Back to Journals » Orthopedic Research and Reviews » Volume 15
The Risk of Developing Osteosarcoma After Teriparatide Use: A Systematic Review
Authors Abdulelah AA , Haddad BI, Alhajahjeh AA , AlQirem LM , El-amayreh L
Received 15 February 2023
Accepted for publication 13 September 2023
Published 27 September 2023 Volume 2023:15 Pages 191—198
DOI https://doi.org/10.2147/ORR.S408718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Clark Hung
Ahmed A Abdulelah,1,* Bassem I Haddad,2,* Abdulrahman A Alhajahjeh,1 Lina M AlQirem,1 Layla El-amayreh1
1School of Medicine, the University of Jordan, Amman, Jordan; 2Department of Special Surgery, Division of Orthopedics, School of Medicine, the University of Jordan, Amman, Jordan
*These authors contributed equally to this work
Correspondence: Ahmed A Abdulelah, School of Medicine, the University of Jordan, Queen Rania Street, Amman, 11972, Jordan, Tel +962799699348, Email [email protected]
Abstract: Teriparatide is a recombinant human parathyroid hormone analog with anabolic mechanism of action utilized in the treatment of osteoporosis with well-established clinical efficacy. Its use is significantly hindered due to label warnings resulting from pre-clinical rat studies demonstrating an increased risk of osteosarcoma. However, clinical trials and post-marketing surveillance studies did not demonstrate any increased risk of osteosarcoma, even after prolonged periods of surveillance reaching up to 15 years, with most of the identified cases of osteosarcomas being solitary and predominantly attributed to other factors. This systematic review provides a comprehensive overview of the currently available literature and provides the highest level of clinical evidence towards demonstrating the lack of any substantial evidence towards osteosarcoma development in patients utilizing TPTD.
Keywords: recombinant parathyroid hormone, osteoporosis, bone tumor, malignancy
Introduction
Teriparatide (TPTD), a recombinant human parathyroid hormone analog [rhPTH(1–34)], is among the clinically proven medications utilized in the treatment of osteoporosis, with osteoblastic activity stimulation resulting in new bone formation involving both cortical and trabecular bone as the mechanism of action, thus making it the first anabolic anti-osteoporosis medication to be approved.1 Currently, TPTD is utilized in various patient groups with clinically proven efficacy in treating males with hypogonadism or primary osteoporosis, males and females affected by glucocorticoid-induced osteoporosis, and postmenopausal women with osteoporosis who are at high risk of fractures.1 However, TPTD is typically prescribed to patients with profoundly severe osteoporosis in addition to having a non-vertebral or at least one vertebral fracture2 with the recommended dosage and duration of 20 µg/day TPTD and a maximum of 2 years, respectively.1,3
Despite the evidence-based clinical efficacy of TPTD in the treatment of osteoporosis, and even superiority to other treatment modalities in specific cases,4 initial preclinical studies, involving rats, demonstrated a duration and dose dependent risk of developing osteosarcoma5,6 which initially resulted in issuing label warnings that were recently removed.7–9 Osteosarcoma is a primary malignant bone tumor with a bimodal peak incidence in the first 3 decades of life, and after the 6th decade with an estimated incidence ranging from 1.7 per million to 3.9 per million in individuals aged younger than 65 and in those older than 65 years, respectively.10
Additionally, patients who are at increased risk of developing osteosarcoma, whether due to underlying pathologies such as Paget’s disease of bone or due to a previous history of radiation exposure, are substantially warned about the use of TPTD.7 Apart from the preclinical studies, clinical trials did not demonstrate any cases of osteosarcoma development in participants undergoing TPTD treatment.1 Accordingly, due to the potential of developing osteosarcoma, which carries significant morbidity and mortality, TPTD underwent long-term postmarketing surveillance that recently concluded, to evaluate the incidence of osteosarcoma among its users with the latest study, which includes 15-year postmarketing surveillance, demonstrating no increased risk of osteosarcoma.3 Nonetheless, no systematic reviews, with or without meta-analysis, have been conducted previously to accurately address this potential adverse risk.
Consequently, the clinical efficacy and use of TPTD in the treatment of osteoporosis can be potentially hindered by the risk of developing osteosarcoma that is yet to be determined and confirmed. In this systematic review, we assessed the currently available evidence in the literature to evaluate the potential risk of developing osteosarcoma after the utilization of TPTD in order to provide the highest level of clinical evidence on the associated risk of developing this debilitating outcome.
