Back to Journals » International Journal of General Medicine » Volume 16
The Relationship Between the Expression of circFAT1 and Immune Cell in Patients with Non-Small Cell Lung Cancer
Authors Li J, Liu Y, Zeng W, Wu Y, Ao W, Yuan X, Zhou C
Received 5 August 2023
Accepted for publication 25 October 2023
Published 31 October 2023 Volume 2023:16 Pages 4943—4951
DOI https://doi.org/10.2147/IJGM.S434065
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jingjing Li,1,* Yabing Liu,1,* Wenxuan Zeng,2 Yanrun Wu,3 Wei Ao,4 Xiwei Yuan,1 Chuanyi Zhou1
1Department of Oncology, Yueyang People’s Hospital, Yueyang City, Hunan Province, 414000, People’s Republic of China; 2Department of Cardiovascular, Yueyang Central Hospital, Yueyang City, Hunan Province, 414000, People’s Republic of China; 3Department of Ultrasonic, Yueyang Central Hospital, Yueyang City, Hunan Province, 414000, People’s Republic of China; 4Department of Cardiovascular, Yueyang People’s Hospital, Yueyang City, Hunan Province, 414000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiwei Yuan; Chuanyi Zhou, Department of Oncology, Yueyang People’s Hospital, No. 263, Baling East Road, Yueyanglou District, Yueyang City, Hunan Province, People’s Republic of China, Email [email protected]; [email protected]
Objective: To analyze the correlation between the expression of circFAT1 in serum and immune cells in patients with non-small cell lung cancer (NSCLC).
Methods: A total of 96 patients with NSCLC admitted to our hospital from November 2019 to November 2022 were regarded as the study subjects. In the meantime, 96 volunteers who had physical examination in our hospital were regarded as the control group. The expression level of circFAT1 in serum was detected by real-time fluorescence quantitative PCR. NSCLC cancer tissue (NSCLC group) and paracancerous tissue (tissue ≥ 2cm away from the focus) (paracancerous group) were collected during the operation, the expression of CD4+, CD8+ and Foxp3+ in tissues was determined by immunohistochemistry; the expression level of circFAT1 mRNA in NSCLC tissue was analyzed using the Ualcan database. Spearman correlation was applied to analyze the correlation between the expression of circFAT1 and immune cells (CD4+, Foxp3+, CD8+).
Results: The level of circFAT1 in NSCLC tissue was higher than that in normal tissue (P < 0.05). Compared with the control group, the expression level of circFAT1 in serum of NSCLC group was obviously higher (P < 0.05). The expression level of circFAT1 was related to lymph node metastasis, TNM stage and differentiation (P < 0.05). Compared with the paracancerous group, the positive expression rate of CD8+ in NSCLC group was obviously lower, and the positive expression rates of CD4+ and Foxp3+ were obviously higher (P < 0.05). The expression of CD4+, Foxp3+ and CD8+ in NSCLC patients’ cancer tissue was related to lymph node metastasis, TNM stage and differentiation degree (P < 0.05). Spearman correlation analysis showed that circFAT1 was positively correlated with the expression of CD4+ and Foxp3+ and negatively correlated with the expression of CD8+ (P < 0.05).
Conclusion: CircFAT1 is highly expressed in the serum of NSCLC patients and is closely related to immune cells.
Keywords: non-small cell lung cancer, circFAT1, immune microenvironment, correlation
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer cases. For localized stage I or II NSCLC patients, surgical resection brings a good prognosis with a survival rate of up to 75%.1,2 Nevertheless, many patients (>5%) are diagnosed with advanced NSCLC and face a survival rate of below 3%.3 A growing number of studies have shown that circRNAs play an important role in a variety of biological processes, especially in cancer onset and development.4 CircFAT1, which plays an important role in the tumorigenesis of multiple organ systems, is a product of 3283 nucleotides in length, formed by reverse splicing of exon 1 of the pre-FAT2 mRNA. In squamous cell carcinoma, circFAT1 binds and activates STAT3 as a way to regulate both cancer stemness and anti-tumor immunity.5 Studies of osteosarcoma, cervical cancer, hepatocellular carcinoma and colorectal cancer have revealed that circFAT1 regulates the behavior of cancer cells, including cell proliferation, and invasion/migration, through the adjustment of miR-375/YAP1, miR-409-3p/CDK8, and miR-30a-5p/REEP3 axes.6 In recent years, the relevance between tumor immune cell and tumor pathogenesis, progression has aroused great concern. There are insights into NSCLC therapy indicating that leukocytes, neutrophils, lymphocytes, and macrophages directly facilitate the immune response and can be easily and conveniently detected.7 CD4+ T helper (Th) cells play a core role in coordinating the adaptive immune response at epithelial sites.8 After Foxp3+ T cells transfer to tumor sites, CD4+ T cells can inhibit cytotoxic T cell activity through intercellular contacts.9 CD8+ T cells are core players in the adaptive immune response to infections and cancer.10 Despite significant progress in NSCLC treatment strategies, NSCLC still exhibits poor prognosis, metastasis, and recurrence. Therefore, it is important to investigate new therapeutic strategies and potential biomarkers based on the molecular mechanisms of NSCLC development.
