Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
The Relationship of Low-Density-Lipoprotein to Lymphocyte Ratio with Chronic Obstructive Pulmonary Disease
Authors Huang Y , Ding K, Dai Z, Wang J, Hu B, Chen X, Xu Y, Yu B, Huang L, Liu C, Zhang X
Received 2 April 2022
Accepted for publication 16 August 2022
Published 8 September 2022 Volume 2022:17 Pages 2175—2185
DOI https://doi.org/10.2147/COPD.S369161
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Min Zhang
Yiben Huang,1,* Keke Ding,2,* Zicong Dai,2,* Jianing Wang,2 Binbin Hu,2 Xianjing Chen,1 Yage Xu,1 Beibei Yu,1 Lingzhi Huang,1 Chunyan Liu,1 Xiaodiao Zhang1
1Department of Respiratory and Critical Medicine, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2School of the First Clinical Medical Sciences, Wenzhou Medical University, Wenzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodiao Zhang; Chunyan Liu, Department of Respiratory and Critical Medicine, The Third Affiliated Hospital of Wenzhou Medical University, No. 108 Wansong Road, Wenzhou, 325000, People’s Republic of China, Tel +86-577-65866223, Fax +86-577-65866586, Email [email protected]; [email protected]
Background: Chronic Obstructive Pulmonary Disease (COPD) has been a concern all over the world because of its high prevalence and mortality. The ratio of low-density-lipoprotein to lymphocyte (LLR) has been widely used to predict the prognosis of cerebral infarction, but its association with COPD is less known. We aim to explore the relationship between LLR and COPD and to investigate its indicative role in the severity and prognosis of COPD.
Methods: In this study, 279 participants (n = 138 with COPD and n = 138 age- and sex-matched health control) were recruited. COPD patients were divided into two groups according to the optimal cut-off value of LLR determined by the receiver operating characteristic curve (ROC). We collected the clinical characteristics, pulmonary function, LLR, neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR) and other data of all subjects. t-test, Pearson correlation test, logistic regression analysis and other statistical analysis were carried out.
Results: Compared with the healthy control group, COPD patients had a significantly higher LLR level (p < 0.001). The disease was more serious in the high LLR group, which was reflected by Global Initiative for Chronic Obstructive Lung Disease (GOLD) and BMI, airway obstruction, dyspnoea, severe exacerbations (BODE) index and St. George’s Respiratory Questionnaire (SGRQ) index (p = 0.001, p = 0.013, p = 0.011, respectively). The forced expiration volume in 1 second (FEV₁) (p = 0.033) and forced expiratory volume in 1 second in percent of the predicted value (FEV₁%) (p = 0.009) in high LLR group were lower. Univariate and multivariate logistic regression analysis showed that LLR was an independent factor affecting the severity of COPD patients (odds ratio [OR] = 2.599, 95% CI: 1.266-5.337, p = 0.009).
Conclusion: We found that LLR is a novel biomarker in predicting the severity of patients with COPD. Further studies with larger database were recommended to verify our findings.
Keywords: low-density-lipoprotein, lymphocyte, COPD, biomarker
Introduction
Chronic obstructive pulmonary disease (COPD), a common disease all across the world, poses a major health care burden due to its prevalence and mortality.1 COPD affected around 10% of the adult population aged above 40 years.2 In a recent study of 59,906 US veterans with COPD, the mean respiratory-related total health care costs were approximately 10 times higher in patients than controls.3 A systemic review in China showed that COPD was the top three leading causes of death. Before emphysema destruction begins, small conduction airways become narrow and disappear, resulting in increased peripheral airway resistance in patients with COPD.4 COPD is a chronic inflammation characterized by an increase in the number of alveolar macrophages, neutrophils and cytotoxic T lymphocytes, as well as the release of a variety of inflammatory mediators (lipids, chemokines, cytokines, growth factors) and high levels of oxidative stress.5 Güneş et al6 found that the ratio of low-density-lipoprotein to lymphocyte is a new predictor of in-hospital mortality of acute cerebral infarction. Besides, the exacerbation of COPD is associated with a variety of infiltrating cells, especially lymphocytes, as well as a variety of enzymes (neutrophil elastase and MMP-9) and cytokines (CXCL8, TNF-ɑ) in sputum and bronchoalveolar lavage (BAL) fluid.7 In addition, serum low-density lipoprotein (LDL) level might be associated with COPD. A previous study showed that oxidized low-density lipoprotein (ox LDL) is involved in the mechanisms related to oxidative stress and inflammation in COPD.8 Higher average values of cholesterol were recorded significantly in the group of patients with very severe COPD, and the ratio of low-density-lipoprotein to lymphocyte (LLR) is an independent factor affecting cholesterol in patients with severe COPD.9 Although COPD has certain relationships with lymphocytes and LDL, respectively, what role of the LLR index plays in COPD is still unknown. Our previous study discussed the relationship between the ratio of lymphocytes to high-density lipoprotein and COPD patients.10 To further explore the effect of lipids and lymphocytes on COPD and based on the above research on LDL and lymphocytes, we therefore intend to use the ratio of LDL and lymphocytes to study its relationship with COPD.
