Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
The Relationship Between the “Adherence Starts with Knowledge-20” Questionnaire and Clinical Factors in Patients with COPD: A Multi-Center, Cross-Sectional Study
Authors Akimoto K, Hirai K , Matsunaga T, Kaneko K, Mikuni H, Kawahara T, Uno T, Fujiwara A, Miyata Y, Ohta S, Homma T, Inoue H, Yamaguchi F , Kusumoto S, Suzuki S , Tanaka A, Sagara H
Received 7 September 2020
Accepted for publication 16 November 2020
Published 4 December 2020 Volume 2020:15 Pages 3201—3211
DOI https://doi.org/10.2147/COPD.S280464
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Kaho Akimoto,1 Kuniaki Hirai,1 Tomohiro Matsunaga,2 Keisuke Kaneko,3 Hatsuko Mikuni,4 Tomoko Kawahara,5 Tomoki Uno,1 Akiko Fujiwara,6 Yoshito Miyata,1 Shin Ohta,1 Tetsuya Homma,1 Hideki Inoue,1 Fumihiro Yamaguchi,7 Sojiro Kusumoto,1 Shintaro Suzuki,1 Akihiko Tanaka,1 Hironori Sagara1
1Division of Respiratory Medicine and Allergology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan; 2Division of Allergology and Respiratory Medicine, Department of Medicine, Showa University Koto Toyosu Hospital, Tokyo, Japan; 3Department of Pulmonary Medicine, Tokyo Metropolitan Health and Hospitals Corporation Ebara Hospital, Tokyo, Japan; 4Department of Respiratory Medicine, Asahi General Hospital, Chiba, Japan; 5Department of Respiratory Medicine, Yamanashi Red Cross Hospital, Yamanashi, Japan; 6Department of Respiratory, Odawara Municipal Hospital, Kanagawa, Japan; 7Department of Respiratory Medicine, Showa University Fujigaoka Hospital, Kanagawa, Japan
Correspondence: Kuniaki Hirai
Division of Respiratory Medicine and Allergology, Department of Medicine, Showa University School of Medicine, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8666, Japan
Tel +81-3-3784-8000
Fax +81-3-3784-8742
Email [email protected]
Purpose: Inhaler therapy is the mainstay of chronic obstructive pulmonary disease (COPD) management. Poor adherence causes disease exacerbation and affects patient mortality. Although the Adherence Starts with Knowledge-20 (ASK-20) questionnaire is a reliable tool for assessing medication adherence, the relationship between the ASK-20 and clinical factors in patients with COPD remains unknown. We investigated the relationship between the ASK-20 and clinical factors, and assessed real-world inhaler therapy use.
Patients and Methods: A multicenter, cross-sectional study of outpatients with COPD undergoing inhaler treatment who completed the ASK-20 questionnaire was performed. We investigated COPD-related health status using the COPD Assessment Test (CAT), psychological status using the Hospital Anxiety and Depression Scale (HADS-anxiety and HADS-depression), respiratory function, patient satisfaction levels, and real-world inhaler therapy use.
Results: Of the total 319 patients, 87% were male with a median age of 74 years. Most patients had mild or moderate COPD, according to Global Initiative for Chronic Obstructive Lung Disease stage. The total ASK-20 scores correlated significantly with the CAT, HADS-anxiety, and HADS-depression scores (r = 0.27, 0.33, and 0.29, respectively, p < 0.01). Multivariable analysis showed that CAT and HADS-anxiety scores had an independent and significant impact on the ASK-20 scores [β, standardized regression coefficient: 0.18 (95% CI, 0.03– 0.35; p = 0.02), and 0.29 (95% CI, 0.17– 0.42; p < 0.01), respectively]; however, the ASK-20 scores were not correlated with age, sex, body mass index, cohabitation, modified Medical Research Council Dyspnea Scale score, pulmonary function, disease duration, number of COPD exacerbations per year, comorbidities, inhaler numbers, nor inhaler components.
Conclusion: The ASK-20 scores in patients with COPD were significantly associated with CAT and HADS scores. In Japan, Respimat was prescribed to younger patients and patients with lower CAT scores. The ASK-20, a simple evaluation method, is useful for identifying clinical factors affecting adherence in patients with COPD.