Data and Methods
General Data
Ninety-six NSCLC patients admitted to our hospital from November 2019 to November 2022 were selected as the NSCLC group. All the patients were diagnosed with NSCLC in pathological examination. Of these, there were 50 men and 46 women aged 35–75 years, with a mean age of (45.50 ± 5.14) years and a body mass index (BMI) of (22.41 (±3.47) kg/m2. There was smoking history in 45 cases, alcohol consumption history in 47 cases. According to the WHO pathological classification, there were 45 cases of squamous carcinoma and 51 cases of adenocarcinoma. According to TNM staging, there were 21, 28, 26, and 21 cases in stages I, II, III, and IV, respectively. Fifty-two cases had lymph node metastasis, 54 cases had low differentiation as compared to 42 cases with medium and high differentiation in terms of differentiation degree. Ninety-six volunteers receiving physical examination in our hospital during the same period were selected as the control group, including 50 men and 46 women aged 34–76 years, with a mean age of (45.00 ± 5.54) years and a BMI of (22.38 ± 3.84) kg/m2. No significant difference was observed in the general data between the two groups (P > 0.05), so the data were comparable. All study subjects were informed of the study and voluntarily signed the consent form, and the study was approved by the ethics committee of our hospital. Inclusion criteria for NSCLC patients: (1) meet the diagnostic criteria for NSCLC in the “Clinical Guidelines of Chinese Medical Association for the Treatment of Lung Cancer (2018 edition)”; (2) age >18 years. Exclusion criteria: (1) patients with immune diseases; (2) patients with contagious diseases; (3) patients with other malignant tumors; (4) patients with other lung diseases; (5) patients in pregnancy or lactation; (6) patients with a mental disorder who cannot communicate normally, as shown in Figure 1.
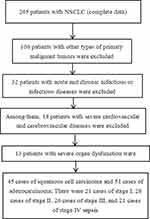 |
Figure 1 Case collection flow chart. |
Methods
Detection of the Expression Level of Serum circFAT1
Ten milliliters of fasting venous blood was drawn from both NSCLC patients and volunteers on the day after hospitalization and the day of the physical examination, respectively, centrifuged at 4200rpm for 20min with a centrifugal radius of 10cm. The supernatant was taken and stored in a −20°C refrigerator for later use. The serum circFAT1 levels were detected by real-time fluorescence quantitative PCR. Total RNA was extracted using TRIzol (Item No. 19201ES60, Yeasen Biotechnology Co., Ltd.), reverse transcription kit (Item No. RP1105, Beijing Solarbio Science & Technology Co., Ltd.) was used to reverse RNA into cDNA, and 2xSYBR GreenFast qPCR Mix (Item No.: IBIO-C141, Jiangxi IBIO Biotechnology Co., Ltd.) was used for real-time fluorescence quantitative PCR reaction. The total reaction system was 20μL, consisting of 2xSYBR Green Fast qPCR Mix 10μL, and upstream and downstream primers of 0.8μL each, cDNA 1μL, ddH2O 7.4μL. The reaction conditions were as follows: 96°C for 4 min, 96°C for 10s, 58°C for 30s, 72°C for 10s, for a total of 40 cycles. The primer sequences were: circFAT1 upstream: 5’-GAGGACGCCAGAAGAGATGG-3’, downstream: 5’-GCCAAATGTCTCCCCATTGC-3. GAPDH upstream: 5’-CACCCACTCCTCCACCTTTG-3, “downstream: 5”-CCACCACCGTTGCTGTAG-3’. The primers were synthesized by Hefei Zhi En Biology Co., Ltd.