Therefore, the purpose of this study is to explore the relationship between the novel indicator LLR and COPD and the role that LLR plays in predicting the severity of COPD.
Materials and Methods
Study Population
The detailed selection criteria of the study are displayed in Figure 1. We conducted a cross-sectional study, in which 138 subjects with COPD from the respiratory department of the Third Affiliated Hospital of Wenzhou Medical University and 138 age- and sex-matched healthy controls between February 2018 and January 2021 were included. Inclusion and exclusion criteria of COPD patients were as follows: Inclusion criteria: 1) age more than 40 years; 2) diagnosis of COPD as defined in the GOLD guidelines;11 Exclusion criteria:12 1) malignant tumor (n = 4); 2) hepatic and renal insufficiency (n = 5); 3) autoimmune diseases or systemic inflammatory diseases (n = 1). And 138 age- and sex-matched healthy controls were included as the control group who met the same inclusion and exclusion criteria as the COPD patients, except for the diagnosis of COPD.
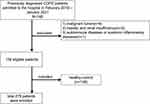 |
Figure 1 Flow chart showing the literature search and selection. Abbreviation: COPD, chronic obstructive pulmonary disease. |
The study was approved by the Ethics Committee of the Third Affiliated Hospital of Wenzhou Medical University, and the registration number of the Ethics Committee was YJ20170015. The study was conducted in accordance with the Declaration of Helsinki. All subjects signed a written informed consent form.
Data Collection
Patients’ demographic information and medical history like age, gender, body mass index (BMI), smoking status, duration of disease and comorbidities were collected by questionnaires at hospital admission. Blood routine indicators such as red blood cells, white blood cells, neutrophils, hemoglobin, platelets and lymphocytes, which were carried out by XT-1800i (Sysmex, Kobe, Japan) were obtained. Blood samples were collected to analyze blood routine parameters, blood biochemistry and arterial blood gas using ARCHITECT c16000 (Abbott Laboratories, Illinois, USA). We also calculated computable parameters such as LLR, neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) in COPD patients and healthy controls. Pulmonary function tests of COPD patients (using spirometer: CareFusion, San Diego, California, USA) at hospital admission were conducted, and parameters of pulmonary function were recorded such as forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, and FEV1 in percent of the predicted value (FEV1%). Besides, we also collected scales of COPD patients, including GOLD, BODE, SGRQ, modified Medical Research Council dyspnea scale (mMRC), COPD assessment test (CAT), and 6-min walk distance (6MWT).
Diagnostic Criteria of COPD
Patients with COPD were categorized into severity grades I–IV using spirometry (GOLD I: FEV1 ≥ 80% predicted; GOLD II: 50% ≤ FEV1 < 80% predicted; GOLD III: 30% ≤ FEV1 < 50% predicted; GOLD IV: FEV1 < 30% predicted).13
Statistical Analysis
All statistical analyses were performed using SPSS (version 26.0, IBM, Armonk, NY). “Shapiro–Wilk” W-test was used to test the normal distribution of measurement data. The data conforming to the normal distribution were described by the mean ± standard deviation, and then the data were tested by the homogeneity of variance. For the data conforming to the homogeneity of variance, the independent sample t-test was used for the comparison between the COPD patients and healthy controls, low LLR group and high LLR group. The one-way analysis of variance (ANOVA) was used for the comparison between multiple groups. Count data described by percentage (%), chi-square test (x2) and Fisher exact probability test were used for comparison between groups. Fisher exact probability test was used if the sample size is ≤5. Based on the results of univariate analysis, multivariate logistic regression analysis was carried out to identify the contribution of the LLR as a predictor of different GOLD stages (Mild: GOLD I + GOLD II; Severe: GOLD III + GOLD IV). Two-sided p values <0.05 were considered significant in all analyses.