Materials and Methods
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 as an assistant tool for the study. The protocol for the study has been successfully registered in the PROSPERO international database under the reference number: CRD42021260949 which thoroughly describes the detailed methodology of the study to ensure the internal validity.
Literature Search
Initially, we searched the existing literature on the presence of any systematic reviews, with or without meta-analysis, evaluating the risk of osteosarcoma following TPTD usage, and found none published or registered in PROSPERO. Subsequently, two authors (Alhajahjeh A. and El-amayreh L.) searched PubMed, Google Scholar, Medline, Research Gate, and SCOPUS databases using the following keywords: (Teriparatide), (Parathyroid analog), (PTH (1–34)), (Recombinant parathyroid), (Forteo), (Forsteo), (Bone cancer), (Bone lesion), (Osteosarcoma), and (Osteogenic sarcoma) without any language restriction while only including human studies. The last literature search was conducted on the 6th of February 2023. References were reviewed manually to ensure that all studies consisting of potential data were screened and evaluated.
Selection Criteria
The inclusion criteria included: teriparatide usage of any dosage; the incidence of osteosarcoma in relation to teriparatide use; randomized controlled trials (RCTs) that screened for osteosarcoma among the study sample’s participants. Exclusion criteria included: systematic reviews and meta-analyses; case report studies; animal studies; if the included data had been reported in previously published studies, or in more inclusive studies; RCTs that did not mention or indicate whether the incidence of osteosarcoma among the study’s participants was evaluated or not; and in vivo studies.
Data Extraction
Two authors (A.A., E.L.) independently extracted the data from the included studies based on a standardized datasheet, and in case of any disagreements, a third author (H.B) was consulted to resolve the disagreement. The following data were extracted: the first author’s name, the year of the study, study design, the population of the study sample, age, gender, mean age at the onset of menopause, number of patients that took teriparatide, number of osteosarcoma patients in each group and any other relevant data regarding the participants and the main outcome of the respective studies.
Quality Assessment
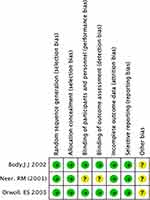
Quality assessment for all of the included studies was conducted by two authors (A.A, A.L) while utilizing the National Institute of Health Study Quality Assessment Tools12 and Cochrane-risk-of-bias tool.13 The quality assessment of the included RCTs was evaluated according to the Cochrane-risk-of-bias tool.13 The categories that were evaluated: (a) random sequence generation; (b) allocation concealment; (c) blinding of participants and study personnel; (d) blinding of outcome assessors; (e) incomplete outcome data; (f) selective outcome reporting; and (g) other bias through the use of the quality assessment tools. Categories were allocated to three different levels according to the risk of bias: low risk, unclear risk, and high risk which was conducted on Review Manager (version 5.3; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, 2014).
On the other hand, the observational cohort and case-series studies that were selected in our study consisted of 14 criteria to evaluate internal validity. The following rating was given for each study; good, fair, or poor quality.
Results
Initially, 243 articles were identified through the searched databases, and after removal of duplicated data and studies, a total of 112 articles were left. Following thorough scanning and screening of these articles, 76 were excluded due to irrelevance after reading the abstracts, and the remaining 36 articles were retrieved in full text for thorough reading and reviewing. Consequently, 18 articles were excluded because their data had been identified as used in other studies or they involved the use of a different type of parathyroid hormone recombinant. Accordingly, 18 articles were included in our study.1,3,14–29 7 out of the 18 studies are prospective studies,14–16,18,24,25,29 5 are cohort studies,1,17,26–28 2 are retrospective case-series studies,3,19 3 are randomized controlled trials,20,21,23 and 1 is a follow up study.22 All of the included articles underwent extensive qualitative and quantitative evidence synthesis to establish their quality.
Risk Assessment
The risk assessment of the randomized clinical trials20,21,23 included in the study was conducted as demonstrated in Figure 1 with all proving to be of very good quality. The risk assessment for the remaining included studies is in the supplement section (1.1). Overall, all of the selected studies were proven to be of good quality following thorough quality assessment.
