Back to Journals » Journal of Pain Research » Volume 12
The relationship between the reporting of euphoria events and early treatment responses to pregabalin: an exploratory post-hoc analysis
Authors Parsons B , Freynhagen R, Schug S , Whalen E, Ortiz M , Bhadra Brown P, Knapp L
Received 21 December 2018
Accepted for publication 2 July 2019
Published 22 August 2019 Volume 2019:12 Pages 2577—2587
DOI https://doi.org/10.2147/JPR.S199203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Michael A Ueberall
Bruce Parsons,1 Rainer Freynhagen,2,3 Stephan Schug,4,5 Ed Whalen,1 Marie Ortiz,1 Pritha Bhadra Brown,1 Lloyd Knapp6
1Pfizer Inc, New York, NY, USA; 2Department of Anesthesiology, Critical Care Medicine, Pain Therapy & Palliative Care, Pain Center Lake Starnberg, Benedictus Hospital, Tutzing, Germany; 3Department of Anesthesiology, Klinikum rechts der Isar, Technische Universität München, Munich, Germany; 4Discipline of Anaesthesiology and Pain Medicine, Medical School, University of Western Australia, Perth, WA, Australia; 5Department of Anaesthesia and Pain Medicine, Royal Perth Hospital, Perth, WA, Australia; 6Pfizer Inc, Groton, CT, USA
Correspondence: Bruce Parsons
Pfizer Inc, 235 East 42nd Street, New York, NY, USA
Tel +1 212 573 1649
Email [email protected]
Background: Euphoria is a complex, multifactorial problem that is reported as an adverse event in clinical trials of analgesics including pregabalin. The relationship between the reporting of euphoria events and pregabalin early treatment responses was examined in this exploratory post-hoc analysis.
Methods: Data were from patients with neuropathic or non-neuropathic chronic pain enrolled in 40 randomized clinical trials, who received pregabalin (75–600 mg/day) or placebo. Reports of treatment-emergent euphoria events were based on the Medical Dictionary of Regulatory Activities preferred term “euphoric mood”. Prevalence rates of euphoria events overall and by indication were assessed. Post-treatment endpoints included ≥30% improvements in pain and sleep scores up to 3 weeks as well as a ≥1-point improvement in daily pain score up to 11 days after treatment.
Results: 13,252 patients were analyzed; 8,501 (64.1%) and 4,751 (35.9%) received pregabalin and placebo, respectively. Overall, 1.7% (n=222) of patients reported euphoria events. Among pregabalin-treated patients, a larger proportion who reported euphoria events achieved an early pain response compared with those who did not report euphoria (30% pain responders in week 1 with euphoria events [43.0%], without euphoria events [24.2%]). Results were similar for weeks 2 and 3. For Days 2–11, a larger proportion of pregabalin-treated patients with (relative to without) euphoria events were 1-point pain responders. Findings were similar in pregabalin-treated patients for sleep endpoints (30% sleep responders in week 1 with euphoria events [50.7%], without euphoria events [36.1%]). Similar results were found for weeks 2 and 3. Patients who received placebo showed similar patterns, although the overall number of them who reported euphoria events was small (n=13).
Conclusion: In patients who received pregabalin for neuropathic or non-neuropathic chronic pain, those who experienced euphoria events may have better early treatment responses than those who did not report euphoria events.
Keywords: euphoria, pain, pregabalin, sleep
Introduction
Euphoria is often perceived as a signal of potential substance abuse or addiction and has been described as an exaggerated feeling of physical and emotional well-being and optimism not consistent with apparent stimuli or events.1–4 It has been reported as an adverse event (AE) in clinical trials of medications that act on the central nervous system including analgesics.2,3 Feelings of euphoria can contribute to the abuse of prescription drugs, as illustrated by the rise in recent years of the misuse of prescription opioids in the US population, where there was a 140% increase in prescription drug misuse from 7.8 million in 1992 to 15.1 million in 2003.5 However, euphoria is a complex multifactorial phenomenon that is not well understood. For instance, euphoria events occur in clinical trials of some medications such as selective serotonin reuptake inhibitors and corticosteroids, which are known not to be abused for psychotropic purposes.6–8 Moreover, many prescription drugs with significant abuse potential, such as benzodiazepines, are not typically associated with euphoria in clinical trials.9 Euphoria may also be reported in patients who receive placebo in some clinical trials.10 One additional complexity that has not been explored is the potential relationship between the reporting of euphoria events and treatment responses.
