Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The Relationship of Fractional Exhaled Nitric Oxide in Patients with AECOPD
Received 5 August 2023
Accepted for publication 5 December 2023
Published 21 December 2023 Volume 2023:18 Pages 3037—3046
DOI https://doi.org/10.2147/COPD.S434040
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Xiaoguang Xu,1,2 Lefei Zhou,3 Zhaohui Tong1
1Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Affiliated Hospital of Jilin Medical University, Jilin, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Ordos Central Hospital, Ordos School of Clinical Medicine, Inner Mongolia Medical University, Inner Mongolia, People’s Republic of China
Correspondence: Zhaohui Tong, Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, No. 8, Gongti South Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +8613089172570, Fax +8601085231217, Email [email protected]
Objective: To identify the relationship between patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and their fractional-exhaled nitric oxide (FeNO) levels.
Methods: Patients diagnosed with AECOPD in the respiratory department of Beijing Chaoyang Hospital from June 2017 to August 2019 were recorded. The demographic data, FeNO value, peripheral blood eosinophil count, number of acute exacerbations in the past year, pulmonary function test, use of inhaled glucocorticoids (ICS) and other data were collected and analyzed. FeNO was measured again three months after discharge, the participants were assessed to determine if the stable period criteria were met.
Results: A total of 214 patients met the requirements of this study. 25ppb for FeNO was used as the cutoff for further analysis. The proportion of males, number of acute exacerbations in the past year, number of ICS users, leukocyte count and eosinophil count in the high FeNO-level group was significantly higher than that in the low-level group (P < 0.05). The results showed that the number of acute exacerbations in the past year, number of ICS users, and eosinophil count were statistically significant in the model (P < 0.05). The study also showed that the level of FeNO in the acute exacerbation phase was significantly higher than that in the stable phase. The ROC curve that the area under the curve used by FeNO to predict ICS used is 0.631 (95% CI: 0.526– 0.736), and the corresponding P value is 0.022.
Conclusion: FeNO is closely related to activated T2 inflammation and eosinophil count in COPD patients. The FeNO levels can be used as an index to evaluate the severity of COPD and predict the recovery of activity after ICS treatment. FeNO can predict the use of ICS and is a beneficial supplement to eosinophils.
Keywords: fractional exhaled nitric oxide, chronic obstructive pulmonary disease, acute exacerbation, airway inflammation
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterized by chronic airway inflammation. Acute exacerbations accelerate the deterioration of lung function in COPD patients, leading to increased mortality and economic burden.1 Therefore, it is essential to evaluate the severity of COPD at an early stage. At present, the gold standard for clinical evaluation and prognosis of COPD is still the pulmonary function test. However, it does not provide reliable treatment guidance and detailed adjustment of the treatment plan for patients. The pulmonary function test has always been an important basis for clinical judgment and assessment of COPD patients, but it does not reflect the degree of airway inflammation in COPD patients. Nitric oxide (NO) is an important reference index of airway inflammation. Fractional exhaled nitric oxide (FeNO) has been recommended by the American Thoracic Society (ATS)2 and the European Respiratory Society (ERS) as a practical and non-invasive method to evaluate airway inflammation and has been proven to be effective in clinical practice. FeNO has been widely used in the diagnosis of asthma,3 the prediction of treatment response and the evaluation of prognosis, but the practical application of FeNO in COPD has not been determined.
The purpose of this study is to conduct statistical analysis of data from patients with acute exacerbation of COPD, in order to further explore whether FeNO levels can assess the severity of COPD, predict the use of ICS, and evaluate the effectiveness of treatment.
Materials and Methods
Subjects
This is a single-center retrospective observational study. This study selected COPD patients with acute exacerbations hospitalized in the respiratory department of Beijing Chaoyang Hospital Affiliated to Capital Medical University from June 2017 to August 2019. Enrolled patients with diagnosed COPD met the following criteria: post-bronchodilator forced expiratory volume in 1s/forced vital capacity (FEV1/FVC) ratio of less than 0.7 according to the GOLD diagnostic criteria.4 Patients with asthma, asthma-COPD overlap syndrome (ACOS) and uncontrolled systemic diseases were excluded. The screening process of subjects is displayed in Figure 1.
 |
Figure 1 The screening process of subjects in this study. |
Clinical Measurements
The individual information of inpatients were collected and recorded, including demographic information, the number of acute exacerbations in the past year, disease duration, smoking history and use of inhaled corticosteroids (ICS).