Detection of Expression of Immune Microenvironment-Related Factors by Immune Cell
Patients were treated surgically, and NSCLC cancer tissues (NSCLC group) and para-carcinoma tissues (tissues ≥2 cm from the lesion) (para-carcinoma group) were collected intraoperatively and preserved for measurement after paraffin embedding. The expression levels of CD4+, CD8+, Foxp3+ in NSCLC cancer tissues were determined using immunohistochemistry. The paraffin sections were routinely dewaxed to water state and antigen repaired to eliminate endogenous peroxidase, incubated at 37°C with primary antibody, incubated with secondary antibody, hematoxylin re-stained for 2 min after DAB coloration, differentiated, dehydrated and sealed. CD4+, CD8+ were interpreted as follows. Positive expression was defined when yellow, tan, or brown particles appeared. Scoring content: high expression was identified if the percentage of stained positive cells in the field of view and the range of epithelial cell infiltration in tumor tissue >5%. Otherwise, it was low expression. Foxp3+ was interpreted as follows: 4 points if the staining color was brownish yellow and positive cells >75%, with 3 points for 51%–75%, 2 points for 26%–50%, 1 point for 6%–25%, 0 point for ≤5%. The staining intensity was scored 3 points if it was tan, with 2 points for pale brown, 1 point for yellow and 0 point for colorless. The product of the two yielded the total score. A score of 0–4 points suggested low expression and a score of 5–12 points indicated high expression.
Database Search
The expression analysis of FAT1 in lung adenocarcinoma was performed by searching the TCGA dataset in the Ualcan database (http://ualcan.path.uab.edu), with the gene symbol set as FAT1 and the dataset set as lung adenocarcinoma.
Statistical Methods
The relevance between circFAT1 expression and immune cell (CD4+, Foxp3+, CD8+) was analyzed by Spearman correlation analysis. P<0.05 indicated statistical significance.
Results
Comparison of circFAT1 Expression Levels in NSCLC Tissues and Normal Tissues in the Ualcan Database
In the Ualcan database, the expression level of circFAT1 was higher in NSCLC tissues than in normal tissues (P<0.05), as shown in Figure 2.
 |
Figure 2 The expression level of circFAT1 in NSCLC tissues was analyzed by Ualcan database. |
Comparison of the Expression Levels of Serum circFAT1 Between the Two Groups
Compared with the control group, the expression level of serum circFAT1 was significantly higher in the NSCLC group (t = 47.089, P < 0.05).
Relationship Between circFAT1 Expression and Clinicopathological Characteristics
With mean circFAT1 levels as the boundary, the cases were divided into high expression group (≥2.43) and low expression group (<2.43). circFAT1 expression levels were not correlated with age, gender, smoking history, alcohol consumption history, and pathological staging of NSCLC patients (P > 0.05) but were correlated with lymph node metastasis, TNM staging, and differentiation degree (P < 0.05), as shown in Table 1.
 |
Table 1 Relationship Between circFAT1 Expression and Clinicopathological Features |
Positive Expression of CD4+, CD8+, Foxp3+ in Tissues
Compared with the para-carcinoma group, the NSCLC group had significantly lower positive expression rate of CD8+ and significantly higher positive expression rate of CD4+ and Foxp3+ (P < 0.05), as shown in Table 2.
 |
Table 2 CD4+, CD8+ and Foxp3+ Were Positively Expressed in the Tissues [n (%)] |
Correlation Between the Expression of CD4+, Foxp3+ and CD8+ and Clinicopathological Characteristics
The expression of CD4+, Foxp3+ and CD8+ in cancer tissues of NSCLC patients was irrelevant with age, gender, smoking history, alcohol consumption history and pathological staging (P > 0.05) but was relevant with lymph node metastasis, TNM staging and differentiation degree (P < 0.05), as shown in Table 3 and Table 4.
 |
Table 3 Correlation Between the Expression of CD4+, Foxp3+ and CD8+ and Clinicopathological Characteristics |
 |
Table 4 Association of CD8+ Expression with Clinicopathological Features |
Correlation Between circFAT1 and CD4+, CD8+, Foxp3+ Expression
Spearman correlation analysis was performed on circFAT1 expression and CD4+, CD8+, and Foxp3+ expression levels. It was found that circFAT1 was positively correlated with CD4+ and Foxp3+ expression (r = 0.414, 0.432 P < 0.01) and negatively correlated with CD8+ expression (r=−0.408 P < 0.01).