Pregabalin is an α2δ calcium channel subunit ligand and analgesic,11–14 indicated in multiple countries for the treatment of peripheral or central neuropathic pain (NeP) and fibromyalgia, as well as generalized anxiety disorder (GAD) and partial-onset seizures.10,15 In placebo-controlled randomized clinical trials, 4% of patients who received pregabalin reported euphoria events (defined by the preferred term “euphoric mood” using the Medical Dictionary for Regulatory Activities [MedDRA], version 19.0), with incidences varying across indications, while 1% of patients who received placebo also reported euphoria events.6
Because of the large number of patients who have participated in clinical studies of pregabalin, an opportunity exists to examine possible links between the reporting of euphoria events and treatment responses. In this exploratory post-hoc analysis of data from 40 placebo-controlled clinical trials, the objective was to assess the relationship between reported euphoria events and early treatment responses (pain and sleep) to pregabalin in patients with NeP or non-NeP.
Materials and methods
Included studies
The Pfizer database of pregabalin clinical studies was searched for studies conducted in patients with peripheral or central NeP, or non-NeP. Studies were excluded if they analyzed patients who: were healthy volunteers, had postoperative pain, underwent experimental procedures that may have induced pain, or were primarily enrolled for functional magnetic resonance imaging. Across all studies, patients were excluded if they experienced any severe acute or chronic medical or psychiatric condition(s), were receiving prespecified concomitant medication(s)—that may have been different in the individual studies—or illicit drug(s), or exhibited any other factors that could potentially increase the risk associated with study participation or would interfere with the interpretation of the study results. In total, 40 randomized, placebo-controlled clinical studies of pregabalin efficacy and safety were identified (Table 1). Pain conditions in these analyses included diabetic peripheral neuropathy (DPN, 13 studies);16–24 postherpetic neuralgia (PHN, eight studies);25–30 posttraumatic peripheral NeP (PT pNeP, two studies);31,32 combined DPN/PHN (two studies);33,34 combined DPN/PHN/PT pNeP (one study);35 NeP due to spinal cord injury (SCI, two studies);36,37 fibromyalgia (FM, seven studies);38–44 chronic low back pain (CLBP, two studies); osteoarthritis (OA, one study); and NeP associated with HIV neuropathy (HIV NeP, two studies).45,46 Nine studies are unpublished (four in DPN, two in PHN, two in CLBP, one in OA). Twenty-three studies have ClinicalTrial.gov identifiers: NCT00156078; NCT00159679;20 NCT00143156; NCT00553475;21 NCT01332149;22 NCT01455415;23 NCT01474772;24 NCT00159666;28 NCT00394901;29 NCT01455428;30 NCT00292188;31 NCT01701362;32 NCT00301223;34 NCT00141219;35 NCT00407745;37 NCT00645398;39 NCT00230776;40 NCT00333866;41 NCT00883740;42 NCT00830167;43 NCT01432236;44 NCT00232141;45 NCT01049217.46 The remaining studies were not registered at ClinicalTrials.gov because they pre-dated the requirement for clinical trial registration.
 |
Table 1 Studies included in the analysis |
The dates of the studies ranged between March 1998 and August 2015. Pregabalin was administered in doses of 75–600 mg/day (either fixed or flexible dosing). Treatment duration ranged from 4 to 16 weeks. All of the original studies were conducted in accordance with the Declaration of Helsinki and/or Good Clinical Practice Guidelines from the International Conference on Harmonisation and were approved by the institutional review board or ethics committee of each participating center. Each patient or their legal guardian or representative provided written informed consent to participate in the study. No new patients were recruited for this analysis.