Peripheral blood eosinophil count, IgE count, leukocyte count, neutrophil count and arterial blood gas analysis results were recorded.
FEV1%, FEV1/FVC were tested using the MasterScreen spirometer (VIASYS Healthcare GmbN, Germany) before and after inhaled salbutamol 400ug via MDI following the guideline. mMRC and CAT scores were also calculated.
FeNO was measured by a nitric oxide analyzer (NIOXMINO, Aerocrine, Sweden), the procedure was carried out according to the clinical application guidelines of exhaled nitric oxide measurement.5 The expiratory flow rate was 50mL⋅s−1. Before performing the FeNO examination, the participants were required to stop smoking for 24 hours. FeNO was expressed in ppb. All patients were tested for FeNO within 24 hours of admission for an acute exacerbation of COPD. The FeNO levels were measured again three months after discharge to confirm whether the participants met the stable period criteria.
Statistical Analysis
The data were analyzed by IBM SPSS Statistics 26.0. The measurement data fitting a normal distribution were represented by mean ± Standard deviation, and a t-test was used for inter-group analysis. The measurement data which did not fit a normal distribution were expressed by median (quartile range). The Mann Whitney U-test was used for inter-group comparison, and the Wilcoxon signed-rank test was used for intragroup comparison in different periods. The count data were represented by composition ratio and rate, and the χ2 test was used for comparison between groups with disordered classification data. The Mann–Whitney U-test was used for the comparison between groups with ordered classification data. Logistic regression was used to analyze the related factors affecting FeNO. Receiver operating characteristic (ROC) curve was used to analyze the value of FeNO and EOS in predicting ICS treatment. All tests of statistical significance were two-sided, and P < 0.05 was considered statistically significant.
Results
Comparison of Clinical Data of Different FeNO Groups in Acute Phase
A total of 214 patients (n=214) met the requirements of this study. In the acute phase, 25ppb for FeNO was used as the cutoff for further analysis. The comparison results of clinical data of different FeNO groups are shown in Table 1. The proportion of males, current smokers and ICS users in the two groups was adjusted, and the χ2 test results showed no significant difference in the proportion of current smokers between the two groups (P > 0.05). However, the proportion of males and ICS users in the high FeNO-level group was significantly higher than that in the low-level group (P < 0.05). This study also suggests that ICS use is more common in the high FeNO group (93.6%).
 |
Table 1 Comparison of Clinical Data of Different FeNO-Level Groups in Acute Phase |
The results of the independent sample t-test showed that there was no significant difference in the levels of age, BMI, FEV1/FVC, CAT score, PaO2, PaCO2 and PH value between the two groups (P > 0.05). However, the leukocyte count of the high FeNO-level group was significantly higher than that of the low-level group (P < 0.05). The Mann Whitney U-test showed that there was no significant difference between the two groups in the disease time, FEV1/pre, mMRC score, GOLD stage, neutrophil count and IgE levels (P > 0.05), but the number of acute exacerbations in the past year (Figure 2) and eosinophil count (Figure 3) in the high FeNO-level group were significantly higher than those in the low-level FeNO group (P < 0.05). In the high FeNO group, COPD patients had experienced more acute exacerbations in the past year than patients in the low FeNO group. Patients in the high FeNO group accounted for about 50.9% and were characterized by elevated eosinophil counts.