Keywords: COPD, adherence, ASK-20, inhaler
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality.1,2 A wide variety of treatment strategies, such as pharmacotherapy, smoking cessation, and respiratory rehabilitation have been reported; however, inhaler therapy remains the mainstay of COPD management.3,4 There have been numerous reports of poor adherence to medication and incorrect use of inhalers in patients with COPD and asthma.5–13 In previous studies, 28‒60% of patients with COPD used their inhalers incorrectly, and 40‒60% of patients did not adhere to the prescribed regimen.8,9 Previous reports have demonstrated that adherence to inhaled medication is worse than oral, injected, and transdermal medicines.14–17 Poor adherence often causes disease exacerbation and affects mortality in patients with COPD and asthma.18,19
Previous studies have shown that the Adherence Starts with Knowledge-20 (ASK-20) questionnaire is a reliable tool for assessing possible medication adherence barriers and behavior in patients with asthma.5,18,20,21 However, the relationships between the ASK-20 scores and inhaler device use, patient satisfaction, respiratory function, quality of life, and mood disorders in patients with COPD remain unclear. Although many previous studies have reported comparisons among a wide range of inhaler devices available to treat COPD,8–11,13,17,22,23 less is known about the characteristics of patients using these devices for COPD and the differences in clinical factors for patients treated with a single maintenance inhaler or multiple maintenance inhalers.
We, therefore, conducted a multicenter, cross-sectional trial aimed at investigating the relationship between the ASK-20 and clinical factors among patients, as well as inhaler therapy characteristics in a real-world setting.
Patients and Methods
This multi-center, cross-sectional study was conducted among patients older than 40 years, who were diagnosed with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria, in Japan between March 2018 and November 2019. Patients were excluded if they experienced an acute exacerbation within the previous four weeks or suffered from other pulmonary diseases. In total, 371 outpatients who were evaluated at regular 1- to 3-month intervals in a secondary- or tertiary-care setting were initially considered eligible for inclusion in this study. Of these patients, 16 were excluded because they did not have inhaler prescriptions, and 36 were excluded because they did not submit or complete the questionnaire. Patients were classified as single or multiple long-acting inhaler users based on their treatment regimen. Patients who used two or more long-acting bronchodilator inhalers concomitantly were defined as multiple-inhaler users. Inhaler devices that were used by fewer than 10 patients were excluded from the analysis of the relationship between inhaler devices and clinical factors. We also analyzed the number of moderate or severe acute exacerbations per year using data extracted from electronic medical records. A moderate COPD exacerbation was defined as an exacerbation that required the administration of antibiotics and/or systemic steroids but no hospitalization. A severe COPD exacerbation was defined as an exacerbation requiring hospitalization.
All participating patients provided written informed consent. The enrolled patients were able to withdraw at any time. This study was carried out in accordance with the Declaration of Helsinki guidelines and was approved by the ethics committee of Showa University School of Medicine (approval number: 2375) and by an independent ethics committee.
Physical and Psychological Measurements
We assessed dyspnea using the modified Medical Research Council (mMRC) Dyspnea Scale,24 COPD-related health status using the COPD Assessment Test (CAT),25 and psychological status using the Hospital Anxiety and Depression Scale (HADS-anxiety and HADS-depression).26 Higher scores indicate the presence of symptoms. Pulmonary function data were collected from the most recent medical records. Patient satisfaction level with their respiratory status was classified using a 4-point scale indicating either satisfaction or dissatisfaction.