Capturing of euphoria events as adverse events
Investigators were responsible for reporting treatment-emergent AEs in the original studies, including severity and potential relationship to study medication. For the purpose of this analysis, the reporting of euphoria events was coded using the preferred term “euphoric mood” (code 10015535 of the MedDRA, version 19.0). The present study did not consider the severity of reported euphoria events or the relationship to study medication. Events were captured separately for pregabalin and placebo. Most studies were of parallel group design, and incidences of euphoria events were captured throughout the duration of the treatment period. Four studies were of 2-period, 2-way crossover design,23,24,42,44 and the incidences of euphoria events in these studies were captured during the first treatment period only.
Prevalence of euphoria events
For all of the analyses, all doses of pregabalin treatment (75–600 mg/day, fixed or flexible dosing) were pooled because (i) the number of patients reporting euphoria for a given dose was low (fixed dosing: 75 mg/day =2; 150 mg/day =7; 300 mg/day =62; 450 mg/day =45; 600 mg/day =62; flexible dosing =31), and (ii) most studies included a dose titration period; therefore, the occurrence of euphoric events may have occurred at a different dose than the randomized dose. The prevalence of reported euphoria events was determined for pregabalin and placebo using data from all identified studies. Prevalence rates were calculated overall and by indication. For the remaining analyses, only studies were included in which euphoria events had been reported (n=26 studies). The time to onset and duration were determined for the reported euphoria events. The number of patients who reported multiple euphoria events was calculated, as was the number of patients in which euphoria events were ongoing at the end of the study.
Pain scores
For the analysis of pain scores, all indications were grouped together. An 11-point numeric rating scale (NRS, where 0= no pain and 10= worst possible pain) was used to assess pain. Early pain responses were assessed in pregabalin- and placebo-treated patients who did or did not report euphoria events. These pain responses were classified as: (i) a ≥30% improvement in mean pain score from baseline for Weeks 1, 2, and 3 after treatment initiation;47,48 and (ii) a ≥1-point reduction in daily pain score compared with baseline for Days 2–11 after treatment initiation.49 Additional pain measures were evaluated only in pregabalin- and placebo-treated patients who reported euphoria events. Mean pain scores were determined before and after the onset of the euphoria events with the day of onset of the event as the cutoff date. Pre-onset data could include baseline scores and the day of onset, when available. Post-onset data were determined for Weeks 1 and 2 after the onset of the event, and excluded the day of onset. The change in mean pain score was also measured from baseline to the days that euphoria events were reported. Change in pain score was calculated as the difference between mean baseline pain score and daily pain score.
Sleep scores
For the analysis of sleep scores, all indications were grouped together. In studies of patients with DPN, PHN, PT pNeP, DPN/PHN, SCI, and CLBP, sleep was assessed using an 11-point pain-related sleep interference (PRSI) score where 0 = pain does not interfere with sleep to 10 = pain completely interferes with sleep. In FM and OA studies, sleep was assessed using an 11-point NRS sleep quality score. For 6 of these studies, the NRS was scored from 0 = best possible sleep to 10 = worst possible sleep. For 2 of the FM studies, the NRS was scored from 0 = worst possible sleep to 10 = best possible sleep. Early sleep responses were assessed in pregabalin- and placebo-treated patients who did and did not report euphoria events. These responses were classified as a ≥30% improvement from baseline in PRSI or mean sleep quality score for Weeks 1, 2, and 3 after treatment initiation.
Statistical analysis
Data are descriptive only; no statistical comparisons were made.
Results
A total of 13,252 patients were included in the analysis. Of these, 8,501 (64.1%) patients received pregabalin and 4,751 (35.9%) patients received placebo. Overall, 222 (1.7%) patients reported euphoria events, comprising 209 (2.5%) with pregabalin and 13 (0.3%) with placebo. The majority of patients were female and aged 18 to 64 years. No notable differences were observed in most demographic characteristics between those who did versus did not experience euphoria events (Table 2). In general, female patients reported euphoria events at a higher prevalence than non-euphoria; however, prevalence of euphoria events in females was comparable between treatment groups (with euphoria: pregabalin [72.2%] and placebo [69.2%]; without euphoria: pregabalin [60.0%] and placebo [56.2%]). The number of euphoria events in each pregabalin dose group were as follows: 75 mg/day (n=2), 150 mg/day (n=7), 300 mg/day (n=62), 450 mg/day (n=45), 600 mg/day (n=62), flexible dosing (n=31), and placebo (n=13) (Table 2). Prevalence rates of euphoria events by indication are shown in Table 3. For pregabalin, euphoria events varied from 0.5% in patients with PT pNeP to 9.3% in patients with OA. The highest rates occurred in patients with non-NeP conditions. For placebo, prevalence varied from 0% in patients with PHN, PT pNeP, or OA to 1.0% in patients with CLBP.
