)
STAMP2 is a transmembrane protein with oxidoreductase-metaloreductase activity associated with inflammation and metabolic disorders,51 and has been shown to be a counter-regulator of inflammation and insulin resistance.52 STAMPs are closely related to the inflammatory and metabolic state of human obesity, Moreno-Navarrete et al53 found that STAMP protein expression was significantly negatively associated with obesity phenotype measures (body mass index, waist circumference, hip and fat mass) and obesity-related metabolic disorders, and positively associated with adipogenic gene expression, after investigating visceral fat in 171 obese subjects. At the same time, it was found that the adipose tissue of obese subjects with low expression of STAMP protein was infiltrated by macrophages and showed an inflammatory state accompanied by increased circulating monocytes. This suggests that STAMPs are involved in the regulation of obesity metabolism and play an important role in obesity-associated adipose tissue inflammation. To explore the mechanism by which obesity affects the expression of STAMP2, Sikkeland et al54 found that ERS significantly reduced adipocyte STAMP2 mRNA and protein expression after in vitro treatment of 3T3-L1 adipocytes with ERS inducer, while genetic analysis and chromatin immunoprecipitation of the STAMP2 promoter revealed that ERS induction disrupted transcription factor CCAAT/ enhancer binding protein α (C/EBPα) mediated STAMP2 expression, which in turn affects total iron reductase activity and leads to the secretion of inflammatory factors such as TNF-α in adipocytes. In contrast, adenovirus overexpression of STAMP2 in a HFD-induced obese mouse model significantly improved adipose tissue inflammation and significantly reduced FFA-induced abnormal lipid accumulation.55 These studies suggest that STAMP2 is a key regulator of the inflammatory and metabolic integrated response system in obese adipose tissue and that ERS under obese conditions can stimulate the secretion of adipocyte inflammatory factors and lead to disruption of lipid metabolism by suppressing the expression of STAMP2.
Lpin1
LPIN1 acts as a phosphatidic acid phosphatase in adipocytes to catalyze the formation of diacylglycerol from phosphatidic acid, thereby promoting triglyceride synthesis.56 In recent years, it has been found that LPIN1 interacts with multiple transcription factors such as peroxisome proliferator-activated receptor-γ (PPAR-γ) as a co-activator to regulate lipid metabolism and regulate the expression of pro-inflammatory cytokines in adipocytes by acting as a co-repressor.57,58 By studying the effect of ERS on the expression of LPIN1 in 3T3-L1 adipocytes in vitro, it was found that ERS in adipocytes suppressed the expression of LPIN1 at the transcriptional level and induced the formation of MCP-1. These evidences suggest that ERS is associated with the expression of LPIN1 in adipocytes and the secretion of adipose inflammatory cytokines. In contrast, the abnormal lipolysis of epididymal fat in LPIN1-overexpressing transgenic mice was significantly attenuated and the expression of lipolytic enzymes including p-HSL and ATGL was reduced compared with normal mice, accompanied by the downregulation of pro-inflammatory cytokines and chemokines including TNF-α, IL-6 and MCP-1 in adipose tissue.59 This suggests that the overexpression of LPIN1 in adipocytes can alleviate the abnormal lipid metabolism and inflammatory response induced by ERS, and LPIN1 may be an important regulator of abnormal metabolism of adipose tissue caused by ERS.To further investigate the effect of LPIN1 on adipose tissue metabolism in obese patients, Miranda et al60 found that the expression level of LPIN1 significantly decreased and was accompanied by an increase in the expression level of ERS genes (CHOP 10, XBP 1, etc.) as the obesity level increased in the subjects by quantifying the gene expression in the abdominal adipose tissue of 62 subjects, and found that LPIN1 mRNA expression was negatively correlated with the level of pro-inflammatory cytokines secreted by adipose tissue, and these associations suggest that ERS may play a role in the inflammation of adipose tissue by regulating LPIN1 under chronic obesity conditions.
TRIP-Br2
TRIP-Br2 is a newly discovered transcriptional co-regulator that, in addition to its regulatory role in the cell cycle, regulates multiple processes including lipolysis, thermogenesis and oxidative metabolism to regulate fat energy metabolism in obesity.61,62 To determine the relationship between obesity ERS and obesity-related metabolic dysfunction and TRIP-Br2 high expression, Qiang et al63 found in HFD-induced obese mice that TRIP-Br2 was highly expressed in visceral fat such as retroperitoneal, perirenal and mesenteric fat and was accompanied by elevated expression of inflammatory markers such as IL-6, TNF-α and Mcp-1, while reduced expression of macrophage markers and MCP-1 mRNA was found in the visceral fat of TRIP-Br2 knockout obese transgenic mice; in contrast, after treatment with the ERS inhibitor TUDCA in normal obese mice, it was found that the expression of TRIP-Br2 and inflammatory markers were attenuated with the inhibition of ERS. This suggests that obesity-mediated ERS can induce high expression of TRIP-Br2 in adipose tissue, and TRIP-Br2 may be a key molecular mediator of ERS-induced visceral fat inflammation and acute phase response. TRIP-Br2 also plays an important role in the abnormal energy metabolism of obesity, as specific upregulation of the transcriptional regulator TRIP-Br2 was found in brown fat of obese ERS mice, and increased TRIP-Br2 significantly reduced the expression of genes involved in thermogenesis, oxidative metabolism and mitochondrial biogenesis, such as Ucp1, peroxisome proliferator-activated receptorγcoactivator-1 (PGC1α), enoyl Coenzyme A hydratase 1 (ECH1) and cytochrome c1 (Cyc1); in contrast, silencing of TRIP-Br2 showed higher oxygen consumption rates in brown adipose tissue and restored energy metabolism in brown adipocytes, suggesting that obese ERS regulates energy metabolism in obese adipose tissue by upregulating TRIP-Br2 to inhibit adipocyte thermogenesis and oxidative metabolism.62,64
Nuclear Transcription Factor Y (NF-Y)
NF-Y is a ubiquitous heterotrimeric transcription factor, NF-Y can regulate adipocyte energy metabolism by affecting adipocyte differentiation and fatty acid synthesis.65 Lu66 used qPCR to detect mRNA levels of various adipocyte-specific genes in NF-Y knockout mice and found that mRNA levels of lipocalin and leptin were significantly reduced, as were RNA levels of other adipose marker RNAs including FABP4, PPARg, CEBPs, CD36, enyl - coa dehydrogenase, hydroxycoa dehydrogenase, and Glut4, and the general downregulation of these adipocyte-specific gene levels indicated that NF-Y knockout caused significant effects on adipocyte mass. Liu67 discovered that obesity-induced endoplasmic reticulum stress can inhibit the transcription of NF-Y mRNA by participating in the synthesis of PPARγ, thereby affecting the mass of adipose tissue and causing abnormal expansion of obese adipose tissue. Therefore, we speculate that NF-Y is a new intermediate product between ERS and obesity, and ERS may participate in the pathogenesis of obesity by inhibiting the expression of NF-Y.