 |
Figure 2 Comparison of AE between the two groups in the past year in acute phase. *P<0.05. |
 |
Figure 3 Comparison of eosinophil count between the two groups in acute phase. ***P<0.001. |
Logistic Regression Analysis of FeNO in Acute Phase
The results of logistic regression analysis affecting FeNO in the acute phase are shown in Table 2 and Figure 4. Taking FeNO as dependent variable (<25ppb = 1, ≥25ppb = 2) and statistically significant indexes in the univariate analysis as independent variables (including male, number of acute exacerbations in the past year, ICS use, leukocyte count and eosinophil count), a binary logistic regression was established by entry method. The results showed that the number of acute exacerbations in the past year, ICS users and eosinophil count were statistically significant in the model (P < 0.05), and they were all risk factors for abnormal levels of FeNO. For each additional acute exacerbation in the past year, the risk of abnormal FeNO increased by 1.265 times. ICS use was associated with a 2.865 times higher risk of abnormal FeNO. For every 100 units increase in eosinophil count, the risk of abnormal FeNO increased by 1.175 time.
 |
Table 2 Logistic Regression Affecting FeNO Level in Acute Phase |
 |
Figure 4 Forest map of each index affecting FeNO level in acute phase. |
Comparison of FeNO in Different Phases
The comparison results of FeNO in different phases are shown in Table 3 and Figure 5. The Wilcoxon signed-rank test showed that the level of FeNO in the acute exacerbation phase was significantly higher than that in the stable phase (P < 0.05).
 |
Table 3 Comparison of FeNO in Different Phase |
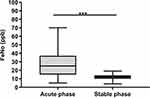 |
Figure 5 Comparison results of FeNO in different phase. ***P<0.001. |
Correlation Between FeNO and Eosinophil Count in Acute Phase
The correlation between FeNO and eosinophil count in the acute phase is shown in Figure 6. According to Spearman correlation analysis, there is a significant positive correlation between FeNO and eosinophil levels (r=0.205, P=0.003).
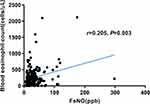 |
Figure 6 Scatter diagram between FeNO and eosinophil count in acute phase. |
Comparison of FeNO Levels Treated with ICS in Different Phases
The FeNO levels were grouped and compared based on whether ICS drugs were used, as shown in Table 4. The FeNO during acute exacerbation phase, stable phase, and the variation of FeNO after ICS treatment in two groups of patients were shown by Mann Whitney U-test results. There were no statistically significant difference in FeNO levels during the stable phase and the variation of FeNO after ICS treatment (P>0.05), while the FeNO in the group used ICS drugs during acute exacerbation phase were significantly higher than those in the group not using ICS drugs, with a statistically significant difference (P<0.05). The results of Wilcoxon signed rank test showed that levels of FeNO in the acute exacerbation and stable phases, both groups of patients had significantly lower levels of FeNO in the stable phase compared to those in the acute exacerbation phase, with statistically significant differences (P<0.05).
 |
Table 4 Comparison of FeNO Levels Treated with ICS in Different Phases |
Prediction of ICS Treatment by ROC Curve of FeNO, EOS and FeNO Combined with EOS in Acute Phase
The use of ROC curve to analyze the value of FeNO, EOS and FeNO combined with EOS in predicting the use of ICS during the acute phase, as shown in Figure 7. It can be seen from the ROC curve that the area under the curve used by FeNO to predict ICS is 0.631 (95% CI: 0.526–0.736), and the corresponding P value is 0.022, indicating that the value of FeNO to predict ICS use is statistically significant. The threshold used by FeNO to predict ICS is 24.5, corresponding sensitivity is 55.4% and the specificity is 76.7%. The area under the curve used for EOS prediction of ICS is 0.605 (95% CI: 0.498–0.711), corresponding to a P value of 0.067, indicating that the value of EOS prediction of ICS use is not statistically significant. The threshold used for EOS prediction of ICS is 175, corresponding sensitivity is 53.3% and the specificity is 76.7%. The area under the curve used by FeNO combined with EOS for predicting ICS is 0.639 (95% CI: 0.536–0.741), corresponding to a P value of 0.015, indicating that the value of FeNO combined with EOS for predicting ICS is statistically significant. The corresponding threshold is 0.8571, sensitivity is 53.8%, and the specificity is 73.3%.