 |
Table 2 Baseline demographics of patients in early pain responders (the euphoria and non-euphoria groups) |
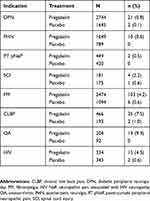 |
Table 3 Prevalence rates of euphoria events by indication and treatment group |
Across all indications, the majority of patients reported only one euphoria event. Eleven patients reported ≥2 events. The median (range) time to the onset of the first event was 2.0 (1–84) days for pregabalin and 2.0 (1–6) days for placebo. The median (range) duration of each event was 10.0 (1–118) days for pregabalin and 3.0 (1–121) days for placebo. Twenty-five patients had ongoing euphoria events at the end date of their studies. The duration of euphoria events (using the study end date as censor) ranged from 11–101 days.
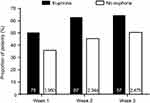
The 30% pain responder rates were compared between pregabalin-treated patients who did and did not report euphoria events (Figure 1). For Weeks 1, 2, and 3 after starting treatment, the proportion of pregabalin-treated patients who were 30% pain responders was numerically higher in patients who reported (vs those who did not report) euphoria events. Placebo patients showed a similar results for Week 1 with a numerically higher percentage of patients achieving responder status with euphoria events (30.0% [n=3]) compared with those with no euphoria events (8.7% [n=244]). One placebo patient who was a 30% pain responder reported a euphoria event in Weeks 2 and 3.
Figure 2 shows the comparison of the 1-point pain responder rates in pregabalin-treated patients who did and did not report euphoria events for Days 2–11 after starting treatment. Similar to the 30% pain responder rate, a numerically higher proportion of pregabalin-treated patients who reported (vs those who did not report) euphoria events were 1-point pain responders for each day. Results for patients who received placebo followed a similar pattern to pregabalin; however, fewer placebo patients were available for analysis (1–5 patients reported euphoria events per day; data not shown).
In patients who reported euphoria events, mean (standard deviation [SD]) pain scores before the onset of the event were numerically similar for pregabalin (6.1 [1.8]) compared with placebo (6.5 [1.9]). For Week 1 after the onset of the event, mean pain scores for pregabalin were numerically lower (4.6 [1.9]) compared with placebo (5.3 [1.9]) suggesting greater pain relief. Findings were similar for Week 2 after the onset of the event (4.4 [2.1] for pregabalin vs 5.3 [2.1] for placebo). The change in mean pain score from baseline was numerically larger for pregabalin versus placebo for the majority of days that euphoria events were reported (Figure 3). The qualitatively greater improvement in mean pain score occurred as early as the first day after starting treatment.
Comparison of the 30% sleep responder rates in pregabalin-treated patients who did and did not report euphoria events is shown in Figure 4. For Weeks 1, 2, and 3 after starting treatment, the proportion of pregabalin-treated patients who were 30% sleep responders was numerically higher in patients who reported (vs those who did not report) euphoria events. This pattern was similar for Weeks 1 and 3 in patients who received placebo (Week 1: euphoria, 30.0% [n=3]; no euphoria, 13.0% [n=366]; Week 3: euphoria, 100% [n=3]; no euphoria, 28.4% [n=752]). Two placebo patients were 30% sleep responders who reported euphoria events for Week 2.