Sirtuin2 (SIRT2)
SIRT2 is a NAD(+)-dependent deacetylase associated with various processes such as neurodegeneration, DNA damage, carcinogenesis, and infection.68–71 Recent studies have found that it also plays an important role in the balance of lipid metabolism in obesity. Leal72 found a concentration-dependent increase in lipid deposition in HepG2 cells treated with the ERS inducer thapsigargin (Tg), suggesting that ERS may directly promote lipid accumulation in hepatocytes. To determine the role of SIRT2 in ERS and lipid overload, investigators incubated SIRT2-silenced or SIRT2 overexpressing hepatocytes with Tg and observed that SIRT2 silencing increased lipid deposition to the same extent as Tg treatment. Therefore, we believe that ERS under obesity will down-regulate SIRT2 and cause lipid overload, which will eventually cause disturbance of liver lipid homeostasis and induce abnormal lipid deposition in liver and hepatic steatosis.
Treatment
miRNA
In recent years, studies have found that the dysregulation of miRNA may affect the state and function of different tissues and organs, such as pancreas, liver, fat and muscle tissue, which may lead to obesity and related metabolic diseases.73 Liu74 found that the expression of miR-320 in 3T3-L1 adipocytes in the obese group was significantly higher than that in the normal somatic group, and miR-320 was positively correlated with the expression of ERS markers and inflammatory factors, ie, the up-regulation of miR-320 could promote the expression of ERS-related markers (GRP78, GRP94, Derlin-1, and CHOP) as well as inflammatory factors (TNF-α, NF-κB, and IL-6 mRNA), and accordingly, we hypothesized that knocking down miR-320 might ameliorate ERS and inflammatory responses in 3T3-L1 adipocytes. Chen75 found that miR-149 expression in hepatocytes of non-alcoholic fatty liver disease (NAFLD) mice was significantly lower than that of the normal group, while the mRNA and protein expression of ATF6 was significantly higher than that of the normal group. When the expression of miR-149 was significantly increased, the mRNA and protein expression of ATF6 was significantly suppressed, suggesting that ATF6 may be a target gene of miR-149 and the expression of miR-149 and ATF6 were negatively correlated. It was also observed that the expression of XBP1 and GRP78 in NAFLD mice was significantly higher than that in the control and normal groups, and the overexpression of miR-149 was found to suppress the protein level of GRP78, which indicated that ERS occurred in the hepatocytes of NAFLD mice, and miR-149 overexpression could alleviate ERS in the hepatocytes of NAFLD mice. In summary, we believe that miR-149 may affect ERS by negatively targeting the ATF6 signaling pathway. In fact, other studies have shown that miRNAs may be a potential mechanism by which the renin-angiotensin system (RAS) regulates molecular signaling pathways.76 Overexpression of angiotensinogen (Agt), a precursor protein of RAS, in adipose tissue or treatment of adipocytes with the RAS bioactive hormone angiotensin II (Ang II) alters miRNAs targeting ERS and inflammation, resulting in adipocyte dysfunction. Menikdiwela77 used small RNA sequencing and microarrays to identify differentially expressed miRNAs and genes in mouse epididymal white adipose tissue (WAT). miR-690 and mitogen-activated protein kinase 3 (MAP2K3) were shown to be significantly upregulated by miR-690 and downregulated by MAP2K3 in Agt overexpressed and Ang II-treated adipocytes, respectively, compared to the corresponding controls. Furthermore, miR-690 directly downregulated the downstream protein targets of MAP2K3, such as p38, NF-κB, IL-6, and CHOP, using mimic, inhibitor, and dual-luciferase reporter assays. This suggests that the critical role of miR-690 in suppressing inflammation (NF-κB, IL-6) and ERS (CHOP) may be achieved by targeting MAP2K3. In conclusion, most studies tend to believe that miRNAs are able to combat obesity by inhibiting adipocyte ERS, reducing the secretion of adipocyte pro-inflammatory factors, improving the chronic inflammatory state of adipocytes in obese patients, and restoring ER homeostasis.