Risk of Osteosarcoma
A total of five prospective studies,1,14–16,18 one of them being a prospective cohort study,1 were conducted in Croatia, Denmark, France, Greece, Italy, Slovenia, Sweden, USA, Denmark, and Japan with the total number of enrolled patients that received TPTD being 234,017. Langdahl et al14 conducted a two phase, multicenter prospective observational study involving patients across several European countries, namely Croatia, Denmark, France, Greece, Italy, Slovenia, and Sweden with the initial phase consisting of evaluating patients receiving TPTD while the second phase involved following up the patients, a total of 1611, for a period of 24 months, except in France and Sweden where the follow up period was 18 months. The mean age of the enrolled patients was 70.2 ± 9.8 years. 43 out of the 1611 patients had been treated with bisphosphonates and 19 were treated with other anti-osteoporosis drug regimens during the follow up period after the discontinuation of TPTD. There were no reported cases of osteosarcoma, neither in the initial phase nor in the second phase.
Gilsenan et al15 conducted a prospective study focused on following up a total of 75,247 patients across several states in the USA who were registered in Forteo Registry during the time period of 2009 to 2019. The study’s population ranged from age 18–104 years with the mean age being 69 years. There was a predominant female population which accounted for 88.6% of the study’s population. Subsequently, Gilsenan et al15 cross checked and compared the data from the Forteo Registry with a US based research database that covers approximately 93% of the USA population, which included 6180 confirmed cases of osteosarcoma since January 1st, 2009, to determine if there is any overlap or matching between the two databases, which resulted in the absence of any overlap or match.
Nishikawa et al16 conducted a prospective study in Japan to evaluate the clinical effectiveness and safety of TPTD among 1847 participants who took 20µg for a total period of 18–24 months with 60.8% of the participants receiving TPTD for 18 months and 39.2% for a total of 24 months. Consequently, there were no identified cases of osteosarcoma in either group. Supplementary Table 1 details the incidence of other reported adverse events. Soen et al18 also evaluated the clinical effectiveness and safety of TPTD among a total of 1996 enrolled patients of whom only 1181 completed the allocated 24 months follow-up period. Despite the fact that there were no reported cases of osteosarcoma, 3 patients developed serious adverse events which was attributed to TPTD by the investigators. With regards to the included cohort studies, Gilsenan et al1 conducted a prospective cohort study in the USA which demonstrated that when the 153,316 patients treated with TPTD of an unknown dose and a mean age of 76.9 ± 7.64 years were compared with the control group which was composed of 613,247 patients with a mean age of 77 ± 7.85 years, there was no significant statistical difference between the two groups. The mean follow-up period, which was 38.2 ± 28.69 months and 38 ± 27.65 months for the TPTD group and the control group, respectively, showed no significant statistical difference. Additionally, the study’s results demonstrated that 11 patients of the control group had developed osteosarcoma in comparison to no reported cases in the TPTD group. Bang et al17 utilized the Danish National Patient Register in conducting a retrospective cohort study consisting of 4104 patients who underwent rPTH (PTH 1–34 and PTH1-84) therapy during the time period 1995–2010 with the mean age at the time of initiating the treatment being 70.9 ± 9.7 years and a median duration of exposure of 477 (Q1-Q3, 238–530) days. This group of patients were compared to a control group consisting of 40,953 patients who did not receive rPTH therapy to evaluate the risk of developing osteosarcoma. Accordingly, Bang et al’s17 findings demonstrated that there were no osteosarcoma cases among the rPTH group in contrast to the control group which had 9 confirmed cases.
Two case series studies were identified and selected which evaluated the risk of osteosarcoma among patients utilizing TPTD as an anti-osteoporosis treatment regimen. Midkiff et al conducted a case series across multiple European countries to evaluate if there was any history of TPTD exposure among 112 confirmed cases of osteosarcoma that were diagnosed between the 1st of January 2004 and 31st of December 2013, which determined that none of the osteosarcoma cases had prior TPTD exposure. Gilsenan et al3 conducted a similar study in the US to investigate for any prior exposure to TPTD in confirmed osteosarcoma cases. Gilsenan et al3 identified 3 cases of osteosarcoma that had prior exposure to TPTD out of 1173 osteosarcoma cases with the first case developing osteosarcoma approximately 3 months after exposure, the second after 8 months, and the third and final case after 2 years of exposure. However, the site of the tumor varied between the 3 patients.