 |
Figure 7 ROC curve used by FeNO, EOS and FeNO+EOS in the acute phase to predict ICS treatment. |
Discussion
COPD is a chronic airway inflammatory disease involving many cell types. Its main characteristics are chronic respiratory symptoms, including difficulty breathing, cough, and expectoration. If COPD is not diagnosed and treated early, the disease will gradually worsen, resulting in the deterioration of the patients’ health, a sharp decline in the quality of life, and may even lead to death. As one of the chronic diseases that are difficult to treat in clinical practice, the prevention and treatment of COPD are focused on alleviating the patients’ condition and preventing further deterioration of the disease. However, even with the current COPD treatment strategy, some patients still can not accurately evaluate their condition. For most patients, disease progress is assessed by measuring the pulmonary function test, but there is a lack of discussion on the influencing factors of the pulmonary function test. Therefore, it is important to find an early and rapid method to evaluate the control of COPD for treatment and prognosis. FeNO measurement is characterized by distinct features. It is simple and convenient, non-invasive, sensitive and causes no trauma to patients. Compared with the traditional CAT score, the evaluation result is more objective.
After Plamer6 et al first proved that NO was, in essence, an endothelium-derived relaxing factor in 1987, a series of subsequent studies found that NO in the respiratory tract is a multifunctional small-molecule substance, which is related to the respiratory inflammatory response.7,8 NO is an important signal molecule that is catalyzed by nitric oxide synthase (NOS)9,10 and can be synthesized and released by a variety of cells. When the lung receives inflammatory lesions caused by a virus or a bacterial infection, it induces the production of inducible nitric oxide synthase11,12 (iNOS). Inflammatory factors affect the concentration of FeNO through the effect of iNOS, resulting in the increase of FeNO.13
GOLD2023 states that COPD is a heterogeneous disease.14 Some studies have shown that the increase in the number of neutrophils, macrophages and the expression of type 1 helper T cell (Th1) cytokines are the main characteristics of COPD airway inflammation. A subgroup of COPD patients tends to have Th2 type inflammation which including elevated sputum and blood eosinophil counts, suggesting that Th2 inflammation may also play a progressive role in a certain proportion of COPD patients.15 A report states that NO plays a crucial role in regulating type 2 inflammation and regulating type 2 immune response.16
With the in-depth study of COPD, it was found that the blood eosinophils of some patients with AECOPD would also increase, and eosinophils could be used as an inflammatory index for the treatment and prognosis of AECOPD. It is reported that patients with stable and acute exacerbation have good sensitivity when the level of eosinophils in peripheral blood increases.17 GOLD2019 recommend using peripheral blood eosinophil count as a biomarker for identifying COPD phenotype. Many studies concluded that the concentration of FeNO is highly correlated with the number of inflammatory cells and can be used as a marker of airway inflammation. A study reported that FeNO can distinguish different phenotypes of COPD.18 In this study, it was found that there is a positive correlation between FeNO levels and peripheral blood eosinophil levels in patients with AECOPD. We believe that FeNO is closely related to activated T2 inflammation and eosinophil count, consistent with previous report.16
In this study, the mean FeNO value was 25.0 (15.0, 37.0) ppb, consistent with that reported by Taylor et al.19 We used the 25 ppb as the cutoff of FeNO based on the mean level of FeNO in the recruited patients with AECOPD. Higher eosinophil levels were seen in the high FeNO group. This study shows that some patients have asthma-like traits, such as high blood eosinophils15,20 and high FeNO levels. Some other studies have reported similar findings. One of the typical manifestations of COPD or asthma is chronic airway inflammation. However, there are differences in effector cells between COPD and asthma, which are neutrophils and eosinophils. FeNO may have a stronger relationship with eosinophils.21 Therefore, FeNO has a certain guiding value in the differentiation of COPD and asthma.
Glucocorticoids can induce the production of anti-inflammatory factors, inhibit the expansion of capillaries and reduce exudation. They are mostly used in the treatment of severe acute infection and inflammation. Many patients with AECOPD have significantly improved symptoms after using glucocorticoids, which may be related to how glucocorticoids can improve airway eosinophilic inflammation. It can be seen that ICS is a risk factor affecting FeNO in this study. Patients with COPD generally have chronic airway inflammation, and ICS drugs are commonly used to improve inflammatory symptoms. Therefore, the guiding value of glucocorticoid drugs in a clinical setting needs to be systematically assessed. The detection of FeNO in COPD patients can evaluate the extent of airway inflammation and guide inhaled glucocorticoid therapy. This study found that the sensitivity and specificity of FeNO in predicting the use of ICS were 55.4% and 76.7%, respectively. The results indicate that measuring FeNO levels plays a crucial role in the treatment of COPD using ICS. However, the value of EOS in predicting ICS use in this study was not statistically significant, possibly because the FeNO measurement results were influenced by multiple factors, including age, diet, smoking, infection, medication, and exercise. FeNO provides additional biomarkers for the diagnosis and management of Th2 type COPD, and its combination with eosinophil can significantly improve the diagnostic accuracy.