Renin-Angiotensin System Inhibitors
Excessive activation of Renin-angiotensin system (RAS) in obesity disrupts the metabolic homeostasis of adipocytes and induces ERS and inflammation, but the underlying mechanisms have not been elucidated. Ang II is a key bioactive component of RAS and one of several pro-inflammatory adipokines secreted by adipocytes, mainly involved in the regulation of blood pressure and fluid balance. Telmisartan is an angiotensin II receptor blocker and a partial agonist of PPARγ. Several studies have confirmed the beneficial effects of telmisartan in glucose and lipid metabolism.78 AMPK is a ubiquitous serine/threonine protein kinase involved in almost all branches of cellular metabolism and plays a key role in the regulation of fatty acid metabolism, adipose tissue thermogenesis and development.79 AMPK activation improves insulin sensitivity, improves obesity. Telmisartan was able to reduce mRNA expression of ERS-related genes (such as Xbp1s, Grp78, Chop and Atf6) in mouse adipose tissue, and AMPK activation was observed both in mouse adipose tissue and in 3T3-L1 adipocytes. The researchers used AMPK inhibitor compound C to further validate that telmisartan inhibits ERS by activating the AMPK signaling pathway. Telmisartan significantly decreased the expression of TNF-α, MCP-1, and IL-18 in the epididymal adipose tissue of mice compared with the control group, and reduced the infiltration of macrophages, which indicated that telmisartan relieved visceral adipose tissue Inflammation of tissue.80 It is well known that long-term application of angiotensin receptor inhibitors will significantly increase the level of Ang (1–7). Ma81 found that Ang (1-7)-treated mice had reduced visceral adipose tissue, decreased leptin levels, reduced adipocyte volume, adipogenesis in epididymal adipose tissue, and downregulated ERS-related proteins compared with mice in the saline group, suggesting that Ang (1–7) alleviates adipocyte hypertrophy and adipose tissue expansion and inhibits ERS in mice with visceral adipose tissue. Furthermore when Mas knockout mice showed increased visceral adiposity, elevated leptin levels, adipocyte enlargement, adipogenesis in epididymal adipose tissue, and upregulation of ERS-related protein expression, we therefore hypothesized that Ang (1–7) inhibits adipose tissue ERS by targeting the Mas gene, thereby reducing visceral adipose tissue expansion and adipocyte hypertrophy. In conclusion, we believe that inhibitors of the renin-angiotensin system inhibit ERS by targeting specific genes or signaling pathways in mouse adipose tissue, reducing adipocyte size and ameliorating the abnormal expansion of adipose tissue, as well as being able to inhibit the expression of inflammatory factors, reducing the infiltration of pro-inflammatory macrophages, and restoring metabolic homeostasis in adipose tissue, thus ameliorating obesity.
Chinese Herbal Extract
Chinese herbal medicine has a long history of clinical application in improving obesity and metabolic disorders. In recent years, many studies have shown that it can improve the dysfunction of obese adipose tissue by inhibiting ERS in adipose tissue. Although traditional Chinese medicine extracts have not been fully used for new drug development, it has great potential in improving obesity. Traditional Chinese medicine believes that panax notoginseng (PNE) has the functions of promoting blood circulation, removing blood stasis, stopping bleeding, reducing swelling and pain, etc. Modern pharmacological analysis of PNE contains a variety of components such as saponins, flavonoids, etc,82 among them, Panax Notoginseng Saponins (PNS) plays an important role in improving metabolic disorders.83 Tan et al84 found that PNS and PNE reversed the upregulation of GRP78, ATF6, CHOP, cystatin-3 and JNK in obese mice, suggesting that PNS and PNE inhibited ERS in adipose tissue of obese mice. Activation of the p38/JNK signaling pathway is involved in regulating inflammation in addition to mediating apoptosis, in which PNE exhibits an inhibitory effect on p38 phosphorylation, so we hypothesized that PNE not only inhibits ERS, but also participates in the regulation of ERS-induced adipose tissue inflammation, thereby alleviating adipose tissue dysfunction and controlling obesity. Quercetin is a powerful antioxidant found in a wide range of sources, such as North Radix Ginseng, Coix Seed, Asparagus, Blueberry, and more.85 Perdicaro et al86 observed that quercetin reduced the levels of proteins involved in adipose inflammation (TLR-4, CD68, MCP-1, JNK) and ERS-related proteins (ATF-6, XBP-1) and plasma concentrations of TNF-α in high-fat diet-induced obese rats, while increasing the expression of anti-inflammatory and insulin-sensitive cytokines (ADPN). This suggests that quercetin can also prevent and control obesity by improving high-fat diet-induced ERS, ameliorating adipose tissue inflammation, increasing ADPN synthesis, and alleviating adipose tissue dysfunction. Mangiferin is a glucosyl flavonoid, which has been proven to improve blood lipid disorders,87 anti-diabetes,88 anti-cancer,89 anti-infection,90 neuroprotection91 and other effects. Xu et al92 found that palmitate (PA) stimulation induced IRE1α and eIF-2α phosphorylation in perivascular adipose tissue (PVAT), but its phosphorylation was inhibited by treatment with mangiferin (concentrations in the range of 0.1 to 10.0 μM), except that mangiferin inhibited IL-6 production by promoting ADPN secretion. The “silencing” of AMPKα1/α2 and specific siRNA in adipocytes attenuated the effect of mangiferin on the dephosphorylation of IRE1α and eIF-2α, this suggests that mangiferin inhibits ERS by activating AMPK, and similar to quercetin mangiferin is also able to inhibit adipose tissue inflammation and improve adipose tissue dysfunction by promoting ADPN secretion for obesity control. Adipose tissue secretes a variety of soluble proteins, commonly referred to as adipokines, and the release of large amounts of inflammatory adipokines and free fatty acids can exacerbate ectopic fat deposition in muscle and liver.93 Chen et al94 found that resveratrol (RES) treatment suppressed the mRNA expression of ERS-related molecules (eg, IRE1α, CHOP, GRP78) in adipose tissue as well as 3T3-L1 cells in mice, and decreased the levels of three inflammatory factors (TNF-α, IL-6, MCP-1) and significantly increased the mRNA of ADPN, SIRT1, and PPARα expression, suggesting that RES ameliorated adipocyte hypertrophy by attenuating ERS and inflammatory responses in adipose tissue as well as in 3T3-L1 cells, reduced adipokine expression, reversed adipose tissue dysfunction, and further increase the expression of SIRT1 in vitro and in vivo to restore insulin sensitivity in mice, and also improve adipose tissue thermogenesis defects by increasing the expression of PPARα, which increases thermogenesis, burns energy and controls obesity. Curcumin is a natural polyphenol with anti-inflammatory, anti-oxidant and other effects, which has been shown to improve obesity and related syndromes in recent years.95 Curcumin treatment effectively reduced the accumulation of cAMP and PKA 62KDa substrate phosphorylation in adipose tissue of HFD-fed mice, and inhibited the phosphorylation of IRE1αand eIF2α, and reduced the accumulation of triglycerides and diglycerides in hepatocytes, which suggests that curcumin alleviates ERS, inhibits lipolysis, and reduces the deposition of aberrant lipids in the liver through the cAMP/PKA pathway, thus altering obesity.96 STAT5 signaling is activated by metabolic hormones and growth factors and is primarily involved in the regulation of food intake, body fat, and glucose homeostasis.97 PPARγ is a major transcription factor induced by the STAT5 signaling pathway during adipogenesis.98 Lee et al99 confirmed for the first time that ginsenoside Rg3 can improve obesity-induced insulin resistance and lipotoxicity through the STAT5-PPARγ pathway in vitro and in vivo. The size of adipocytes can indirectly reflect lipid metabolism. Yao et al100 found that ginsenoside (GS) administration significantly reduced feeding as well as liver weight, abdominal fat, epididymal fat and perirenal fat in mice, suggesting that GS may improved obesity by suppressing appetite in mice. It was also observed that GS inhibited the expression of GRP78, CHOP, and ATF4 in hepatic adipocytes and reversed hepatic steatosis in high-fat diet-fed mice, suggesting that inhibition of ERS may be an effective way for GS to reverse steatosis. The above studies found that herbal monomers improve obesity by inhibiting ERS in various ways, including inhibiting the inflammatory response of adipose tissue, ameliorating the defect of adipose tissue thermogenesis, and inhibiting the abnormal catabolism of adipose tissue.