Adherence barriers to inhaled medicines were evaluated using the ASK-20 questionnaire. The ASK-20 questionnaire includes the domains of lifestyles (items Q1‒Q6), attitude and behavior (Q7‒Q8), support from others or communication with the healthcare team (Q9‒Q12), barriers to medicine use (Q13‒Q15), and adherence to medicines (Q16‒Q20). Responses for each item were scored on a 5-point scale: strongly disagree (1 point) to strongly agree (5 points). For items Q1‒Q6 and Q13‒Q15, higher scores suggested poor adherence. Items Q7–Q12 were reversely scored so that their final scores were in the same direction as the other 14 items. Items Q16‒Q20, related to past experiences with adherence, were scored on a 5-point scale: never (1 point) to in the last week (5 points). Higher scores represented poor adherence to inhaled medications. In addition, we considered a score of 5 in Q6; a score of 4 or more in Q1, Q4, Q5, Q9, Q13‒Q15, and Q20; and a score of 3 or more in Q2, Q3, Q7, Q8, Q10‒Q12, and Q16‒Q19 as barriers. Further, each item of the ASK-20 was recoded into “barrier present” or “barrier absent.” Barriers were indicated by a score of 5 in Q6; a score of 4 or more in Q1, Q4, Q5, Q9, Q13‒Q15, and Q20; and a score of 3 or more in Q2, Q3, Q7, Q8, Q10‒Q12, and Q16‒Q19. The total barrier counts (TBCs), the total number of barriers for all items, were calculated as a summary score, with a potential range of 0 (no barrier) to 20 (maximum number of barriers), and higher TBCs indicating more barriers.18,20
Statistical Analysis
Distributions were checked using box plots, and outliers were excluded if input was incorrect. However, outliers without incorrect input were not excluded. The validity of the normal distribution was assessed using the Shapiro–Wilk test. Vital capacity (VC) and the percent predicted forced expiratory volume in one second (%FEV1) followed a normal distribution; none of the other continuous variables followed a normal distribution. Categorical variables were presented as numbers (%), while continuous variables were reported as the mean (standard deviation ± SD) for normally distributed data and median (interquartile range) for non-normally distributed data. The non-parametric Wilcoxon signed-rank test was used for comparisons. The Pearson’s Χ2 test or Fisher’s exact test was used to assess the relationship between categorical variables. In order to investigate the relationships between inhaler devices and clinical factors, ANOVA, Kruskal–Wallis, chi-square, and Wilcoxon rank sum tests were employed. Correlation analysis was performed using Spearman’s rank test.
We developed a linear regression model to determine factors influencing the ASK-20 score, using the following candidate predictors: age, sex, CAT, HADS-anxiety, and patient satisfaction. Statistical analyses were performed using JMP version 14 software (SAS Institute, Cary, NC). Differences of p < 0.05 were considered statistically significant. All authors had full access to all data and take responsibility for the decision to submit for publication.
Results
Characteristics of the Participants
Table 1 shows the characteristics of the participants. Of the total 371 outpatients with COPD who used inhalers, 319 were ultimately analyzed in this study. The median patient age was 74 years, and the majority of patients were male (87%, n=278). Overall, 31% of study patients had mild COPD (GOLD stage Ⅰ), 45% had moderate COPD (GOLD stage II), and 24% had severe or very severe COPD (GOLD stages III–IV). The ASK-20 score distribution is illustrated in Figure 1, showing a median total ASK-20 score of 38 (33–44). About one-third of patients were satisfied with their disease control (33%, n=106). Among participants in this study, there were markedly more single-inhaler users than multiple-inhaler users (82%, n=262). Six inhaler devices were used by more than 1% of the single-inhaler group. Among inhaler devices, the main device prescribed for patients with COPD was Respimat. Long‐acting muscarinic antagonist (LAMA)/long‐acting beta2‐agonist (LABA) fixed-dose combinations were used by more than half of patients, followed by LAMA and LAMA/LABA/inhaled corticosteroids (ICS).
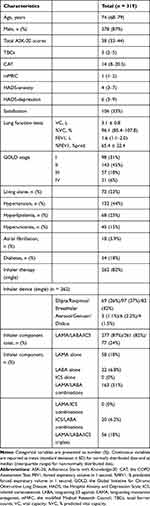 |
Table 1 Characteristics of the Included Studies |
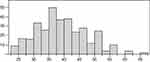 |
Figure 1 Distribution of Adherence Starts with Knowledge-20 (ASK-20) score. The median total ASK-20 score was 38 (33–44). The x-axis shows the total ASK-20 scores and the y-axis shows the frequency. |
Association Between the Adherence Starts with Knowledge-20 Questionnaire, Total Barrier Counts, and Clinical Factors
Figure 2 shows the correlation between total ASK-20 scores and TBCs with patients’ clinical factors and inhaler therapy. The total ASK-20 scores were significantly correlated with CAT, HADS-anxiety, and HADS-depression (r = 0.27, r = 0.33, and r = 0.29, respectively; all p < 0.01). Similarly, TBCs showed a significant correlation with CAT, HADS-anxiety, and HADS-depression (r = 0.24, r = 0.22, and r = 0.24; all p < 0.01). There was also a significant difference in the total ASK-20 scores or TBCs between the satisfied and non-satisfied groups (both p < 0.01). There was no correlation between ASK-20 scores or TBCs with age, sex, mMRC, or pulmonary function. The ASK-20 score or TBCs were not correlated with disease duration (r = −0.10, p = 0.17 and r = −0.11, p = 0.13, respectively) and number of COPD exacerbations per year (r = 0.02, p = 0.71 and r = 0.01, p = 0.85, respectively). Comorbidities were also not associated with the ASK-20 score and TBCs (p > 0.05) (Table 2).