Patients with COPD often have vascular endothelial cell damage. In addition, the reduction of NO synthesis can reduce the relaxation of vascular smooth muscle cells, resulting in enhanced pulmonary vasoconstriction and structural remodeling. NO plays an important role in the occurrence and development of COPD. The acute exacerbation of COPD is often caused by pulmonary infection. During pulmonary infection, a variety of cells express iNOS under the action of stimulating factors, resulting in increased NO synthesis. A large amount of NO can form oxygen radicals after transformation, which can result in tissue damage and airway inflammation. In the remission stage of COPD, NO synthesis decreased with the control of infection and inflammatory reaction. The results show a significant difference in FeNO between the acute exacerbation and stable phases in COPD patients. As COPD patients reach a stable condition, FeNO decreases significantly, proving that FeNO can reflect the condition of COPD patients. The FeNO value in AECOPD is higher than that in the stable phase, which is consistent with the results of a large sample follow-up study.22 Similarly, Antus et al23 reported that patients with relatively high FeNO levels are more likely to have an acute exacerbation. FeNO levels are significantly lower in stable COPD patients, which proves that FeNO can reflect the condition of COPD patients. The level of airway inflammation can be reflected by FeNO concentration, and the therapeutic effect of COPD can be evaluated by the change of FeNO concentration. The recent study24 reports that the change in FeNO from stable state visit to AECOPD was positively correlated with the probability of viral infections and negatively correlated with the probability of bacterial infections. Therefore, changes of FeNO levels from stable state to AECOPD may help doctors determine the aetiology of AECOPD and accelerate effective treatment decisions for COPD patients.
At present, the main detection indexes of COPD include the pulmonary function test and arterial blood gas analysis, but the above two indexes lack the function of quantifying airway inflammation. In this study, we demonstrate that the pulmonary function test is not an influencing factor of FeNO, as previously concluded by other studies. It is considered that the change of FeNO in COPD patients does not play an important role in evaluating pulmonary function. The results of this study indicate that FeNO, as an emerging inflammatory marker, can not only effectively reflect the degree of respiratory acidophilic inflammation, but also differentiate the types of respiratory inflammation, evaluate the level of respiratory inflammation, and predict the use of ICS drugs. In this study, it can be inferred that blood eosinophils and FeNO can be used to evaluate airway inflammation in patients with AECOPD. This relationship has potential application in assessing the acute exacerbation of airway inflammation in patients with COPD, guiding the use of glucocorticoid therapy and evaluating the prognosis. It indicates that FeNO is a beneficial supplement to blood eosinophil count. Its accuracy has been clinically verified and has prominent clinical significance for the detection of COPD. However, there are still many limitations in the practical clinical application of FeNO detection. To address some of these limitations, a large sample, multi-factor and multi-mode experiment should be carried out. Comprehensive testing is required to explore the value and prospect of FeNO in clinical application.
Conclusion
FeNO is a marker of airway inflammation level, which can evaluate airway inflammation in patients with AECOPD and effectively reflect the severity of the disease. FeNO has certain significance in assessing the acute exacerbation of airway inflammation in patients with COPD, guiding the treatment and prognosis of glucocorticoid. At the same time, the detection of FeNO concentration can be used as an index to evaluate the severity of COPD and predict the recovery of activity after treatment. The combination of FeNO and eosinophils can improve the prediction of ICS use in COPD patients. FeNO is a beneficial supplement to eosinophils.
Ethical Statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing ChaoYang Hospital (NO.: NCT05059873). Informed consent was waived by our Ethics Committee because of the retrospective nature of our study. Our research involves patient data information, which is kept confidential under ethical regulations.