Other Treatment Avenues
Some studies believe that vitamin deficiency is closely related to obesity and related metabolic disorders.101 After human preadipocytes and adipocytes were co-treated with the pro-inflammatory cytokine TNFα (10 ng/mL) and saturated fatty acid palmitate (300 μM) for 24 hr, Luo et al102 found that the expression of ERS-associated markers, sXBP1, CHOP, and GRP78 mRNAs in preadipocytes was significantly increased by 6- to 20-fold, whereas vitamin C co-treatment not only reduced these ERS-associated markers to the baseline values, but also promoted the secretion of ADPN. This suggests that vitamin C may improve adipose tissue dysfunction and restore adipose tissue metabolic homeostasis by inhibiting ERS and increasing ADPN secretion. In addition, pro-inflammatory macrophages are thought to be critical in the development of obesity, blocking pro-inflammatory macrophage polarization through pharmacological intervention has become a research hotspot in the prevention and treatment of obesity, and Mcardle et al103 revealed that vitamin C can regulate macrophage pro-inflammatory properties. The application of non-thermal atmospheric plasma (NTP) is gradually expanding from biomedical to various other fields, and to clarify the inhibitory effect of non-thermal plasma treatment solution (NTS) on adipocyte differentiation, researchers used NTS to treat 3T3-L1 cells and found that the expression of GRP78, CHOP, p-PERK and p-eIF2α within them was significantly inhibition and significantly reduced the formation of lipid droplets within adipocytes and decreased the mRNA levels of lipogenic factors PPARγ and C/EBPα, suggesting that the inhibition of lipogenic differentiation of preadipocytes by NTS may be related to its significant inhibition of ERS and UPR activation.104 Accordingly, we hypothesize that NTS prevents obesity by inhibiting ERS and UPR activation, reducing lipid droplet formation and lipogenic differentiation of preadipocytes, and inhibiting abnormal adipocyte catabolism and ectopic lipid deposition. Fibroblast growth factor 21 (FGF21) is a novel endocrine hormone. Guo et al105 administered FGF21 in high-fat diet-induced obese mice and membranomycin-induced 3T3-L1 adipocytes and assessed metabolic parameters, ERS indexes, etc., by protein blotting, and found that FGF21 treatment resulted in the down-regulation of phosphorylated expression of CHOP, ATF4, and eIF2α, while up-regulation of ADPN expression in adipose tissues of obese mice, and that FGF 21’s effect of increasing insulin sensitivity was significantly inhibited by knocking down ADPN. This suggest that FGF21 treatment reverses the inhibition of ADPN expression, improves insulin sensitivity, adjusts the metabolic disordered state of adipose tissue and adipocytes, and ameliorates their dysfunction by inhibiting ERS in the adipose tissue of obese mice in order to combat obesity. Diacylglycerol acyltransferase 1 (DGAT1), a member of the membrane-bound O-acyltransferase gene family, is a multifunctional ER membrane protein whose likely active site is located on the luminal side of the ER membrane.106 Xu et al107 investigators found that DGAT1 overexpression in adipose tissue of ketotic cows attenuated adrenaline-induced phosphorylation of IRE1 and PERK and inhibited the secretion of TNF-α and IL-6, suggesting that DGAT1 may be a potential target for inhibiting ERS and attenuating the inflammatory response. Chitraju108 demonstrated that DGAT1 mRNA levels in human adipose tissue are inversely correlated with many genes associated with ERS. In the future, it may be possible to improve the inflammatory state of adipose tissue and alleviate adipose tissue dysfunction by targeting DGAT1 to inhibit ERS in order to control obesity progression. In vivo injection of CYP2J3 gene has been proven to have anti-inflammatory, anti-hypertensive, and improved insulin resistance effects.109 Xu et al further observed in 3T3-L1 adipocytes that overexpression of CYP2J3 significantly prevented TG-induced ADPN downregulation, accompanied by attenuated ERS characterized by reduced CHOP and GRP78 expression. This suggests that CYP2J3 overexpression may ameliorate adipocyte dysfunction and combat obesity by inhibiting the 3T3-L1 adipocyte ERS and upregulating ADPN expression.110
Conclusion
From the above analysis, it is clear that ERS is closely related to adipose tissue dysfunction in obese conditions, and ER homeostasis is involved in regulating adipose metabolism through ER stress as well as UPR, promoting the secretion of inflammatory factors and macrophage infiltration in adipose tissue, inducing abnormal lipolysis in adipose tissue to cause increased circulating FFA and ectopic deposition of lipids, and inhibiting the browning of white fat and the thermogenic capacity of brown fat, ultimately leading to abnormal metabolism of adipose tissue in obesity. Under obesity conditions, the effects of ERS on molecules such as NF-κB, ADPN, STAMP2, LPIN1, TRIP-Br2, NF-Y, and SIRT2 are important causes of adipose tissue dysfunction. miRNA, renin-angiotensin system inhibitors, and traditional Chinese medicine extracts have been widely used to inhibit ERS in adipose tissue. However, the molecular mechanism of adipose tissue dysfunction in obesity caused by ERS still needs further study, and this review expects to provide new ideas and theoretical basis for the treatment of human obesity-related metabolic abnormal diseases.