 |
Table 2 Relationship Between Comorbidities and Adherence Starts with Knowledge-20 (ASK-20) Score and Total Barrier Counts (TBCs) |
Multivariable analysis showed that CAT and HADS-anxiety had an independent and significant impact on the ASK-20 score [β, standardized regression coefficient: 0.18 (95% CI, 0.03-0.35; p = 0.02), and 0.29 (95% CI, 0.17-0.42; p < 0.01), respectively] (Table 3).
 |
Table 3 Multivariable Analysis of Factors Associated with the Adherence Starts with Knowledge-20 (ASK-20) Score |
Association Between Inhaler Components and Clinical Factors
Table 4 shows the relationship between inhaler devices and clinical factors. Patients who were Respimat users were significantly younger than Breezhaler users (p = 0.03). The CAT scores were significantly different between Ellipta users and Respimat users (p = 0.02). Among the three devices, there was no significant association with the total ASK-20 scores, TBCs, sex, mood disorder distribution, satisfaction, or pulmonary function.
 |
Table 4 Relationship Between Inhaler Devices and Clinical Factors |
Supplementary Table 1 presents a comparison of the characteristics between users of LAMA or LABA alone and users of LAMA/LABA combinations. Users of LAMA/LABA combinations had significantly higher CAT and mMRC (both p < 0.01), and lower %FEV1 (p < 0.01) than users of LAMA or LABA alone. Satisfaction was significantly higher in patients who used LAMA or LABA alone than in patients who used LAMA/LABA combinations (p < 0.01). There were no significant differences in the total ASK-20 score or TBCs between users of LAMA or LABA alone and users of LAMA/LABA combinations (p > 0.05).
Additionally, short-acting beta2-agonist (SABA) users had significantly higher CAT and mMRC scores and lower %FEV1 than non-SABA users (p < 0.01, p = 0.03, and p < 0.01, respectively) (Supplementary Table 2).
Clinical Factors Associated with Single and Multiple Inhaler Use
Table 5 shows the differences in the clinical factors of patients treated with single or multiple maintenance inhalers. Single inhalers were used significantly more often by patients living alone (p = 0.01). Although there were no significant differences in the total ASK-20 scores or TBCs between single-inhaler and multiple-inhaler users, TBCs in patients using a single inhaler tended to be higher than in those using multiple inhalers (p = 0.055).
 |
Table 5 Differences in Clinical Factors Between Patients Treated with Single or Multiple Maintenance Inhalers |
Discussion
Our investigation of ASK-20 for assessing medication adherence in patients with COPD and the characteristics in a real-world setting found that the ASK-20 score was related to the CAT and mood disorders (anxiety and depression). Moreover, Respimat users were significantly younger than Breezhaler users and had lower CAT scores than Ellipta users. Additionally, LAMA/LABA combinations and SABA users had significantly higher CAT and mMRC, and lower %FEV1, and their use was not associated with mood disorders.