Furthermore, a few RCTs also demonstrated further evidence on the risk of developing osteosarcoma among patients utilizing TPTD with the total number of patients included in the three selected RCTs20,21,23 being 1211 patients in the intervention group in comparison to a total of 634 patients in the control groups. The dosage of TPTD utilized in the selected RCTs was either one of two different regimens, 20µg or 40µg. There were no reported cases of osteosarcomas in the intervention group. The detailed results are highlighted in Supplementary Table 1. Lindsay et al22 conducted a follow up study for a total of 2 years for the patients enrolled in Neer et al’s23 study to evaluate the degree of fracture prevention among the patients who received TPTD treatment. Their findings also revealed that there was no evidence of osteosarcoma among the treatment group.22 Figure 2 demonstrates the worldwide distribution of study participants and those who developed osteosarcoma.
 |
Figure 2 Worldwide distribution of the regions in which studies have been conducted to evaluate the risk of osteosarcoma among individuals receiving TPTD. |
Discussion
The clinical indications for the utilization of TPTD in the treatment of osteoporosis are considerably extensive due to its anabolic mechanism of action.3 Additionally, there is substantial published evidence in regards to the clinical efficacy of TPTD which has also demonstrated superiority to other anti-osteoporosis treatment regimens such as risedronate in preventing fractures in post-menopausal women with severe osteoporosis, while also demonstrating a similar overall risk of developing adverse events.4,30
Nonetheless, there is significant impediment towards the use of TPTD resulting from the presence of a black box warning, which was just recently removed, and a limitation to the duration of the treatment to only 2 years. This is due to the plausible notion that there is increased risk of developing osteosarcoma following the use of TPTD based on the pre-clinical findings of a dose and duration dependent substantial risk of osteosarcoma in rats that received TPTD.5,6 Due to the generally unfavourable outcomes of osteosarcoma, especially in advanced cases of malignancy and in centers with insufficient experience, thorough investigation for the potential development of this adverse event was warranted. Therefore, this is the first systematic review conducted in order to thoroughly evaluate the risk of osteosarcoma following the use of TPTD, thus providing an unbiased and high level of clinical evidence.
The findings by Gilsenan et al3 were determined to be the only currently available study, other than a scarce number of published case reports, demonstrating the occurrence of osteosarcoma among TPTD users, as 3 patients out of 1173 developed osteosarcoma. However, there was no distinguishable association between the 3 cases of osteosarcoma as they were identified at largely different intervals following the exposure to TPTD, in addition to developing at different sites. Additionally, there were some identifiable risk factors in all of the patients who developed osteosarcoma. This is highly indicative that these reported cases probably had no correlation or association with the exposure to TPTD, thus further reinforcing the continually emerging evidence that TPTD use is not associated with the development of osteosarcoma. Additionally, Gilsenan et al3 extended their postmarketing surveillance to 15 years and did not demonstrate any occurrence of other cases of osteosarcoma. Other conducted studies, including RCTs, did not identify any cases of osteosarcoma, whether in the treatment regimens consisting of 20µg or 40µg, with a follow up period ranging between 18 and 24 months. On the contrary, the control groups in several studies did report the development of osteosarcoma in a number of patients with the overall rates being within the normally expected.1,17 Additionally, further studies24–29conducted also demonstrated the absence of osteosarcoma cases among the patients using TPTD, thus supporting the evidence towards the absence of any causative link between osteosarcoma development and TPTD use in humans.
With regards to the preclinical findings of osteosarcoma development in Fischer 334 rats, there are several postulations towards the pathogenesis that was not reciprocated in humans. Initially, the dosage utilized in animal testing substantially influenced the development of osteosarcomas as the lowest dosage used in rats, 5µg/kg, is significantly higher than the equivalent usual dose in humans.6 Additionally, the duration was also determined to be a major factor which consequently contributed towards the 2 years limit.5 Furthermore, the 2-year treatment duration in Fischer 334 rats constitutes a significant portion of their lifetime, approximately 90%, thus it is postulated that the number of documented osteosarcoma cases during the preclinical trials would be substantially high.6,31 On the contrary, the 2-year treatment duration does not constitute a considerable portion of the life expectancy of humans, thus proposing a plausible argument that supports the lack of an established link correlating TPTD exposure and the development of osteosarcoma. The collective outcome of the factors contributing towards the pathogenesis of TPTD-induced osteosarcoma in Fischer 334 rats that were absent in humans, in addition to the evident absence of reported osteosarcoma cases among human TPTD users in the various studies conducted, support the fact that there is an absence of a fundamental link and causal relationship between TPTD use and osteosarcoma development in humans.