Main Concepts and Learning Points
- Adipocyte endoplasmic reticulum stress leads to adipose tissue dysfunction and metabolic disorders in obese patients.
- ERS induces metabolic disorders such as catabolism, thermogenesis and inflammation in obese adipose tissue by modulating molecules and pathways such as NF-κB, ADPN, STAMP2, LPIN1, TRIP-Br2, NF-Y and SIRT2.
- MiRNAs, Renin-angiotensin system inhibitors, Chinese herbal extract, and other drugs can be used to improve adipose tissue dysfunction in obese states.
Acknowledgments
The Shandong Traditional Chinese Medicine Science and Technology Development Project Fund of China (2019–0108) and Qilu Internal Medicine Academic School of Blood Turbidity Inheritance Project Fund of China (Lu WeiHan [2021] No. 45) funded this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Singh R, Barrios A, Dirakvand G, et al. Human brown adipose tissue and metabolic health: potential for therapeutic avenues. Cells. 2021;10(11):3030. doi:10.3390/cells10113030
2. Alcalá M, Calderon-Dominguez M, Serra D, et al. Mechanisms of impaired brown adipose tissue recruitment in obesity. Front Physiol. 2019;2019:10.
3. Jia W. Obesity, metabolic syndrome and bariatric surgery: a narrative review. J Diabetes Investig. 2019;11(2):294–296. doi:10.1111/jdi.13236
4. Sarma S, Sockalingam S, Dash S. Obesity as a multisystem disease: trends in obesity rates and obesity‐related complications. Diabetes Obes Metab. 2021;23(S1):3–16. doi:10.1111/dom.14290
5. Tremmel M, Gerdtham U, Nilsson P, et al. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14(4):435. doi:10.3390/ijerph14040435
6. Bahia L, Schaan CW, Sparrenberger K, et al. Overview of meta-analysis on prevention and treatment of childhood obesity. J Pediatr. 2019;95(4):385–400. doi:10.1016/j.jped.2018.07.009
7. Boden G, Merali S. Measurement of the increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Methods Enzymol. 2011;489:67–82.
8. Ferro-Novick S, Reggiori F, Brodsky JL. ER-Phagy, ER Homeostasis, and ER quality control: implications for disease. Trends Biochem Sci. 2021;46(8):630–639. doi:10.1016/j.tibs.2020.12.013
9. Scherer PE. The many secret lives of adipocytes: implications for diabetes. Diabetologia. 2019;62(2):223–232. doi:10.1007/s00125-018-4777-x
10. Freyre CA, Rauher PC, Ejsing CS, et al. MIGA2 links mitochondria, the ER, and lipid droplets and promotes de novo lipogenesis in adipocytes. Mol Cell. 2019;76(5):811–825. doi:10.1016/j.molcel.2019.09.011
11. Zhao M, Zang B, Cheng M, et al. Differential responses of hepatic endoplasmic reticulum stress and inflammation in diet-induced obese rats with high-fat diet rich in lard oil or soybean oil. PLoS One. 2013;8(11):e78620. doi:10.1371/journal.pone.0078620
12. Veiga FMS, Graus-Nunes F, Rachid TL, et al. Anti-obesogenic effects of WY14643 (PPAR-alpha agonist): hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice. Biochimie. 2017;140:106–116. doi:10.1016/j.biochi.2017.07.003
13. Almanza A, Carlesso A, Chintha C, et al. Endoplasmic reticulum stress signalling – from basic mechanisms to clinical applications. FEBS J. 2019;286(2):241–278. doi:10.1111/febs.14608
14. van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi:10.1038/nrm2330
15. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi:10.1038/nrm2199
16. Ghemrawi R, Battaglia-Hsu S, Arnold C. Endoplasmic reticulum stress in metabolic disorders. Cells. 2018;7(6):63. doi:10.3390/cells7060063
17. Pavlović N, Heindryckx F. Targeting ER stress in the hepatic tumor microenvironment. FEBS J. 2021;289:7163–7176. doi:10.1111/febs.16145
18. Teske BF, Wek SA, Bunpo P, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22(22):4390–4405. doi:10.1091/mbc.e11-06-0510
19. Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14(8):996–1007. doi:10.1007/s10495-009-0341-y
20. Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69(2):169–181. doi:10.1016/j.molcel.2017.06.017
21. Tabas I, Kitakaze M. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107(7):839–850. doi:10.1161/CIRCRESAHA.110.224766
22. Menikdiwela KR, Ramalingam L, Allen L, et al. Angiotensin II increases endoplasmic reticulum stress in adipose tissue and adipocytes. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-44834-8
23. Boden G, Duan X, Homko C, et al. Increase in endoplasmic reticulum stress–related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57(9):2438–2444. doi:10.2337/db08-0604
24. Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18(1):59–68. doi:10.1016/j.molmed.2011.07.010
25. Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–531. doi:10.1038/nature09968
26. Zha BS, Zhou H, Zhang K. ER Stress and Lipid Metabolism in Adipocytes. Biochem Res Int. 2012;2012:312943–312949. doi:10.1155/2012/312943
27. Lee S, Min KT. The interface between ER and mitochondria: molecular compositions and functions. Mol Cells. 2018;41(12):1000–1007. doi:10.14348/molcells.2018.0438
28. Han J, Kaufman RJ. Measurement of the unfolded protein response to investigate its role in adipogenesis and obesity. Methods Enzymol. 2014;538:135–150.