The CAT and HADS scores were associated with the ASK-20 score and TBCs. This is consistent with the results of previous studies that have shown a significant association between medication adherence and health-related quality of life.6,19 It has been reported that mood disorders may affect the recognition of personal health goals and cause poor adherence.18,27,28 The study showed a link between the HADS and ASK-20 scores, suggesting that mood disorders may increase the ASK-20 scores. In patients with high ASK-20 scores, it may be necessary to consider the possibility that mood disorders may underlie poor adherence.19
We found that satisfaction was related to the ASK-20 score and TBCs in univariate analysis. Improved patient satisfaction may be associated with lower CAT scores, exacerbation of COPD, and healthcare resource use, according to previous studies.19,22,23,29–32 In this study, neither the ASK-20 score nor TBCs was correlated with other patient-level factors, including age, sex, BMI and cohabitation, mMRC scores, pulmonary function, disease duration, number of COPD exacerbations per year, or comorbidities. However, in previous studies, adherence to inhaler therapy was best in patients who were 65 years or older, male, and had more severe COPD based on GOLD criteria.6,28,33 The discrepancy between the findings of this and previous studies may be explained by the fact that most participants in this study were elderly men with mild-to-moderate COPD (GOLDⅠ–Ⅱ). Previous studies have reported that poor adherence appears in the initial year of inhalation therapy and that inhalation education improves adherence.13,22,34 On the other hand, some studies have shown that disease duration may contribute to poor adherence due to an increased risk of cognitive impairment or memory problems over time.35 Therefore, the relationship between disease duration and adherence remains controversial, and, in this study, no association was found between disease duration and the ASK-20 score nor TBCs.
Although no association was identified between exacerbations and the ASK-20 score or TBCs in this study, it has previously been demonstrated that higher rates of exacerbations are associated with poorer medication adherence in patients with COPD.36,37 The ASK-20 questionnaire may, therefore, have limited ability to predict exacerbations. Furthermore, it is possible that mild exacerbations were not picked up due to a retrospective assessment of exacerbation in this study or the overall lower rate of exacerbations in Japan compared to the rest of the world might have affected our results.38
Patients with COPD often have multiple comorbidities, including hypertension, hyperlipidemia, heart diseases, diabetes, and osteoporosis.38 Although comorbidities were not found to be associated with the ASK-20 score nor TBCs in this study, TBCs tended to be higher in patients with diabetes (p = 0.06). Comorbidities have previously been shown to be related to adherence due to the increasing number of medications and complex medication regimens.22,39,40 In addition, previous study showed the association between adherence to medications for comorbidities and COPD, and patients with diabetes were shown to have poor adherence.41 This finding is consistent with the tendency for higher TBCs in patients with diabetes in this study.
In the present study, Respimat was more frequently prescribed to younger patients than Breezehaler and more frequently prescribed than Ellipta to patients with lower CAT scores. Literature indicates that adherence to COPD medication is poor, although little is known about the barriers among different inhaler devices.8,18,19 A previous study reported that critical errors in the use of these inhalers were observed in Respimat (46.9%) and Breezhaler users (15.4%), and most patients felt confident or very confident that they receive the full dose with a Breezhaler.11 Although no significant association was found between the ASK-20 score and inhaler devices in the present study, a prior study found that patient-reported adherence to inhaler treatment was highest with Breezhaler, followed by other devices.8 Patients using Breezhaler in this study were older, and it was considered that the outpatient clinician chose a device that was reported to be associated with better adherence. Respimat users had significantly lower CAT scores, and many patients who were maintained on LAMA used only Respimat. This result may correspond with a previous study, which showed that patients with mild or moderate COPD, who received tiotropium therapy for 24 months, had a higher forced expiratory volume in 1 second (FEV1.0) than those who received the placebo. The annual decrease in FEV1.0 was smaller than in patients who were not on tiotropium therapy.42
In the present study, patients with COPD who had higher mMRC and poor pulmonary function were prescribed LAMA/LABA combinations and rescue medication. Patients with COPD who use SABA before feeling short of breath during activities can improve dyspnea and exercise tolerance, according to previous studies.43–45 We found that inhalers were prescribed as proposed in the GOLD report.46 According to a study investigating the characteristics of patients using inhaler therapy, there were no differences in mood disorders between patients using LAMA only or using LAMA/LABA combinations.47 Similarly, this study showed that there was no association between the use of LAMA/LABA combinations and mood disorders. In a real-world setting, a doctor’s prescription thus may not be impacted by the patient’s mood disorders.