Results
Baseline Characteristics of the Study Subjects
As shown in Table 1, the clinical characteristics of 138 COPD patients and 138 age- and sex-matched healthy controls were compared. Significant differences between the two groups were seen in neutrophils, lymphocytes and LDL (p < 0.001, p < 0.001, p = 0.001 respectively). Neutrophils were higher in COPD group than in control group, while lymphocytes and LDL were higher in healthy group. The levels of NLR (p < 0.001) and PLR (p < 0.001) in patients with COPD were higher than those in healthy people. In addition, we also found significant statistical difference in LLR, a novel marker in COPD, between two groups (p < 0.001).
 |
Table 1 Comparison of COPD Patients and Healthy Controls |
To further explore the relationship between LLR and severity of COPD patients, 138 COPD patients were divided into two groups according to the cut-off value of LLR (LLR ≤ 1.57, n = 63; LLR > 1.57, n = 75).
Demographic variables, such as age, gender, BMI, current smokers, hypertension and diabetes, have no significant difference between the two groups, but we observed significant differences in the duration of disease (p = 0.003). In terms of lung function, FEV1 and FEV1% in the low LLR group were higher than that in the high LLR group (p = 0.033, p = 0.009, respectively). Moreover, it can be seen that there were significant differences between the two groups on GOLD scale, BODE index and SGRQ (p = 0.001, p = 0.013, p = 0.011, respectively), but the meaningful results were not observed in mMRC, CAT, and 6MWT (Table 2). Figure 2 shows that the patients with higher LLR level accounted for more proportion of GOLD III and GOLD IV, which indicated severe condition of COPD patients (52.46% vs 88.23%, p = 0.005).
 |
Table 2 Baseline Characteristics of COPD Patients According to LLR Cutoff |
Association Between LLR and the Severity of COPD
Pearson’s correlation test showed that LLR was negatively correlated with FEV1% (r = −0.191, p = 0.033), while NLR and PLR were not (Figure 3). Because FEV1% represents the severity of pulmonary function in COPD, which means that the lower the FEV1%, the poorer the pulmonary function of COPD. Thus, we can deduce that LLR is positively correlated with the severity of COPD.
In order to further investigate the independent factors affecting COPD severity, univariate logistic regression analysis was performed on the variables. From Table 3, significant differences in BMI (p = 0.012), FEV1 (p < 0.001), FEV1% (p < 0.001), FVC (p < 0.001), FEV1/FVC (p < 0.001), PaCO2 (p < 0.001), lymphocyte (p = 0.034) and LLR (p = 0.025) were observed in severe COPD (Mild: GOLD I, GOLD II; Severe: GOLD III, GOLD IV).
 |
Table 3 Univariate Logistic Regression Analysis Assesses Different Prognostic Predictors of COPD |
In order to control other potential confounding variables, a multivariate logistic regression analysis was carried out. In model 1, there were no adjustments (odds ratio [OR] = 1.706, 95% CI: 1.068–2.725, p = 0.025). After adjusting for age, gender, and BMI in model 2, the association between LLR and GOLD remained significant (odds ratio [OR] = 1.823, 95% CI: 1.056–3.146, p = 0.031). On the basis of model 2, we also revolved FEV1/FVC and PaCO2 in model 3. After adjustment, the link between LLR and GOLD was still significant (odds ratio [OR] = 2.599, 95% CI: 1.266–5.337,p = 0.009). We basically concluded that LLR is a potential influencing factor of COPD severity (Figure 4).