29. Yuzefovych LV, Musiyenko SI, Wilson GL, et al. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8(1):e54059. doi:10.1371/journal.pone.0054059
30. Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700. doi:10.2337/db08-1220
31. Menikdiwela KR, Torres GJ, Ramalingam L, et al. Mechanisms linking endoplasmic reticulum (ER) stress and microRNAs to adipose tissue dysfunction in obesity. Crit Rev Biochem Mol Biol. 2021;56(5):455–481. doi:10.1080/10409238.2021.1925219
32. Fernandes-Da-Silva A, Miranda CS, Santana-Oliveira DA, et al. Endoplasmic reticulum stress as the basis of obesity and metabolic diseases: focus on adipose tissue, liver, and pancreas. Eur J Nutr. 2021;60(6):2949–2960. doi:10.1007/s00394-021-02542-y
33. Kawasaki N, Asada R, Saito A, et al. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep. 2012;2(1). doi:10.1038/srep00799
34. Suzuki T, Gao J, Ishigaki Y, et al. ER stress protein CHOP mediates insulin resistance by modulating adipose tissue macrophage polarity. Cell Rep. 2017;18(8):2045–2057. doi:10.1016/j.celrep.2017.01.076
35. Alicka M, Marycz K. The effect of chronic inflammation and oxidative and endoplasmic reticulum stress in the course of metabolic syndrome and its therapy. Stem Cells Int. 2018;2018:1–13.36. doi:10.1155/2018/4274361
36. Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644–656. doi:10.1016/j.cmet.2013.03.008
37. Bogdanovic E, Kraus N, Patsouris D, et al. Endoplasmic reticulum stress in adipose tissue augments lipolysis. J Cell Mol Med. 2015;19(1):82–91. doi:10.1111/jcmm.12384
38. Torre Villalvazo I, Bunt AE, Alemán G, et al. Adiponectin synthesis and secretion by subcutaneous adipose tissue is impaired during obesity by endoplasmic reticulum stress. J Cell Biochem. 2018;119(7):5970–5984. doi:10.1002/jcb.26794
39. Poher A, Altirriba J, Veyrat-Durebex C, et al. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol. 2015;6:4.
40. Yuliana A, Daijo A, Jheng H, et al. Endoplasmic reticulum stress impaired uncoupling protein 1 expression via the suppression of peroxisome proliferator-activated receptorγbinding activity in mice beige adipocytes. Int J Mol Sci. 2019;20(2):274. doi:10.3390/ijms20020274
41. Shan B, Wang X, Wu Y, et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18(5):519–529. doi:10.1038/ni.3709
42. Wang Y, Cheng Y, Yin X, et al. Anxa2 gene silencing attenuates obesity-induced insulin resistance by suppressing the NF-κB signaling pathway. Am J Physiol Cell Physiol. 2019;316(2):C223–C234. doi:10.1152/ajpcell.00242.2018
43. Lappas M, Yee K, Permezel M, et al. Sulfasalazine and BAY 11-7082 interfere with the nuclear factor-κB and IκB kinase pathway to regulate the release of proinflammatory cytokines from human adipose tissue and skeletal muscle in vitro. Endocrinology. 2005;146(3):1491–1497. doi:10.1210/en.2004-0809
44. Chen Y, Wu Z, Zhao S, et al. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci Rep. 2016;6:1.
45. Yin J, Gu L, Wang Y, et al. Rapamycin improves palmitate-induced ER Stress/NFκB pathways associated with stimulating autophagy in adipocytes. Mediators Inflamm. 2015;2015:1–12.
46. Choi HM, Doss HM, Kim KS. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int J Mol Sci. 2020;21(4):1219. doi:10.3390/ijms21041219
47. Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30(5):234–239. doi:10.1016/j.tips.2009.02.004
48. Altomonte J, Harbaran S, Richter A, et al. Fat depot-specific expression of adiponectin is impaired in zucker fatty rats. Metabolism. 2003;52(8):958–963. doi:10.1016/S0026-0495(03)00092-1
49. Mondal AK, Das SK, Varma V, et al. Effect of endoplasmic reticulum stress on inflammation and adiponectin regulation in human adipocytes. Metab Syndr Relat Disord. 2012;10(4):297–306. doi:10.1089/met.2012.0002
50. Zhou L, Liu M, Zhang J, et al. DsbA-L Alleviates Endoplasmic Reticulum Stress-Induced Adiponectin Downregulation. Diabetes. 2010;59(11):2809–2816.
51. Sikkeland J, Sheng X, Jin Y, et al. STAMPing at the crossroads of normal physiology and disease states. Mol Cell Endocrinol. 2016;425:26–36. doi:10.1016/j.mce.2016.02.013
52. Wellen KE, Fucho R, Gregor MF, et al. Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell. 2007;129(3):537–548. doi:10.1016/j.cell.2007.02.049
53. Moreno-Navarrete JM, Ortega F, Serrano M, et al. DecreasedSTAMP2 expression in association with visceral adipose tissue dysfunction. J Clin Endocrinol Metab. 2011;96(11):E1816–E1825. doi:10.1210/jc.2011-0310
54. Sikkeland J, Lindstad T, Nenseth HZ, et al. Inflammation and ER stress differentially regulate STAMP2 expression and localization in adipocytes. Metabolism. 2019;93:75–85. doi:10.1016/j.metabol.2019.01.014
55. Kim HY, Park SY, Lee MH, et al. Hepatic STAMP2 alleviates high fat diet-induced hepatic steatosis and insulin resistance. J Hepatol. 2015;63(2):477–485. doi:10.1016/j.jhep.2015.01.025
56. Zhou F, Fan X, Miao Y. LPIN1 promotes triglycerides synthesis and is transcriptionally regulated by PPARG in Buffalo mammary epithelial cells. Sci Rep. 2022;12:1.
57. Finck BN, Gropler MC, Chen Z, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 2006;4(3):199–210. doi:10.1016/j.cmet.2006.08.005
58. Kim HB, Kumar A, Wang L, et al. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol Cell Biol. 2010;30(12):3126–3139. doi:10.1128/MCB.01671-09
59. Zhang W, Zhong W, Sun Q, et al. Adipose-specific lipin1 overexpression in mice protects against alcohol-induced liver injury. Sci Rep. 2018;8:1.