This study indicated that single device users tended to demonstrate poorer treatment adherence rates than multiple device users. This was in contrast to several previous studies reporting that multiple-inhaler users were less likely to be adherent than single-inhaler users.19,48,49 We should, however, be aware of the possibility that patients expressing milder symptoms may be more susceptible to lower medication usage, due to lower disease burden. Outpatient clinicians may not prescribe multiple devices for patients who are expected to show poor adherence. In addition, patients living alone were more commonly prescribed a single inhaler and had an increased risk of poor adherence compared to cohabiting patients. Single inhalers are often prescribed to solitary patients, as they cannot obtain inhalation-support from their cohabitants.50
This study had several limitations. First, the ASK-20 scores might have been influenced by selection bias that most patients were male with mild or moderate COPD. In addition, the fact that patients managed in a primary-care setting were not included in this study might have affected our results. In a secondary- or tertiary-care setting, patients managed by respiratory specialists might have fewer adherence barriers than those managed by non-specialized physicians in a primary-care. Second, no patients had been diagnosed with COPD during the preceding year; thus, we could not assess the ASK-20 in the early phase after diagnosis. Third, there was not enough data on single inhaler triple therapy, because single inhalers containing ICS, LAMA and LABA have only recently been developed. In this study, most patients with COPD receiving triple therapy used at least two inhalers, and we could not assess the clinical factors of patients using single inhaler triple therapy. Considering that most of the components of multiple-inhalers, in this study, were ICS/LAMA/LABA, we can expect better adherence in the future.
Conclusion
In conclusion, we showed that the ASK-20 score was significantly associated with the CAT and HADS scores in COPD patients, and that, in Japan, Respimat was prescribed to patients who were younger and had lower CAT scores. Thus, this study showed that the ASK-20 questionnaire, which is a simple evaluation method, is useful for identifying the clinical factors affecting adherence in COPD patients.
Abbreviations
ASK-20, Adherence Starts with Knowledge-20; CAT, the COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1.0, forced expiratory volume in 1 second; %FEV1, % predicted forced expiratory volume in 1 second; GOLD, the Global Initiative for Chronic Obstructive Lung Disease; HADS-anxiety and HADS-depression, the Hospital Anxiety and Depression Scale; ICS, inhaled corticosteroids; LAMA, long‐acting muscarinic antagonist; LABA, long‐acting beta2‐agonist; mMRC, the modified Medical Research Council; SABA, short-acting beta2-agonist; TBCs, the total barrier counts.
Acknowledgments
This work was supported, in part, by the Environment Research and Technology Development Fund (year 2019 to 2021) of the Environmental Restoration.
Disclosure
Shintaro Suzuki reports a grant from KAKENHI (20K03161) and a sponsored research grant from MylanEPD (JAPAN), outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Soriano JB, Kendrick PJ, Paulson KR; GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8:585–596. doi:10.1016/S2213-2600(20)30105-3
2. David M. Epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121:121–126.
3. Terry PD, Dhand R. Inhalation therapy for stable COPD: 20 years of GOLD reports. Adv Ther. 2020;37:1812–1828. doi:10.1007/s12325-020-01289-y
4. Roche N, Aguilaniu B, Li PZ, Hess D; COLIBRI collaborators. Trends over time in COPD treatment choices by respiratory physicians: an analysis from the COLIBRI-COPD French cohort. Respir Med. 2019;156:8–14. doi:10.1016/j.rmed.2019.07.023
5. Atsuta R, To Y, Sakamoto S, et al. Assessing usability of the “Adherence Starts with Knowledge 20” (ASK-20) questionnaire for Japanese adults with bronchial asthma receiving inhaled corticosteroids long term. Allergol Int. 2017;66:411–417. doi:10.1016/j.alit.2016.09.001
6. Rogliani P, Ora J, Puxeddu E, Matera MG, Cazzola M. Adherence to COPD treatment: myth and reality. Respir Med. 2017;129:117–123. doi:10.1016/j.rmed.2017.06.007
7. Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:481–490. doi:10.1016/j.rmed.2013.04.005
8. Price D, Keininger DL, Viswanad B, Gasser M, Walda S, Gutzwiller FS. Factors associated with appropriate inhaler use in patients with COPD - lessons from the REAL survey. Int J Chronic Obstruct Pulmon Dis. 2018;13:695–702. doi:10.2147/COPD.S149404
9. Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28:219–228. doi:10.1089/jamp.2014.1142
10. Ahn JH, Chung JH, Shin KC, et al. Critical inhaler handling error is an independent risk factor for frequent exacerbations of chronic obstructive pulmonary disease: interim results of a single center prospective study. Int J Chron Obstruct Pulmon Dis. 2019;14:2767–2775. doi:10.2147/COPD.S234774
11. Müller T, Müller A, Hübel C, et al. Optimizing inhalation technique using web-based videos in obstructive lung diseases. Respir Med. 2017;129:140–144.
12. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi:10.1016/j.rmed.2011.01.005
13. Serra-Batlles J, Plaza V, Badiola C, Morejón E; Inhalation Devices Study Group. Patient perception and acceptability of multidose dry powder inhalers: a randomized crossover comparison of Diskus/Accuhaler with Turbuhaler. J Aerosol Med. 2002;15:59–64. doi:10.1089/08942680252908584
14. Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. J Asthma. 2003;40:93–101. doi:10.1081/JAS-120017212
15. Rand C, Bilderback A, Schiller K, Edelman JM, Hustad CM, Zeiger RS. Adherences with montelukast or fluticasone in a long-term clinical trial: results from the mild asthma montelukast versus inhaled corticosteroid trial. J Allergy Clin Immunol. 2007;119:916–923. doi:10.1016/j.jaci.2006.12.664
16. Broder MS, Chang EY, Ory C, Kamath T, Sapra S. Adherence and persistence with omalizumab and fluticasone/salmeterol within a managed care population. Allergy Asthma Proc. 2009;30:148–157. doi:10.2500/aap.2009.30.3190
17. Tamura G, Ohta K. Adherence to treatment by patients with asthma or COPD: comparison between inhaled drugs and transdermal patch. Respir Med. 2007;101:1895–1902. doi:10.1016/j.rmed.2007.05.001
18. Toyama T, Kawayama T, Kinoshita T, et al. Differences in adherence barriers to inhaled medicines between Japanese patients with chronic obstructive pulmonary disease and asthma evaluated using the “Adherence Starts with Knowledge 20” (ASK-20) questionnaire. Intern Med. 2019;58:175–185. doi:10.2169/internalmedicine.0488-17
19. Pierobon A, Sini Bottelli E, Ranzini L, et al. COPD patients’ self-reported adherence, psychosocial factors and mild cognitive impairment in pulmonary rehabilitation. Int J Chron Obstruct Pulmon Dis. 2017;12:2059–2067. doi:10.2147/COPD.S133586
20. Hahn SR, Park J, Skinner EP, et al. Development of the ASK-20 adherence barrier survey. Curr Med Res Opin. 2008;24:2127–2138. doi:10.1185/03007990802174769
21. Sasaki J, Kawayama T, Yoshida M, et al. Adherence barriers to inhaled medicines in Japanese older patients with asthma evaluated using the “Adherence Starts with Knowledge 20” (ASK-20) questionnaire. J Asthma. 2019;56:632–641. doi:10.1080/02770903.2018.1484132
22. López-Campos JL, Quintana Gallego E, Carrasco Hernández L. Status of and strategies for improving adherence to COPD treatment. Int J Chron Obstruct Pulmon Dis. 2019;14:1503–1515. doi:10.2147/COPD.S170848
23. Miravitlles M, Montero-Caballero J, Richard F, et al. A cross-sectional study to assess inhalation device handling and patient satisfaction in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:407–415. doi:10.2147/COPD.S91118
24. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi:10.1136/thx.54.7.581
25. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi:10.1183/09031936.00102509
26. Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287–1295. doi:10.2147/COPD.S101459
27. Andriana I. The impact of depressive symptoms on recovery and outcome of hospitalized COPD exacerbations. Eur Respir J. 2013;41:815–823. doi:10.1183/09031936.00013112
28. Humenberger M, Horner A, Labek A, et al. Adherence to inhaled therapy and its impact on chronic obstructive pulmonary disease (COPD). BMC Pulm Med. 2018;18:163. doi:10.1186/s12890-018-0724-3
29. Bjermer L. The importance of continuity in inhaler device choice for asthma and chronic obstructive pulmonary disease. Respiration. 2014;88:346–352. doi:10.1159/000363771
30. van der Palen J, Cerveri I, Roche N, et al. DuoResp Spiromax adherence, satisfaction and ease of use: findings from a multi-country observational study in patients with asthma and COPD in Europe (SPRINT). J Asthma. 2019:1–9.