Discussion
In this exploratory post-hoc pooled analysis of clinical trial data for peripheral or central NeP or non-NeP, pregabalin-treated patients who reported euphoria events may experience early improvements in pain and sleep. These findings also extend to patients who received placebo, although few placebo-treated patients reported euphoria events limiting the ability to draw definitive conclusions. Pregabalin-treated (compared with placebo) patients had numerically greater improvements in pain (absolute and change from baseline) after the onset of the euphoria event. Most of the patients in this analysis were female and in the 18–64 year age group; however there were no notable differences in demographic characteristics between pregabalin and placebo treatment groups. This is in line with a previous pooled analysis of 27 randomized clinical trials that showed that age and sex were among a group of potential predictors of efficacy of pregabalin that had no effect on outcome.50
Although this was an exploratory analysis with a relatively small sample of patients who reported euphoria events, this study raises interesting questions about the possible relationship between euphoria and early treatment responses. Patients may be reporting euphoria events because they are experiencing rapid and clinically relevant pain relief and/or sleep improvement after long-standing pain and/or sleep disturbance, respectively. However, we cannot rule out the possibility that patients may report better treatment responses because they have experienced a euphoric event. A causal link between euphoria events and positive treatment responses is difficult to demonstrate, and this study cannot distinguish between these two possibilities. Future research should investigate the potential causal relationship between positive treatment responses and euphoria.
The question of whether treatment-emergent AEs are a direct adverse effect of a study drug, or whether the relationship between reported AEs and active treatment is a more complex interaction, is a difficult question to answer. In a pooled analysis of seven randomized controlled studies that examined triptan therapy for migraine attacks, the treatment-emergent AEs somnolence and asthenia were shown to occur due to the unmasking of CNS-related neurological symptoms that were part of the migraine attack, rather than being direct side effects of triptan treatment.51 The relationship between treatment-emergent AEs and active treatment may not be as clear-cut as first thought, and is an area for possible future research.
In the US and Europe, up to one-third of individuals have chronic pain, equivalent to >100 million people in the US.52–54 Chronic pain is a debilitating condition associated with a considerable individual burden.54–56 An improvement of 2 points on an 11-point NRS (equivalent to a 30% improvement) may be considered potentially beneficial.47,48 Moreover, pain relief can occur quickly, with estimated times to improvement of pain with pregabalin of 1–2 days for patients with FM,57 DPN/PHN,49 or NeP due to SCI.58 For pregabalin-treated patients who reported euphoria in these analyses, the time to onset of pain relief is similar to the median time to onset of euphoria events (2.0 days). The improvements in sleep in patients who reported euphoria events mirrored the improvements in pain. This finding may not be surprising because the majority of studies in this pooled analyses evaluated PRSI, a measure of how much pain interfered with sleep. However, the findings here reinforce how closely connected pain and sleep are in patients with NeP or non-NeP conditions. Path analysis (a method used to assess direct and indirect contributions of individual variables to efficacy outcomes) has suggested that a considerable proportion (~60–80%) of the improvements in pain seen with pregabalin in patients with DPN or PHN are due to indirect improvements in sleep.59 Moreover, in patients with FM,57 DPN/PHN,60 and NeP due to SCI,58 the time to onset of improvements in sleep (1–2 days) are almost identical to the time to onset of pain improvement (1–2 days).49,57,58
The relationship between euphoria and the potential abuse of a drug is complex and not fully understood. Reports of euphoria are associated with some centrally acting drugs with no history of abuse.6–8 On the other hand, some centrally acting drugs with a well-established history of abuse are not associated with reports of euphoria.9 In the US, pregabalin is classified as a Schedule V drug (ie, it has the lowest potential for abuse compared with other controlled [Schedules I to IV] substances).61 Nonetheless, reports have been made of pregabalin misuse and abuse.62,63 One factor that may affect whether individual patients may misuse or abuse pregabalin is previous substance abuse.63,64 The US Prescribing Information and European Summary of Product Characteristics both state that patients should be carefully evaluated for history of drug abuse and observed for signs of pregabalin misuse or abuse.10,15 The findings from the current study should be viewed in light of reports of misuse and abuse of pregabalin.