60. Miranda M, Escoté X, Ceperuelo-Mallafré V, et al. Relation between human LPIN1, hypoxia and endoplasmic reticulum stress genes in subcutaneous and visceral adipose tissue. Int J Obes. 2010;34(4):679–686. doi:10.1038/ijo.2009.290
61. Tseng Y, Butte AJ, Kokkotou E, et al. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat Cell Biol. 2005;7(6):601–611. doi:10.1038/ncb1259
62. Liew CW, Boucher J, Cheong JK, et al. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nat Med. 2013;19(2):217–226. doi:10.1038/nm.3056
63. Qiang G, Kong HW, Fang D, et al. The obesity-induced transcriptional regulator TRIP-Br2 mediates visceral fat endoplasmic reticulum stress-induced inflammation. Nat Commun. 2016;7:11378. doi:10.1038/ncomms11378
64. Qiang G, Whang Kong H, Gil V, et al. Transcription regulator TRIP-Br2 mediates ER stress-induced brown adipocytes dysfunction. Sci Rep. 2017;7(1). doi:10.1038/srep40215
65. Nishi-Tatsumi M, Yahagi N, Takeuchi Y, et al. A key role of nuclear factor Y in the refeeding response of fatty acid synthase in adipocytes. FEBS Lett. 2017;591(7):965–978.
66. Lu Y, Dallner OS, Birsoy K, et al. Nuclear Factor-Y is an adipogenic factor that regulates leptin gene expression. Mol Metab. 2015;4(5):392–405. doi:10.1016/j.molmet.2015.02.002
67. Liu Y, Zhang Y, Zhang Y, et al. Obesity-induced endoplasmic reticulum stress suppresses nuclear factor-Y expression. Mol Cell Biochem. 2017;426(1–2):47–54.
68. Liu S, Zhou Z, Zhang L, et al. Inhibition of SIRT2 by Targeting GSK3β-mediated phosphorylation alleviates SIRT2 Toxicity in SH-SY5Y cells. Front Cell Neurosci. 2019;13:13. doi:10.3389/fncel.2019.00013
69. Nguyen P, Shukla S, Liu R, et al. Sirt2 regulates radiation-induced injury. Radiat Res. 2019;191(5):398. doi:10.1667/RR15282.1
70. Minten EV, Kapoor-Vazirani P, Li C, et al. SIRT2 promotes BRCA1-BARD1 heterodimerization through deacetylation. Cell Rep. 2021;34(13):108921. doi:10.1016/j.celrep.2021.108921
71. Pereira JM, Chevalier C, Chaze T, et al. Infection reveals a modification of SIRT2 critical for chromatin association. Cell Rep. 2018;23(4):1124–1137. doi:10.1016/j.celrep.2018.03.116
72. Leal H, Cardoso J, Valério P, et al. SIRT2 deficiency exacerbates hepatic steatosis via a putative role of the ER stress pathway. Int J Mol Sci. 2022;23(12):6790. doi:10.3390/ijms23126790
73. Mennigen JA. Micromanaging metabolism—a role for miRNAs in teleost energy metabolism. Comparative biochemistry and physiology part B. Biochem Mol Biol. 2016;199:115–125. doi:10.1016/j.cbpb.2015.09.001
74. Liu L, Li X. Downregulation of miR-320 alleviates endoplasmic reticulum stress and inflammatory response in 3T3-L1 adipocytes. Exp Clin Endocrinol Diabetes. 2021;129(2):131–137. doi:10.1055/a-1012-8420
75. Chen Z, Liu Y, Yang L, et al. MiR-149 attenuates endoplasmic reticulum stress-induced inflammation and apoptosis in nonalcoholic fatty liver disease by negatively targeting ATF6 pathway. Immunol Lett. 2020;222:40–48. doi:10.1016/j.imlet.2020.03.003
76. Barte DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51.
77. Menikdiwela KR, Ramalingam L, Abbas MM, et al. Role of microRNA 690 in mediating angiotensin II effects on inflammation and endoplasmic reticulum stress. Cells. 2020;9(6):1327. doi:10.3390/cells9061327
78. Fang T, Di Y, Li G, et al. Effects of telmisartan on TNFα induced PPARγ phosphorylation and insulin resistance in adipocytes. Biochem Biophys Res Commun. 2018;503(4):3044–3049. doi:10.1016/j.bbrc.2018.08.091
79. Wu L, Zhang L, Li B, et al. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front Physiol. 2018;9:9. doi:10.3389/fphys.2018.00009
80. Huang Y, Li Y, Liu Q, et al. Telmisartan attenuates obesity-induced insulin resistance via suppression of AMPK mediated ER stress. Biochem Biophys Res Commun. 2020;523(3):787–794. doi:10.1016/j.bbrc.2019.12.111
81. Ma C, Shi T, Song L, et al. Angiotensin(1–7) attenuates visceral adipose tissue expansion and lipogenesis by suppression of endoplasmic reticulum stress via Mas receptor. Nutr Metab. 2022;19(1). doi:10.1186/s12986-022-00716-x
82. Yang F, Ma Q, Matsabisa MG, et al. Panax notoginseng for cerebral ischemia: a systematic review. Am J Chin Med. 2020;48(06):1331–1351. doi:10.1142/S0192415X20500652
83. Zhang C, Zhang B, Zhang X, et al. Panax notoginseng saponin protects against diabetic cardiomyopathy through lipid metabolism modulation. J Am Heart Assoc. 2022;11(4). doi:10.1161/JAHA.121.023540
84. Tan Y, Zhang X, Zhou Y, et al. Panax notoginseng extract and total saponin suppress diet-induced obesity and endoplasmic reticulum stress in epididymal white adipose tissue in mice. Chin Med. 2022;17(1). doi:10.1186/s13020-022-00629-0
85. Hosseini A, Razavi BM, Banach M, et al. Quercetin and metabolic syndrome: a review. Phytother Res. 2021;35(10):5352–5364. doi:10.1002/ptr.7144
86. Perdicaro DJ, Rodriguez Lanzi C, Gambarte Tudela J, et al. Quercetin attenuates adipose hypertrophy, in part through activation of adipogenesis in rats fed a high-fat diet. J Nutr Biochem. 2020;79:108352. doi:10.1016/j.jnutbio.2020.108352