Discussion
The tumor microenvironment mainly consists of immune cells, inflammatory cells, mesenchymal cells, tumor cells, stromal cells, inflammatory mediators and cytokines. It supports tumor biological behavior of pathogenesis, progression, invasion and metastasis.11 The novel and meaningful immune cell-related genes found in NSCLC can be used as important clinical targets.
circRNA is expressed in a highly tissue- and cell-specific manner. circFAT1 plays an essential role in several types of cancers, including cervical cancer, head and neck squamous cell carcinoma, hepatocellular carcinoma, osteosarcoma, colorectal cancer, papillary thyroid cancer, breast cancer, and NSCLC, which suggests the importance of circFAT1 in tumorigenesis.12 circFAT1 has been proved to be highly expressed in the exosomes of colorectal cancer, indicating its potential role in tumorigenesis.13 CircFAT1 was found to be highly expressed in osteosarcoma cell lines and tissues. Silencing of circFAT1 inhibited proliferation and migration and induced apoptosis in osteosarcoma cells.14 In gastric cancer, circFAT1 is directly bound to YBX1 in the nucleus as a sponge for miR-548 to suppress protein function and regulated the level of oncogene RUNX family transcription factor 1 in the cytoplasm, thereby hindering cancer progression. circFAT1 overexpression inhibited proliferation, migration and invasion of gastric cancer cells and correlated with overall survival of gastric cancer patients.6 Compared with normal tissues, circFAT1 was highly expressed in NSCLC tissues.15 CircFAT1 can promote the progression of HCC through sponge miR-30a-5p and enhancing REEP3.16 In this study, the expression level of serum circFAT1 was significantly higher in the NSCLC group than in the control group, which coincided with the Ualcan database data and Dong et al16 conducted basic research, suggesting that high circFAT1 expression accelerated the proliferation, invasion and growth of NSCLC cells, increased oncogenic activity, and promoted the progression of NSCLC carcinogenesis. circFAT1 expression level was correlated with lymph node metastasis, TNM staging, and differentiation degree, which further suggests that circFAT1 expression correlates with NSCLC progression.
The presence of excessive lymphocyte infiltration during tumor progression indicates that T cells cannot provide an effective immune response to control tumor growth.17 A growing body of evidence suggests that tumor-infiltrating T lymphocytes are functionally defective and incompletely activated.18 CD8+ T lymphocytes are recognized as effector T cells with powerful cytotoxic effects in cancer.8 Another important factor affecting the clinical outcome of NSCLC is tumor infiltration of Foxp3+, CD4+ T cells. By producing immunomodulatory cytokines, these cells can influence cytolytic CD8+ T cell responses in lung cancer.9,19 This study found that the NSCLC group had significantly lower positive expression of CD8+ and significantly higher positive expression of CD4+ and Foxp3+ than the para-carcinoma group. The expression of CD4+, Foxp3+, and CD8+ in cancer tissues of NSCLC patients was associated with lymph node metastasis, TNM staging, and differentiation degree, suggesting that CD8+ promoted immune response in NSCLC patients, while Foxp3+, and CD4+ suppressed the immune response.
Spearman correlation analysis revealed that circFAT1 was positively correlated with CD4+ and Foxp3+ expression and negatively correlated with CD8+ expression, indicating that circFAT1 may influence the immune cell by indirectly inhibiting CD8+ expression and promoting CD4+ and Foxp3+ expressions.
To sum up, circFAT1 was highly expressed in the serum of NSCLC patients and could indirectly influence the immune cells. Nonetheless, this study has a small sample size, NSCLC has a more complex pathogenesis and a greater test sample size is needed later for more in-depth study.