This study had several potential limitations. Data are from clinical trials only, although this permits a systematic analysis of available data using defined terms. Additional reports of euphoria events may have occurred through ongoing postmarketing pharmacovigilance efforts, but collection of these data may not have been as rigorous as in clinical trials and efficacy outcomes would not necessarily have been captured in postmarketing reports. Moreover, participants in clinical studies are restricted to those who meet inclusion and exclusion criteria resulting in a relatively more homogeneous study population compared with the heterogeneous patients in real world postmarketing data. In addition, only chronic pain indications were evaluated, and other non-pain indications (eg, GAD or partial onset seizures) were not included in the analysis and this study thus cannot draw conclusions on potential efficacy and euphoria interactions in those populations. Relatively few euphoria events were reported with pregabalin, and even fewer with placebo. Because of this, individual indications could not be analyzed separately and individual pregabalin doses had to be pooled. Discerning the potential euphoric effects and efficacy by dose or by individual therapeutic indication would need to occur in a future study. No statistical comparisons were made because this was an exploratory analysis and few patients reported euphoria events limiting the statistical power for an inferential analysis. Thus, all results are intended to be interpreted qualitatively and do not support any causal or statistically significant relationships.
Conclusion
In summary, the reports of euphoria events in these clinical trials may be related to early treatment responses in some pregabalin-treated patients with chronic pain. More studies are needed to explore the relationship between the euphoria events and pregabalin treatment responses for non-pain indications. These data may also help to inform the design of future clinical trials of analgesics and other centrally acting drugs.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Acknowledgments
We thank Regina Behar from Pfizer for comments on a previous version of the manuscript. Medical writing support was provided by David Cope PhD, of Engage Scientific Solutions. This study was sponsored by Pfizer.
Disclosure
RF reports consultancy and speaker fees in the past 2 years from AOP Orphan Pharmaceuticals, Eli Lilly, Grünenthal, Merck Selbstmedikation, Mitsubishi Tanabe Pharma, Pfizer Inc., and Scilex Pharmaceuticals, outside of the submitted work. SS is an employee of the University of Western Australia (UWA); within the last 2 years his employer has received honoraria, consulting fees and travel support from Andros Pharmaceuticals, Aspen, bioCSL, Eli Lilly, Grünenthal, Invidior, Janssen, Luye Pharma, Mundipharma, Pfizer Inc., Pierre Fabre, Seqirus, and iX Biopharma. BP, EW, MO, PBB, and LK are employees of Pfizer and have stock or stock options with Pfizer. The authors report no other conflicts of interest in this work.
References
1. de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev. 2012;36(6):1565–1576. doi:10.1016/j.neubiorev.2012.04.005
2. Mansbach RS, Schoedel KA, Kittrelle JP, Sellers EM. The role of adverse events and related safety data in the pre-market evaluation of drug abuse potential. Drug Alcohol Depend. 2010;112(3):173–177. doi:10.1016/j.drugalcdep.2010.07.001
3. O’Connor AB, Turk DC, Dworkin RH, et al. Abuse liability measures for use in analgesic clinical trials in patients with pain: IMMPACT recommendations. Pain. 2013;154(11):2324–2334. doi:10.1016/j.pain.2013.06.035
4. Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39(2):254–262. doi:10.1038/npp.2013.261
5. Voon P, Kerr T. “Nonmedical” prescription opioid use in North America: a call for priority action. Subst Abuse Treat Prev Policy. 2013;8:39. doi:10.1186/1747-597X-8-39
6. Pfizer Inc [Internet]. Zoloft® Prescribing Information. New York, NY: Pfizer Inc; 2017. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=517#page=1.
7. Lilly USA, LLC [Internet]. Prozac® Prescribing Information. Indianapolis, IN: Lilly USA, LLC; 2016. Available from: http://pi.lilly.com/us/prozac.pdf.
8. Horizon Pharma USA, Inc [Internet]. Rayos® Prescribing Information. Deerfield IL: Horizon Pharma USA, Inc; 2016. Available from: http://www.rayosrx.com/pi/RAYOS-Prescribing-Information.pdf.
9. Pfizer Inc [Internet]. Xanax® Prescribing Information. New York, NY: Pfizer Inc; 2016. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=547.
10. Pfizer Inc [Internet]. Lyrica® Prescribing Information. New York, NY: Pfizer Inc; 2016. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=561.