Function of LLR in Different Subgroups
To further explore the indication function of LLR in different subgroups, we divided 138 COPD patients into 8 subgroups according to their age (whether they were over 65 years old), sex, smoking history, disease course (whether the disease course was over 5 years), whether they had hypertension or diabetes, whether their BMI >24 and whether they suffered from cerebral infarction. It can be seen from Table 4 that there is no significant difference in LLR among the subgroups of age, sex, duration of disease, hypertension, diabetes, and BMI. However, in the nonsmoker group, the results were statistically significant, reflecting that smoking may be closely related to LLR (odds ratio [OR] = 1.741, 95% CI: 1.017–2.982, p = 0.043). Moreover, statistical significance was also seen in the cerebral infarction group, while not seen in the patients without cerebral infarction (odds ratio [OR] = 1.888, 95% CI: 1.093–3.201, p = 0.023).
 |
Table 4 Subgroup Analysis Between COPD Patients and Healthy Controls |
Discussion
To the best of our knowledge, this study is the first study to investigate the associations between LLR and COPD. Our data showed that COPD patients had higher LLR levels than healthy controls. Moreover, higher LLR levels were associated with severe COPD. Besides, elevated LLR can be seen significantly in non-smoking and cerebral infarction subgroups.
In this study, we found that the lymphocyte count of COPD patients was lower than that of healthy controls. The findings are further supported by the studies that found that blood lymphocyte number was lower in COPD than in non-COPD patients.14 It was also found that a lower lymphocyte proportion in peripheral blood was associated with poorer exercise capacity, worse quality of life and higher mortality in elderly patients with severe COPD.15–17 The potential mechanism is that lymphopenia, as a sign of impaired immunity, increases the risk of infection, which is related to the deterioration of fatal COPD and early mortality.18 Moreover, chronic inflammation is a key pathogenic element in COPD,19 which leads to increased production of cortisol, catecholamines, and proinflammatory cytokines which results in decreasing the production of lymphocytes, increasing the apoptosis, or making redistribution of lymphocytes.20,21
Our data showed that LDL was slightly lower in COPD patients than that of healthy controls, which was consistent with previous research results.22 However, previous conclusions of numerous studies on the relationship between COPD and blood lipid profiles remain conflicting and contradictory.23 Jiayu et al24 reported that there was no difference between serum TG, TC, and LDL levels of patients and controlled individuals, the same results were also found by Basili et al.25 Nevertheless, a previous study showed that increased oxidized low-density lipoprotein (ox LDL) is correlated with lung function, inflammation, and oxidative stress in COPD.8 Compared with the control group, plasma levels of chemerin and LDL in the COPD group in acute exacerbation and remission stage were elevated.26 The mechanism of LDL in COPD is still unclear and needs further research. However, it was reported that a more pro-inflammatory diet was related to higher small dense LDL and lower large LDL in a cross-sectional sample of 1992 adults.27 There may be a potential correlation between different types of LDL and contradictory results. Besides, the plasma level of LDL resulted to be significantly lower in patients that used a violent method compared to patients who attempted suicide with a nonviolent method, which indicated that low-level LDL may be related to mental factors in COPD patients.28 This may also have a potential correlation with the long-term changes in eating habits.29
Our article is the first to use LLR as a predictive biomarker for the severity of COPD. In this study, we found that the LLR level of COPD patients in severe group (Severe: GOLD III, GOLD IV) was higher than that of COPD patients in mild group (Mild: GOLD I, GOLD II). The higher the LLR level, the higher the GOLD and BODE indexes and the SGRQ score. In addition, our data showed that elevated LLR is associated with COPD pulmonary function indicators, especially low-level FEV1, which means that patients with higher LLR have worse pulmonary function and more serious disease. Univariate and multivariate logistic regression analyses showed that elevated LLR was an independent indicator of the severity of COPD and was associated with adverse outcomes of COPD. Previously, LLR was used as a new predictor of in-hospital mortality of acute cerebral infarction.6 A few previous population-based studies have found that cerebrovascular disease is a common comorbidity in patients with COPD.30–32 Therefore, we consider applying LLR, a meaningful index in cerebral infarction, to COPD to observe whether it has indicative significance. The possible mechanism is the increased risk of atherosclerosis in patients with COPD or the result of common risk factors between stroke and COPD.32 This is consistent with the results of subgroup analysis showing that LLR has significance in COPD patients with cerebral infarction.