It may be argued that the lack of a meta-analysis in this systematic review is a considerable limitation to the study. However, due to the absence of reported cases of osteosarcoma in virtually all of the included studies except one, statistical analysis, and hence meta-analysis, could not be conducted. Additionally, the different study designs of the involved investigations might have influenced the findings due to the divergence in the studies’ methodology. However, the inclusion of all of the relevant studies currently available in the literature in this review, despite being only 18 studies, unquestionably provides a significantly high level of clinical evidence demonstrating that there is no reported evidence of osteosarcoma among human users of TPTD. Additionally, all of the included studies were determined to be of good quality and thus further support the quality of evidence provided. Case reports were not included due to the fact that they do not reflect or prove any actual causal relationship between TPTD and osteosarcoma. Furthermore, our findings are consistent with, and support the recent findings reported by Krege et al32 which indicate the lack of evidence towards increased risk of osteosarcoma among teriparatide users in the currently available literature. Therefore, the currently presented findings indicate that TPTD can be safely utilized in patients and thus further support the removal of the black box warning initially imposed.
Conclusion
The currently available literature demonstrates the lack of any substantial evidence pointing towards any increased risk of osteosarcoma formation and development in patients who used TPTD. Our study demonstrates the highest level of clinical evidence in this matter given that all of the pre-existing literature had been systematically reviewed and analysed. These findings coupled with the clinical efficacy of TPTD can certainly alter the current treatment guidelines for osteoporosis given its clinical superiority to other anti-osteoporosis treatment modalities.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gilsenan A, Midkiff K, Harris D, et al. Assessing the incidence of osteosarcoma among teriparatide users based on Medicare Part D and US State Cancer Registry Data. Pharmacoepidemiol Drug Saf. 2020;29(12):1616–1626. doi:10.1002/pds.5103
2. Langdahl BL, Silverman S, Fujiwara S, et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: integrated analysis of 4 prospective observational studies. Bone. 2018;116:58–66. doi:10.1016/j.bone.2018.07.013
3. Gilsenan A, Midkiff K, Harris D, Kellier-Steele N, McSorley D, Andrews EB. Teriparatide Did Not Increase Adult Osteosarcoma Incidence in a 15-Year US Postmarketing Surveillance Study. J Bone Mineral Res. 2021;36(2):244–251. doi:10.1002/jbmr.4188
4. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–240. doi:10.1016/S0140-6736(17)32137-2
5. Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone Neoplasms in F344 Rats Given Teriparatide [rhPTH(1-34)] Are Dependent on Duration of Treatment and Dose. Toxicol Pathol. 2004;32(4):426–438. doi:10.1080/01926230490462138
6. Vahle JL, Sato M, Long GG, et al. Skeletal Changes in Rats Given Daily Subcutaneous Injections of Recombinant Human Parathyroid Hormone (1-34) for 2 Years and Relevance to Human Safety. Toxicologic Pathology. 2002;30(3):312–321. doi:10.1080/01926230252929882
7. FDA, CDER. Forteo[Packageinsert].Indianapolis, IN:EliLillyandCo; 2020. Available from: Https://Www.Access-Data.Fda.Gov/Drugsatfda_docs/Label/2020/021318Orig1s054lbl.Pdf.