Acknowledgments
The source of support for the study was no help from others. The research was carried out without funding.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
1. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi:10.1164/rccm.201204-0596PP
2. Chang D, Yao W, Tiller CJ, et al. Exhaled nitric oxide during infancy as a risk factor for asthma and airway hyperreactivity. Eur Respir J. 2015;45(1):98–106. doi:10.1183/09031936.00034614
3. Zietkowski Z, Bodzenta-Lukaszyk A, Tomasiak MM, et al. Comparison of exhaled nitric oxide measurement with conventional tests in steroid-naive asthma patients. J Investig Allergol Clin Immunol. 2006;16(4):239–246.
4. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2019.
5. Exhaled NO. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi:10.1164/rccm.200406-710ST
6. Fleming I, Busse R. NO: the primary EDRF. J Mol Cell Cardiol. 1999;31(1):5–14. doi:10.1006/jmcc.1998.0839
7. Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70(2):115–120. doi:10.1136/thoraxjnl-2014-205634
8. Kim HB, Eckel SP, Kim JH, et al. Exhaled NO: determinants and clinical application in children with allergic airway disease. Allergy Asthma Immunol Res. 2016;8(1):12–21. doi:10.4168/aair.2016.8.1.12
9. Geller DA, Lowenstein CJ, Shapiro RA, et al. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993;90(8):3491–3495. doi:10.1073/pnas.90.8.3491
10. Marsden PA, Heng HH, Scherer SW, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem. 1993;268(23):17478–17488. doi:10.1016/S0021-9258(19)85359-0
11. Gaston B, Drazen JM, Loscalzo J, et al. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149(2 Pt 1):538–551. doi:10.1164/ajrccm.149.2.7508323
12. Barnes PJ, Belvisi MG. Nitric oxide and lung disease. Thorax. 1993;48(10):1034–1043. doi:10.1136/thx.48.10.1034
13. Ferrante G, Malizia V, Antona R, et al. The value of FeNO measurement in childhood asthma: uncertainties and perspectives. Multidiscip Respir Med. 2013;8(1):50. doi:10.1186/2049-6958-8-50
14. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report.; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
15. Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(12):956–967. doi:10.1016/S2213-2600(17)30432-0
16. Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J. 2020;55(3):1901633. doi:10.1183/13993003.01633-2019
17. Singh D, Kolsum U, Brightling CE, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414
18. Alcázar-Navarrete B, Romero-Palacios PJ, Ruiz-Sancho A, Ruiz-Rodriguez O. Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COPD phenotypes. Nitric Oxide. 2016;54:67–72. doi:10.1016/j.niox.2016.02.003
19. Taylor DR, Pijnenburg MW, Smith AD, et al. Exhaled nitric oxide measurements: clinical application and interpretation. Thorax. 2006;61(9):817–827. doi:10.1136/thx.2005.056093
20. Çolak Y, Afzal S, Nordestgaard BG, et al. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the Copenhagen general population study. Eur Respir J. 2018;52(2):1800616. doi:10.1183/13993003.00616-2018
21. Feng JX, Lin Y, Lin J, et al. Relationship between fractional exhaled nitric oxide level and efficacy of inhaled corticosteroid in Asthma-COPD overlap syndrome patients with different disease severity. J Korean Med Sci. 2017;32(3):439–447. doi:10.3346/jkms.2017.32.3.439
22. Alcázar-Navarrete B, Ruiz Rodríguez O, Conde Baena P, et al. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur Respir J. 2018;51(1):1701457. doi:10.1183/13993003.01457-2017
23. Antus B, Barta I, Horvath I, et al. Relationship between exhaled nitric oxide and treatment response in COPD patients with exacerbations. Respirology. 2010;15(3):472–477. doi:10.1111/j.1440-1843.2010.01711.x
24. Schumann DM, Papakonstantinou E, Kostikas K, Grize L, Tamm M, Stolz D. Variability of fractional exhaled nitric oxide is associated with the risk and aetiology of COPD exacerbations. Respirology. 2023;28(5):445–454. doi:10.1111/resp.14439
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