In our previous study, lymphocyte to high-density lipoprotein ratio (LHR) has higher accuracy for predicting pulmonary function in COPD in contrast to NLR and PLR, and lower LHR level is independently associated with poorer pulmonary function.10 This result can also be partially confirmed by our experiment as the study showed that elevated LLR is associated with COPD pulmonary function indicators, especially low-level FEV1, which indicated that lipids and lymphocytes may be related to the pulmonary function of COPD. However, compared to the previous study, exploring the relationship between LLR and COPD severity is the main purpose of our current study. We found that the higher the LLR ratio, the worse the outcome, and the more serious the condition of COPD. The current study supplemented the blank of previous research.
We also found that LLR was higher in non-smoking COPD patients. This result is interesting. Cigarette smoking can cause a series of changes in the trachea, lung tissue, pulmonary blood vessels, and promotes the occurrence and development, as it contains a large number of toxic substances.33 Previous studies found that oxidants in cigarette smoke can increase the oxidative modification of plasma LDL.34–36 And the average LDL level of smokers was significantly higher than that of non-smokers.37 Besides, exposure to cigarette smoke is perceived to facilitate sensibility to self-antigens and the consequent autoreactive T cells.38 It was also found that there is an increase in Th17 cells in the lung and circulation in a mouse model with short-term smoke exposure, suggesting that smoking is associated with increased lymphocytes. Considering all the above research, we can know that smoking is related to increased LDL and increased lymphocytes. However, in our experiment, we explored the role of the ratio of LDL to lymphocytes in the smoking subgroup. This may lead to the result that when calculated, respectively, two indicators were meaningful while after calculating the ratio of them, the ratio becomes meaningless. In the non-smoking group, without the complex influence of smoking, the significance of LLR itself can be expressed.
However, there were still some limitations in our study. First, this study was limited as a retrospective cohort of cross-sectional surveys; therefore, further study should include the correlation between LLR and longitudinal results to verify its causality. Second, our research is limited to small samples. More studies with larger samples should be recruited to verify our findings. In addition, patients from the same hospital in the same area have limitations. Some factors related to LDL and lymphocytes cannot be completely removed, which gave rise to bias.
In conclusion, as a simple, repeatable, widely used, and cheap laboratory index, LLR can be calculated and has uniform and strong indicative significance for the severity of COPD. In clinical work, blood routine and blood biochemical results are easy to obtain, but some COPD patients may be difficult to measure their pulmonary function due to the limitation of physical function. Therefore, LLR has certain practical value and is worth popularizing in clinical application. Greater attention should be attached to LLR for its indicative role in COPD severity.
Conclusion
The findings of this study suggest that LLR may be a novel, valuable and easily obtainable biomarker to predict the severity of COPD. High-level LLR is closely related to high severity in patients with COPD. Multidisciplinary studies based on larger cohorts are required to validate the utility of LLR.
Abbreviations
COPD, chronic obstructive pulmonary disease; LDL, low-density-lipoprotein; LLR, low-density-lipoprotein to lymphocyte ratio; ROC, receiver-operating characteristics; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; BMI, body mass index; GOLD, Global Initiative for Chronic Obstructive Lung Disease; BODE, body mass index, airway obstruction, dyspnoea, severe exacerbations; SGRQ, St. George’s Respiratory Questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FEV1%, forced expiratory volume in 1 second in percent of the predicted value; mMRC, modified Medical Research Council dyspnea scale; CAT, COPD assessment test; 6MWT, 6-min walk distance; PaCO2, partial pressure of carbon dioxide in arterial blood; AUC, the area under the curve; PaO2, partial pressure of oxygen in arterial blood; WBC, white blood cell; RBC, red blood cell.