Data Sharing Statement
The original contributions presented in the study are included in the article.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Yueyang People’s Hospital in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent to participate in this study was provided by the patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Petrek H, Yu A-M. MicroRNAs in non-small cell lung cancer: gene regulation, impact on cancer cellular processes, and therapeutic potential. Pharmacol Res Perspect. 2019;7(6):e00528. doi:10.1002/prp2.528
2. Parker AL, Bowman E, Zingone A, et al. Extracellular matrix profiles determine risk and prognosis of the squamous cell carcinoma subtype of non-small cell lung carcinoma. Genome Med. 2022;14(1):126. doi:10.1186/s13073-022-01127-6
3. Parakh S, Ernst M, Poh AR. Multicellular effects of stat3 in non-small cell lung cancer: mechanistic insights and therapeutic opportunities. Cancers. 2021;13:24. doi:10.3390/cancers13246228
4. Zhang Y, Zhang X, Xu Y, et al. Circular RNA and its roles in the occurrence, development, diagnosis of cancer. Front Oncol. 2022;12:845703. doi:10.3389/fonc.2022.845703
5. Jia L, Wang Y, Wang C-Y. circFAT1 promotes cancer stemness and immune evasion by promoting STAT3 activation. Adv Sci. 2021;8(13):2003376. doi:10.1002/advs.202003376
6. Peng H, Zhang W, Dong H, et al. CircFAT1 promotes lung adenocarcinoma progression by sequestering miR-7 from repressing IRS2-ERK-mediated CCND1 expression. Int J Biol Sci. 2022;18(10):3944–3960. doi:10.7150/ijbs.70889
7. Peña-Romero AC, Orenes-Piñero E. Dual effect of immune cells within tumour microenvironment: pro- and anti-tumour effects and their triggers. Cancers. 2022;14:7. doi:10.3390/cancers14071681
8. Boieri M, Malishkevich A, Guennoun R, et al. CD4+ T helper 2 cells suppress breast cancer by inducing terminal differentiation. J Exp Med. 2022;219:7. doi:10.1084/jem.20201963
9. Ménoret S, Tesson L, Remy S, et al. CD4+ and CD8+ regulatory T cell characterization in the rat using a unique transgenic Foxp3-EGFP model. BMC Biol. 2023;21(1):8. doi:10.1186/s12915-022-01502-0
10. Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–168. doi:10.1016/j.immuni.2011.07.010
11. Che L, Yu C, Chen G, et al. The inflammatory response induced by RELMβ Upregulates IL-8 and IL-1β expression in bronchial epithelial cells in COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:2503–2513. doi:10.2147/COPD.S321877
12. Yao Y, Li X, Cheng L, Wu X, Wu B. Circular RNA FAT atypical cadherin 1 (circFAT1)/microRNA-525-5p/spindle and kinetochore-associated complex subunit 1 (SKA1) axis regulates oxaliplatin resistance in breast cancer by activating the notch and Wnt signaling pathway. Bioengineered. 2021;12(1):4032–4043. doi:10.1080/21655979.2021.1951929
13. Pan F, Zhang D, Li N, Liu M. Circular RNA circFAT1(e2) Promotes Colorectal Cancer Tumorigenesis via the miR-30e-5p/ITGA6 Axis. Comput Math Methods Med. 2021;2021:9980459. doi:10.1155/2021/9980459
14. Liu G, Huang K, Jie Z, et al. CircFAT1 sponges miR-375 to promote the expression of Yes-associated protein 1 in osteosarcoma cells. Mol Cancer. 2018;17(1):170. doi:10.1186/s12943-018-0917-7
15. Dong W, Zhang H, Dai Y, et al. circRNA circFAT1(e2) elevates the development of non-small-cell lung cancer by regulating miR-30e-5p and USP22. Biomed Res Int. 2021;2021:6653387. doi:10.1155/2021/6653387
16. Wei H, Yan S, Hui Y, et al. CircFAT1 promotes hepatocellular carcinoma progression via miR-30a-5p/REEP3 pathway. J Cell Mol Med. 2020;24(24):14561–14570. doi:10.1111/jcmm.16085
17. Marschner N, Zacharias S, Lordick F, et al. Association of disease progression with health-related quality of life among adults with breast, lung, pancreatic, and colorectal cancer. JAMA Netw Open. 2020;3(3):e200643. doi:10.1001/jamanetworkopen.2020.0643
18. Verma NK, Wong BHS, Poh ZS, et al. Obstacles for T-lymphocytes in the tumour microenvironment: therapeutic challenges, advances and opportunities beyond immune checkpoint. EBioMedicine. 2022;83:104216. doi:10.1016/j.ebiom.2022.104216
19. Schulze AB, Evers G, Görlich D, et al. Tumor infiltrating T cells influence prognosis in stage I-III non-small cell lung cancer. J Thorac Dis. 2020;12(5):1824–1842. doi:10.21037/jtd-19-3414a
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