8. Miller PD, Lewiecki EM, Krohn K, Schwartz E. Teriparatide: label changes and identifying patients for long-term use. Cleve Clin J Med. 2021;88(9):489–493. doi:10.3949/ccjm.88a.21011
9. de Nigris F, Ruosi C, Napoli C. Clinical efficiency of epigenetic drugs therapy in bone malignancies. Bone. 2021;143. doi:10.1016/j.bone.2020.115605
10. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi:10.1002/cncr.24121
11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. doi:10.1136/bmj.n71
12. National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
13. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(7829). doi:10.1136/bmj.d5928
14. Langdahl BL, Ljunggren Ö, Benhamou CL, et al. Fracture Rate, Quality of Life and Back Pain in Patients with Osteoporosis Treated with Teriparatide: 24-Month Results from the Extended Forsteo Observational Study (ExFOS). Calcif Tissue Int. 2016;99(3):259–271. doi:10.1007/s00223-016-0143-5
15. Gilsenan A, Harris D, Reynolds M, et al. Long-term cancer surveillance: results from the Forteo Patient Registry Surveillance Study. Osteoporosis Int. 2021;32(4):645–651. doi:10.1007/s00198-020-05718-0
16. Nishikawa A, Ishida T, Taketsuna M, Yoshiki F, Enomoto H. Safety and effectiveness of daily teriparatide in a prospective observational study in patients with osteoporosis at high risk of fracture in Japan: final report. Clin Interv Aging. 2016;11:913–925. doi:10.2147/CIA.S107285
17. Bang UC, Hyldstrup L, Jensen JEB. The impact of recombinant parathyroid hormone on malignancies and mortality: 7 Years of experience based on nationwide Danish registers. Osteoporosis Int. 2014;25(2):639–644. doi:10.1007/s00198-013-2470-y
18. Soen S, Fujiwara S, Takayanagi R, et al. Real-world effectiveness of daily teriparatide in Japanese patients with osteoporosis at high risk for fracture: final results from the 24-month Japan Fracture Observational Study (JFOS). Curr Med Res Opin. 2017;33(11):2049–2056. doi:10.1080/03007995.2017.1354826
19. Krohn K, Kellier N, Masica D, Gilsenan A, Harding A, Andrews E. Forteo Voluntary Patient Registry: 4-Year Progress on a Prospective Osteosarcoma Surveillance Study. J Clin Densitometry. 2014;17(3):429. doi:10.1016/j.jocd.2014.04.105
20. Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87(10):4528–4535. doi:10.1210/jc.2002-020334
21. Orwoll ES, Scheele WH, Paul S, et al. The Effect of Teriparatide [Human Parathyroid Hormone (1-34)] Therapy on Bone Density in Men With Osteoporosis. Journal of Bone and Mineral Research. 2003;18(1):9–17. doi:10.1359/jbmr.2003.18.1.9
22. Lindsay R, Scheele WH, Neer R, et al. Sustained Vertebral Fracture Risk Reduction After Withdrawal of Teriparatide in Postmenopausal Women With Osteoporosis. Available from: http://archinte.jamanetwork.com/.
23. Neer R, Arnaud C, Zanchetta J, et al. The New England Journal of Medicine effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England Journal of Medicine. 2001;344(19):1434–1441.
24. Silverman S, Miller P, Sebba A, et al. The Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE) study: 2-year nonvertebral fragility fracture results. Osteoporosis Int. 2013;24(8):2309–2317. doi:10.1007/s00198-013-2284-y
25. Fahrleitner-Pammer A, Langdahl BL, Marin F, et al. Fracture rate and back pain during and after discontinuation of teriparatide: 36-month data from the European Forsteo Observational Study (EFOS). Osteoporosis Int. 2011;22(10):2709–2719. doi:10.1007/s00198-010-1498-5
26. Jakob F, Oertel H, Langdahl B, et al. Effects of teriparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-month results from the European Forsteo Observational Study. Eur J Endocrinol. 2012;166(1):87–97. doi:10.1530/EJE-11-0740
27. Napoli N, Langdahl BL, Ljunggren Ö, et al. Effects of Teriparatide in Patients with Osteoporosis in Clinical Practice: 42-Month Results During and After Discontinuation of Treatment from the European Extended Forsteo® Observational Study (ExFOS). Calcif Tissue Int. 2018;103(4):359–371. doi:10.1007/s00223-018-0437-x
28. Chen CH, Elsalmawy AH, Ish-Shalom S, et al. The Effect of Teriparatide Treatment on the Risk of Fragility Fractures in Postmenopausal Women with Osteoporosis: results from the Asian and Latin America Fracture Observational Study (ALAFOS). Calcif Tissue Int. 2022;110(1):74–86. doi:10.1007/s00223-021-00895-4
29. Kaufman JM, Orwoll E, Goemaere S, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16(5):510–516. doi:10.1007/s00198-004-1713-3
30. Yuan F, Peng W, Yang C, Zheng J. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: a meta-analysis. Int J Surg. 2019;66:1–11. doi:10.1016/j.ijsu.2019.03.004
31. Capriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Mineral Res. 2012;27(12):2419–2428. doi:10.1002/jbmr.1800
32. Krege JH, Gilsenan AW, Komacko JL, Kellier-Steele N. Teriparatide and Osteosarcoma Risk: history, Science, Elimination of Boxed Warning, and Other Label Updates. JBMR Plus. 2022;6(9). doi:10.1002/jbm4.10665
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.