31. Chrystyn H, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108:358–365. doi:10.1016/j.rmed.2013.09.021
32. Plaza V, Giner J, Curto E, et al. Determinants and differences in satisfaction with the inhaler among patients with asthma or COPD. J Allergy Clin Immunol Pract. 2020;8:645–653. doi:10.1016/j.jaip.2019.09.020
33. Ingebrigtsen TS, Marott JL, Nordestgaard BG, et al. Low use and adherence to maintenance medication in chronic obstructive pulmonary disease in the general population. J Gen Intern Med. 2015;30:51–59. doi:10.1007/s11606-014-3029-0
34. Rie N, Keiko N, Amelia T, et al. Barriers to medication adherence among patients with non-communicable diseases: Fijian health professionals’ perceptions. J Int Health. 2014;29(4):313–320.
35. Stewart RB, Caranasos GJ. Medication compliance in the elderly. Med Clin North Am. 1989;73(6):1551–1563. doi:10.1016/S0025-7125(16)30616-2
36. Vestbo J, Anderson JA, Calverly PMA, et al. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64(11):939–943. doi:10.1136/thx.2009.113662
37. Davis JR, Wu B, Kern DM, et al. Impact of nonadherence to inhaled corticosteroid/LABA therapy on COPD exacerbation rates and healthcare costs in a commercially insured US population. Am Health Drug Benefits. 2017;10(2):92–102.
38. Ishii T, Nishimura M, Akimoto A, James MH, Jones P. Understanding low COPD exacerbation rates in Japan: a review and comparison with other countries. Int J Chron Obstruct Pulmon Dis. 2018;13:3459–3471. doi:10.2147/COPD.S165187
39. Darbà J, Ramírez R, Sicras A, et al. The importance of inhaler devices: the choice of inhaler device may lead to suboptimal adherence in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:2335–2345. doi:10.2147/COPD.S90155
40. Qian J, Simoni-Wastila L, Rattinger GB, et al. Association between depression and maintenance medication adherence among Medicare beneficiaries with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2014;29(1):49–57. doi:10.1002/gps.3968
41. Rolnick SJ, Pawloski PA, Hedblom BD, et al. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11:54–65. doi:10.3121/cmr.2013.1113
42. Zhou Y, Zhong NS, Li X, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377:923–935. doi:10.1056/NEJMoa1700228
43. Fujimoto K, Yoshiike F, Yasuo M, et al. Effects of bronchodilators on dynamic hyperinflation following hyperventilation in patients with COPD. Respirology. 2007;12:93–99. doi:10.1111/j.1440-1843.2006.00963.x
44. O’Donnell DE, Webb KA. Exercise testing. In: Celli BR, editor. Pharmacotherapy in Chronic Obstructive Pulmonary Disease. New York: Marcel Dekker; 2004:45–71.
45. O’Donnell DE, Hernandez P, Kaplan A, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care. Can Respir J. 2008;15(SupplA):1A–8A.
46. Pauwels RA, Buist AS, Calverley PM, et al.; for GOLD Scientific Committee. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med.2018;163:1256–1276. doi:10.1164/ajrccm.163.5.2101039
47. Bloom CI, Elkin SL, Quint JK. Changes in COPD inhaler prescriptions in the United Kingdom, 2000 to 2016. Int J Chron Obstruct Pulmon Dis. 2019;14:279–287. doi:10.2147/COPD.S190086
48. Yu AP, Guérin A, Ponce de Leon D, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14:486–496. doi:10.3111/13696998.2011.594123
49. van der Palen J, Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER. Multiple inhalers confuse asthma patients. Eur Respir J. 1999;14:1034–1037. doi:10.1183/09031936.99.14510349
50. Tøttenborg SS, Lange P, Johnsen SP, Nielsen H, Ingebrigtsen TS, Thomsen RW. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir Med. 2016;119:160–167. doi:10.1016/j.rmed.2016.09.007
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