Availability of Date and Materials
Data included in the current study are not publicly available to ensure confidentiality of the patients but are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank all the participants in the study and the staff at the Third Affiliated Hospital of Wenzhou Medical University for their contribution in obtaining the data and assisting in the successful completion of the research. Yiben Huang, Keke Ding, and Zicong Dai are co-first authors for this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by the Wenzhou Municipal Sci-Tech Bureau Program (Y20210842). The Funding body had no role in the study design, data collection, analysis, interpretation of data or writing of the manuscript.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Rabe K, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9
2. Zhu B, Wang Y, Ming J, Chen W, Zhang L. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis. 2018;13:1353–1364. doi:10.2147/COPD.S161555
3. Sharafkhaneh A, Petersen N, Yu H, Dalal A, Johnson M, Hanania N. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125–132. doi:10.2147/copd.s8047
4. McDonough J, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi:10.1056/NEJMoa1106955
5. Barnes P, Shapiro S, Pauwels R. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(4):672–688. doi:10.1183/09031936.03.00040703
6. Güneş M, Büyükgöl H, Novel Predictive A. Marker for in-hospital mortality in acute cerebral infarction: low-density lipoprotein cholesterol to lymphocyte ratio. Cureus. 2020;12(8):e9986. doi:10.7759/cureus.9986
7. Sethi S, Murphy T. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi:10.1056/NEJMra0800353
8. Shen Y, Yang T, Guo S, et al. Increased serum ox-LDL levels correlated with lung function, inflammation, and oxidative stress in COPD. Mediators Inflamm. 2013;2013:972347. doi:10.1155/2013/972347
9. Zafirova-Ivanovska B, Stojkovikj J, Dokikj D, et al. The level of cholesterol in COPD patients with severe and very severe stage of the disease. Open Access Maced J Med Sci. 2016;4(2):277–282. doi:10.3889/oamjms.2016.063
10. Huang Y, Jiang B, Miao X, et al. The relationship of lymphocyte to high-density lipoprotein ratio with pulmonary function in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:3159–3169. doi:10.2147/COPD.S276372
11. Vogelmeier C, Criner G, Martinez F, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
12. Chen H, Xiong C, Shao X, et al. Lymphocyte to high-density lipoprotein ratio as a new indicator of inflammation and metabolic syndrome. Diabetes Metab Syndr Obes. 2019;12:2117–2123. doi:10.2147/DMSO.S219363
13. Mirza S, Clay R, Koslow M, Scanlon P. COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc. 2018;93(10):1488–1502. doi:10.1016/j.mayocp.2018.05.026
14. Halper-Stromberg E, Yun J, Parker M, et al. Systemic markers of adaptive and innate immunity are associated with chronic obstructive pulmonary disease severity and spirometric disease progression. Am J Respir Cell Mol Biol. 2018;58(4):500–509. doi:10.1165/rcmb.2017-0373OC
15. Acanfora D, Scicchitano P, Carone M, et al.; American Journal of Respiratory Cell and Molecular Biology. Relative lymphocyte count as an indicator of 3-year mortality in elderly people with severe COPD. BMC Pulm Med. 2018;18(1):116. doi:10.1186/s12890-018-0685-6
16. Moon S, Leem A, Kim Y, et al. Low serum lymphocyte level is associated with poor exercise capacity and quality of life in chronic obstructive pulmonary disease. Sci Rep. 2020;10(1):11700. doi:10.1038/s41598-020-68670-3
17. Tinè M, Bazzan E, Semenzato U, et al. Heart failure is highly prevalent and difficult to diagnose in severe exacerbations of COPD presenting to the emergency department. J Clin Med. 2020;9(8):2644. doi:10.3390/jcm9082644
18. Fuso L, Incalzi R, Pistelli R, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. 1995;98(3):272–277. doi:10.1016/S0002-9343(99)80374-X
19. Baraldo S, Lokar Oliani K, Turato G, Zuin R, Saetta M. The role of lymphocytes in the pathogenesis of asthma and COPD. Curr Med Chem. 2007;14(21):2250–2256. doi:10.2174/092986707781696573
