Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
The Relationship Between Peripartum Cardiomyopathy and Preeclampsia – Pathogenesis, Diagnosis and Management
Authors Kuć A , Kubik D , Kościelecka K , Szymanek W , Męcik-Kronenberg T
Received 11 January 2022
Accepted for publication 14 March 2022
Published 23 April 2022 Volume 2022:15 Pages 857—867
DOI https://doi.org/10.2147/JMDH.S357872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Aleksandra Kuć,1 Daria Kubik,1 Klaudia Kościelecka,1 Wojciech Szymanek,2,3 Tomasz Męcik-Kronenberg4
1Student Research Group at the Chair and Department of Pathomorphology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, Zabrze, Poland; 2Hospital Emergency Department, St. John Paul II Mazovia Regional Hospital in Siedlce, Siedlce, Poland; 3Nursing at Collegium Mazovia Innovative University in Siedlce, Siedlce, Poland; 4Chair and Department of Pathomorphology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, Zabrze, Poland
Correspondence: Aleksandra Kuć, 1E Street, Siedlce, 08-110, Poland, Tel +48 504 188 178, Email [email protected]
Abstract: Peripartum cardiomyopathy (PPCM) is a condition with an incompletely understood etiology, although many risk factors for this disorder have been mentioned. Preeclampsia (PE) is a rare but undoubtedly very important cause of PPCM. Early recognition and prompt treatment of preeclampsia and peripartum cardiomyopathy are essential to optimize pregnancy outcomes. An extensive manual search of major electronic databases was conducted in November 2021. The following literature review provides a comprehensive discussion of peripartum cardiomyopathy and preeclampsia and quantifies the prevalence of PE in women with PPCM. The authors highlighted aspects such as epidemiology, risk factors, cardiovascular changes, diagnosis and clinical presentation, and management and complications. Accumulating data indicate that both conditions have a similar pathogenesis characterized by vascular abnormalities. In both conditions we can observe an increase in interleukin-6 and gamma interferon, CCL2/MCP1, and decreased SOD activity. sFLT1 (a soluble form of fms-like tyrosine kinase 1), a substance with antiangiogenic and probably cardiotoxic effects, may be important. Preeclampsia and peripartum cardiomyopathy are characterized by recurrence rates that follow a similar pattern in subsequent pregnancies, and mortality remains a concern. Our analysis highlights the need to better understand the co-morbidity of PE and PPCM, and the need to qualify patients for the same clinical trials because of the common origin of these conditions.
Keywords: pregnancy, PE, PPCM, conditions
Introduction
Peripartum cardiomyopathy (PPCM) was defined in the 1990s as heart failure developing in the last month of pregnancy or up to 5 months after delivery characterized by left ventricular systolic dysfunction.1,2 As reported by Sliwa et al, in 2010 European Society of Cardiology (ESC) modified the definition of peripartum cardiomyopathy perinatal cardiomyopathy, noting that the condition occurs “at the end of pregnancy or within end of pregnancy or within a few months after delivery, when no other cause of heart failure is fund heart failure”.3 The revisions incorporated a broader time frame without being more specific about the month, minimizing the risk of confusion and overlooking the condition, which was very likely before when strict time criteria were followed. Elkayam et al report that peripartum cardiomyopathy is usually seen in the early postpartum period - about 75% within the first month and about 45% within the first week.4
Elevated blood pressure at week 20 of pregnancy and beyond is defined as gestational hypertension, while hypertension (systolic blood pressure values ≥140 mm Hg and diastolic blood pressure values ≥90 mm Hg) with proteinuria ≥300 mg in a daily urine collection present (a score of at least +1 on a strip test or based on a protein-creatinine ratio value ≥ 0.3) is closely associated with preeclampsia (PE).5
The association between hypertensive disease and postpartum heart failure was first described in 1938, when the authors concluded that more than 85% of cases of peripartum cardiomyopathy were related to hypertension and this was twice as common as in the control group.6 Whereas Demakis and Rahimtoola in 1971, reported that preeclampsia (PE), was detected in 22% of women affected by cardiomyopathy.7 Since then, peripartum cardiomyopathy is often combined with preeclampsia and at the same time recognized as its severe complication, while hypertension is rarely the cause of heart failure itself.1,8,9 It is worth mentioning that National Heart, Lung and Blood Institute (NHLBI) - 2000 and European Society of Cardiology (2010) indicate idiopathic background of peripartum cardiomyopathy when no other cause of heart failure can be identified. The diagnosis therefore becomes a diagnosis by exclusion.10
The following literature review provides a comprehensive discussion of peripartum cardiomyopathy and preeclampsia and determines the prevalence of PE in women with PPCM. The authors focused on epidemiology, risk factors, clinical features and management during pregnancy and prognosis, and wanted to summarize recent scientific reports.
Materials and Methods
An extensive manual search of major electronic databases (PubMed, EMBASE, Web of Science, and Google Scholar) was conducted in November 2021 to identify relevant studies published on the association of peripartum cardiomyopathy with preeclampsia. No lower date limit was specified. Articles were limited to those published in English and Polish. The following search terms were used: “preeclamspia”, “peripartum cardiomyopathy”, “pre-eclampsia” in various combinations. Articles were analyzed first by title, then by abstract, and finally by full text. All articles selected were the most relevant available for this review.