11. Field MJ, Cox PJ, Stott E, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103(46):17537–17542. doi:10.1073/pnas.0409066103
12. Li Z, Taylor CP, Weber M, et al. Pregabalin is a potent and selective ligand for alpha(2)delta-1 and alpha(2)delta-2 calcium channel subunits. Eur J Pharmacol. 2011;667(1–3):80–90. doi:10.1016/j.ejphar.2011.05.054
13. Mico JA, Prieto R. Elucidating the mechanism of action of pregabalin: alpha(2)delta as a therapeutic target in anxiety. CNS Drugs. 2012;26(8):637–648. doi:10.2165/11634510-000000000-00000
14. Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73(2):137–150. doi:10.1016/j.eplepsyres.2006.09.008
15. Pfizer Ltd [Internet]. Lyrica® Summary of Product Characteristics. Sandwich, UK: Pfizer Ltd; 2016. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000546/WC500046602.pdf.
16. Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253–260. doi:10.1016/j.jpain.2004.12.007
17. Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–2110. doi:10.1212/01.wnl.0000145767.36287.a1
18. Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–638. doi:10.1016/j.pain.2004.05.001
19. Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP
20. Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. doi:10.1186/1471-2377-8-33
21. Satoh J, Yagihashi S, Baba M, et al. Efficacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week, randomized, double-blind, placebo-controlled trial. Diabet Med. 2011;28(1):109–116. doi:10.1111/j.1464-5491.2010.03152.x
22. Mu Y, Liu X, Li Q, et al. Efficacy and safety of pregabalin for painful diabetic peripheral neuropathy in a population of Chinese patients: A randomized placebo-controlled trial. J Diabetes. 2018;10(3):256–265. doi:10.1111/1753-0407.12585
23. Raskin P, Huffman C, Yurkewicz L, et al. Pregabalin in patients with painful diabetic peripheral neuropathy using an NSAID for other pain conditions: a double-blind crossover study. Clin J Pain. 2016;32(3):203–210. doi:10.1097/AJP.0000000000000254
24. Huffman C, Stacey BR, Tuchman M, et al. Efficacy and safety of pregabalin in the treatment of patients with painful diabetic peripheral neuropathy and pain on walking. Clin J Pain. 2015;31(11):946–958. doi:10.1097/AJP.0000000000000198
25. Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004;109(1–2):26–35. doi:10.1016/j.pain.2004.01.001
26. Dworkin RH, Corbin AE, Young JP
27. van Seventer R, Feister HA, Young JP
28. Stacey BR, Barrett JA, Whalen E, Phillips KF, Rowbotham MC. Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain. 2008;9(11):1006–1017. doi:10.1016/j.jpain.2008.05.014
29. Ogawa S, Suzuki M, Arakawa A, Araki S, Yoshiyama T. Efficacy and tolerability of pregabalin for postherpetic neuralgia: a multicenter, randomized, double-blind, placebo-controlled clinical trial. J Jpn Soc Pain Clin. 2010;17:141–152.
30. Liu Q, Chen H, Xi L, et al. A randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of pregabalin for postherpetic neuralgia in a population of chinese patients. Pain Pract. 2017;17(1):62–69. doi:10.1111/papr.12413
31. van Seventer R, Bach FW, Toth CC, et al. Pregabalin in the treatment of post-traumatic peripheral neuropathic pain: a randomized double-blind trial. Eur J Neurol. 2010;17(8):1082–1089. doi:10.1111/j.1468-1331.2010.02979.x
32. Markman J, Resnick M, Greenburg S, et al. Efficacy of pregabalin in post-traumatic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase 3 trial. J Neurol. 2018;265(12):2815–2824. doi:10.1007/s00415-018-9063-9
33. Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115(3):254–263. doi:10.1016/j.pain.2005.02.032
34. Guan Y, Ding X, Cheng Y, et al. Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin Ther. 2011;33(2):159–166. doi:10.1016/j.clinthera.2011.02.007
35. Moon DE, Lee DI, Lee SC, et al. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin Ther. 2010;32(14):2370–2385. doi:10.1016/j.clinthera.2011.01.014
36. Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67(10):1792–1800. doi:10.1212/01.wnl.0000244422.45278.ff
37. Cardenas DD, Nieshoff EC, Suda K, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013;80(6):533–539. doi:10.1212/WNL.0b013e318281546b
38. Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(4):1264–1273. doi:10.1002/art.20983