20. Gupta S, Agrawal A, Agrawal S, Su H, Gollapudi S. A paradox of immunodeficiency and inflammation in human aging: lessons learned from apoptosis. Immun Ageing. 2006;3:5. doi:10.1186/1742-4933-3-5
21. Shah A, Denaxas S, Nicholas O, Hingorani A, Hemingway H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: a CALIBER cohort study. Open Heart. 2016;3(2):e000477. doi:10.1136/openhrt-2016-000477
22. Markelić I, Hlapčić I, Rogić D, et al. Lipid profile and atherogenic indices in patients with stable chronic obstructive pulmonary disease. Nutr Metab Cardiovasc Dis. 2021;31(1):153–161. doi:10.1016/j.numecd.2020.07.039
23. Xuan L, Han F, Gong L, et al. Association between chronic obstructive pulmonary disease and serum lipid levels: a meta-analysis. Lipids Health Dis. 2018;17(1):263. doi:10.1186/s12944-018-0904-4
24. Can U, Yerlikaya F, Yosunkaya S. Role of oxidative stress and serum lipid levels in stable chronic obstructive pulmonary disease. J Chin Med Assoc. 2015;78(12):702–708. doi:10.1016/j.jcma.2015.08.004
25. Basili S, Ferroni P, Vieri M, et al. Lipoprotein(a) serum levels in patients affected by chronic obstructive pulmonary disease. Atherosclerosis. 1999;147(2):249–252. doi:10.1016/S0021-9150(99)00192-6
26. Li C, Yan L, Song J. [COPD 患者血浆 chemerin 水平的变化及其与患者脂代谢的关系]. Zhong Nan da Xue Xue Bao Yi Xue Ban. 2016;41(7):676–683. Chinese. doi:10.11817/j.issn.1672-7347.2016.07.003
27. Phillips C, Shivappa N, Hébert J, Perry I. Dietary inflammatory index and biomarkers of lipoprotein metabolism, inflammation and glucose homeostasis in adults. Nutrients. 2018;10(8):1033. doi:10.3390/nu10081033
28. Capuzzi E, Caldiroli A, Capellazzi M, et al. Exploring the role of serum lipid profile and neutrophil-to-lymphocyte ratio in violent suicide attempters: a cross sectional study. CNS Spectr. 2020;27(3):1–7.
29. Meng H, Matthan N, Wu D, et al. Comparison of diets enriched in stearic, oleic, and palmitic acids on inflammation, immune response, cardiometabolic risk factors, and fecal bile acid concentrations in mildly hypercholesterolemic postmenopausal women-randomized crossover trial. Am J Clin Nutr. 2019;110(2):305–315. doi:10.1093/ajcn/nqz095
30. Divo M, Cote C, de Torres J, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi:10.1164/rccm.201201-0034OC
31. Hansell A, Walk J, Soriano J. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003;22(5):809–814. doi:10.1183/09031936.03.00031403
32. Portegies M, Lahousse L, Joos G, et al. Chronic obstructive pulmonary disease and the risk of stroke. The Rotterdam Study. Am J Respir Crit Care Med. 2016;193(3):251–258. doi:10.1164/rccm.201505-0962OC
33. Song Q, Chen P, Liu X. The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir Res. 2021;22(1):39. doi:10.1186/s12931-021-01630-1
34. Ambrose J, Barua R. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–1737. doi:10.1016/j.jacc.2003.12.047
35. Chen C, Loo G. Cigarette smoke extract inhibits oxidative modification of low density lipoprotein. Atherosclerosis. 1995;112(2):177–185. doi:10.1016/0021-9150(94)05412-C
36. Yamaguchi Y, Matsuno S, Kagota S, Haginaka J, Kunitomo M. Peroxynitrite-mediated oxidative modification of low-density lipoprotein by aqueous extracts of cigarette smoke and the preventive effect of fluvastatin. Atherosclerosis. 2004;172(2):259–265. doi:10.1016/j.atherosclerosis.2003.09.030
37. Schuitemaker G, Dinant G, van der Pol G, van Wersch J. Relationship between smoking habits and low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, and triglycerides in a hypercholesterolemic adult cohort, in relation to gender and age. Clin Exp Med. 2002;2(2):83–88. doi:10.1007/s102380200011
38. Zhou J, Li Z, Xu X, et al. Cigarette smoke-initiated autoimmunity facilitates sensitisation to elastin-induced COPD-like pathologies in mice. Eur Respir J. 2020;56(3):2000404. doi:10.1183/13993003.00404-2020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