87. Ardakanian A, Ghasemzadeh RM, Omidkhoda F, et al. Effect of alpha-mangostin on olanzapine-induced metabolic disorders in rats. Iran J Basic Med Sci. 2022;25(2):198–207. doi:10.22038/IJBMS.2022.58734.13047
88. Liu Z, Apontes P, Fomenko E, et al. Mangiferin accelerates glycolysis and enhances mitochondrial bioenergetics. Int J Mol Sci. 2018;19(1):201. doi:10.3390/ijms19010201
89. Zeng Z, Lin C, Wang S, et al. Suppressive activities of mangiferin on human epithelial ovarian cancer. Phytomedicine. 2020;76:153267. doi:10.1016/j.phymed.2020.153267
90. Shen J, Lu R, Cai Q, et al. Mangiferin enhances the antifungal activities of caspofungin by destroying polyamine accumulation. Virulence. 2021;12(1):217–230. doi:10.1080/21505594.2020.1870079
91. Liu T, Song Y, Hu A. Neuroprotective mechanisms of mangiferin in neurodegenerative diseases. Drug Dev Res. 2021;82(4):494–502. doi:10.1002/ddr.21783
92. Xu X, Chen Y, Song J, et al. Mangiferin suppresses endoplasmic reticulum stress in perivascular adipose tissue and prevents insulin resistance in the endothelium. Eur J Nutr. 2018;57(4):1563–1575. doi:10.1007/s00394-017-1441-z
93. Cusi K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr Diab Rep. 2010;10(4):306–315. doi:10.1007/s11892-010-0122-6
94. Chen L, Wang T, Chen G, et al. Influence of resveratrol on endoplasmic reticulum stress and expression of adipokines in adipose tissues/adipocytes induced by high-calorie diet or palmitic acid. Endocrine. 2017;55(3):773–785. doi:10.1007/s12020-016-1212-2
95. Alalaiwe A, Fang J, Lee H, et al. The demethoxy derivatives of curcumin exhibit greater differentiation suppression in 3T3-L1 adipocytes than curcumin: a mechanistic study of adipogenesis and molecular docking. Biomolecules. 2021;11(7):1025. doi:10.3390/biom11071025
96. Wang L, Zhang B, Huang F, et al. Curcumin inhibits lipolysis via suppression of ER stress in adipose tissue and prevents hepatic insulin resistance. J Lipid Res. 2016;57(7):1243–1255. doi:10.1194/jlr.M067397
97. Furigo IC, Teixeira PDS, Quaresma PGF, et al. STAT5 ablation in AgRP neurons increases female adiposity and blunts food restriction adaptations. J Mol Endocrinol. 2020;64(1):13–27. doi:10.1530/JME-19-0158
98. Cho Y, Park J, Kang HJ, et al. Ginkgetin, a biflavone from Ginkgo biloba leaves, prevents adipogenesis through STAT5-mediated PPARγ and C/EBPα regulation. Pharmacol Res. 2019;139:325–336. doi:10.1016/j.phrs.2018.11.027
99. Lee J, Yoon S, Lee S, et al. Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARγ. J Endocrinol. 2017;235(3):223–235. doi:10.1530/JOE-17-0233
100. Yao Y. Ginsenosides reduce body weight and ameliorate hepatic steatosis in high fat diet‑induced obese mice via endoplasmic reticulum stress and p‑STAT3/STAT3 signaling. Mol Med Rep. 2020;21(3):1059–1070. doi:10.3892/mmr.2020.10935
101. Thomas-Valdés S, Tostes MD, Anunciação PC, et al. Association between vitamin deficiency and metabolic disorders related to obesity. Crit Rev Food Sci Nutr. 2017;57(15):3332–3343. doi:10.1080/10408398.2015.1117413
102. Luo X, Ng C, He J, et al. Vitamin C protects against hypoxia, inflammation, and ER stress in primary human preadipocytes and adipocytes. Mol Cell Endocrinol. 2022;556:111740. doi:10.1016/j.mce.2022.111740
103. Mcardle MA, Finucane OM, Connaughton RM, Mcmorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endo. 2013;4:52.
104. Kang SU, Kim HJ, Kim DH, et al. Nonthermal plasma treated solution inhibits adipocyte differentiation and lipogenesis in 3T3-L1 preadipocytes via ER stress signal suppression. Sci Rep. 2018;8:1.
105. Guo Q, Xu L, Liu J, et al. Fibroblast growth factor 21 reverses suppression of adiponectin expression via inhibiting endoplasmic reticulum stress in adipose tissue of obese mice. Exp Biol Med. 2017;242(4):441–447. doi:10.1177/1535370216677354
106. Mcfie PJ, Stone SL, Banman SL, et al. Topological Orientation of Acyl-CoA:Diacylglycerol Acyltransferase-1 (DGAT1) and identification of a putative active site histidine and the role of the N terminus in dimer/tetramer formation. J Biol Chem. 2010;285(48):37377–37387. doi:10.1074/jbc.M110.163691
107. Xu Q, Fan Y, Loor JJ, et al. Effects of diacylglycerol O-acyltransferase 1 (DGAT1) on endoplasmic reticulum stress and inflammatory responses in adipose tissue of ketotic dairy cows. J Dairy Sci. 2022;105(11):9191–9205. doi:10.3168/jds.2022-21989
108. Chitraju C, Mejhert N, Haas JT, et al. Triglyceride Synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell Metab. 2017;26(2):407–418. doi:10.1016/j.cmet.2017.07.012
109. Xu X, Zhao CX, Wang L, et al. IncreasedCYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes. 2010;59(4):997–1005. doi:10.2337/db09-1241
110. Xu X, Tu L, Feng W, et al. CYP2J3 gene delivery up-regulated adiponectin expression via reduced endoplasmic reticulum stress in adipocytes. Endocrinology. 2013;154(5):1743–1753. doi:10.1210/en.2012-2012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.