39. Mease PJ, Russell IJ, Arnold LM, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol. 2008;35(3):502–514.
40. Arnold LM, Russell IJ, Diri EW, et al. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain. 2008;9(9):792–805. doi:10.1016/j.jpain.2008.03.013
41. Pauer L, Winkelmann A, Arsenault P, et al. An international, randomized, double-blind, placebo-controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. J Rheumatol. 2011;38(12):2643–2652. doi:10.3899/jrheum.110569
42. Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res (Hoboken). 2012;64(4):597–606. doi:10.1002/acr.21595
43. Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. A randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia. Arthritis Res Ther. 2012;14(5):R217. doi:10.1186/ar4056
44. Arnold LM, Sarzi-Puttini P, Arsenault P, et al. Efficacy and safety of pregabalin in patients with fibromyalgia and comorbid depression taking concurrent antidepressant medication: a randomized, placebo-controlled study. J Rheumatol. 2015;42(7):1237–1244. doi:10.3899/jrheum.141196
45. Simpson DM, Schifitto G, Clifford DB, et al. Pregabalin for painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Neurology. 2010;74(5):413–420. doi:10.1212/WNL.0b013e3181ccc6ef
46. Simpson DM, Rice AS, Emir B, et al. A randomized, double-blind, placebo-controlled trial and open-label extension study to evaluate the efficacy and safety of pregabalin in the treatment of neuropathic pain associated with human immunodeficiency virus neuropathy. Pain. 2014;155(10):1943–1954. doi:10.1016/j.pain.2014.05.027
47. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
48. Farrar JT, Young JP
49. Sharma U, Griesing T, Emir B, Young JP
50. Almas M, Parsons B, Whalen E. Prediction of therapeutic response to pregabalin in subjects with neuropathic pain. Curr Med Res Opin. 2018;34(12):2041–2052. doi:10.1080/03007995.2018.1520694
51. Goadsby PJ, Dodick DW, Almas M, et al. Treatment-emergent CNS symptoms following triptan therapy are part of the attack. Cephalalgia. 2007;27(3):254–262. doi:10.1111/j.1468-2982.2007.01278.x
52. Institute of Medicine (IOM). Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
53. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230–1239. doi:10.1016/j.jpain.2010.07.002
54. Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1229. doi:10.1186/1471-2458-13-1229
55. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. doi:10.1016/j.ejpain.2005.06.009
56. Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non-cancer pain and chronic neuropathic pain. Pain Pract. 2014;14(1):79–94. doi:10.1111/papr.12050
57. Arnold LM, Emir B, Pauer L, Resnick M, Clair A. Time to improvement of pain and sleep quality in clinical trials of pregabalin for the treatment of fibromyalgia. Pain Med. 2015;16(1):176–185. doi:10.1111/pme.12636
58. Cardenas DD, Emir B, Parsons B. Examining the time to therapeutic effect of pregabalin in spinal cord injury patients with neuropathic pain. Clin Ther. 2015;37(5):1081–1090. doi:10.1016/j.clinthera.2015.02.028
59. Vinik A, Emir B, Parsons B, Cheung R. Prediction of pregabalin-mediated pain response by severity of sleep disturbance in patients with painful diabetic neuropathy and post-herpetic neuralgia. Pain Med. 2014;15(4):661–670. doi:10.1111/pme.12310
60. Parsons B, Emir B, Knapp L. Examining the time to improvement of sleep interference with pregabalin in patients with painful diabetic peripheral neuropathy and postherpetic neuralgia. Am J Ther. 2015;22(4):257–268. doi:10.1097/MJT.0000000000000100
61. Drug Enforcement Administration. Schedules of controlled substances: placement of pregabalin into schedule V. Final rule. Fed Regist. 2005;70(144):43633–43635.
62. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403–426. doi:10.1007/s40265-017-0700-x
63. Schjerning O, Rosenzweig M, Pottegard A, Damkier P, Nielsen J. Abuse potential of pregabalin: a systematic review. CNS Drugs. 2016;30(1):9–25. doi:10.1007/s40263-015-0303-6
64. Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118–122. doi:10.1159/000321079
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.