Epidemiology
The incidence of peripartum cardiomyopathy varies from 1 in 1421 to 1 in 9861 births.11 In 2011, it increased to 1 in 849 live births in the United States, where it was 1 in 1200 live births among women aged 20–29 years, 1 in 790 in women aged 30–39 years, and 1 in 270 live births among women aged 40–54 years.12 As reported by other sources, 58% of PPCM cases occurred in women aged >30 years, with 27–33% of women having their first live birth.4 The lowest incidence is seen in Hispanic women in the United States, while the highest incidence is seen in African-American or southern African women. The authors indicate an approximate prevalence in Haiti of 1 case per 299 live births13 and 1 case per 1000 live births in South Africa.14
Similar to perinatal cardiomyopathy, the prevalence of preeclampsia varies by region. Studies in the US indicate 1 in 2367 births, noting that only 4% of diagnoses were made in the antenatal period, 18% in the perinatal period, and 78% in the postpartum period.15 South Korean statistics report an incidence of 1 in 1741 cases, Taiwanese data report an incidence of 1 in 3790 births, and Swedish data report an incidence of 1 in 5719 births.16–18 Observations by Melamed et al showed that 17–46% of women with gestational hypertension experienced preeclampsia, and in patients without hypertension, data indicated 5–8%.19
In 2013, Bello et al conducted a meta-analysis that found the prevalence of preeclampsia in women with peripartum cardiomyopathy to be 22%, which is more than four times the estimated global average (5%).20 Similar data were reported in the PPCM registry of the EURObservational Research Programme - of 411 women with peripartum cardiomyopathy, 22.8% experienced preeclampsia.21 It is unclear whether the association between preeclampsia and PPCM differs between black women (both conditions are more common in black women) and women from other ethnic and racial backgrounds due to limitations in data availability and variation in previous studies from Africa and the Caribbean.20
Risk Factors
The pathophysiological features of peripartum cardiomyopathy and preeclampsia as vascular diseases appear similar.22–24 sFLT1 (a soluble form of fms-like tyrosine kinase 1), a substance with antiangiogenic and probably cardiotoxic effects, may be important. sFLT1 is secreted by the placenta as pregnancy progresses and also in the perinatal period. Subclinical dysfunction of cardiomyocytes may occur as a result of impaired mechanisms protecting the heart against anti-angiogenic factors or as a result of increased secretion of this substance observed in PPCM. Its concentration is also increased in pre-eclampsia early in pregnancy, even before the diagnosis is made.25–27 It has also been noted that angiogenic imbalance in the form of an increase in sFLT1/placental growth factor (PLGF) ratio can lead to heart failure.28 There are reports in the literature that another substance with anti-angiogenic effects, sVEGFR1 (soluble version of vascular endothelial growth factor receptor-1), which disrupts homeostasis in various vascular beds, is also presumed to be important in the pathogenesis of the diseases described.24,29,30
Twin pregnancies are a risk factor for both peripartum cardiomyopathy and preeclampsia. In such pregnancies, the placenta is larger and therefore secretes more antiangiogenic factors into the maternal circulation.31 In both conditions, increased levels of inflammatory factors and mediators such as interleukin-6, interferon gamma and CCL2/MCP1 have also been found.32–36 It is important to mention that changes in SOD activity can lead to the development of PPCM and PE by increasing the amount of reactive oxygen species that exert negative effects on the vascular endothelium. Furthermore, it is worth noting that prolactin under physiological conditions promotes angiogenesis and has a protective effect on the endothelium. However, pregnancy-induced oxidative stress can lead to the formation of a shorter form of prolactin, 16kDa, which has cardiotoxic effects.37 The use of bromocriptine as a prolactin inhibitor is still under investigation.
Preeclampsia as well as peripartum cardiomyopathy is more common in pregnant women with diabetes, obesity, multiple pregnancies and late maternal age.38,39
In conclusion, the data presented above suggest that both conditions have a similar pathogenesis associated with increased release of anti-angiogenic factors from the placenta, increased levels of inflammatory factors, and decreased SOD activity.
Cardiovascular Changes
In preeclampsia, researchers describe two theories regarding cardiac function. One indicates the presence of low cardiac output (CO) and elevated systemic vascular resistance (SVR), and the other states that cardiac output is elevated with slightly increased systemic vascular resistance.40–42 In a 2012 study, untreated preeclamptic patients with increased SVR and higher CO were described.43 The most commonly observed hemodynamic changes in preeclampsia are elevated CO along with a hyperdynamic left ventricle and a subsequent decrease in CO and hypertrophy of the left ventricular wall. Under increased afterload and sustained stress, such a hypertrophied ventricle develops diastolic dysfunction.10 It is worth mentioning that, according to recent reports, preeclampsia can also occur in the newborn, as left ventricular hypertrophy (LVH) has been observed in the offspring of mothers who have experienced PE.44,45
Patients with peripartum cardiomyopathy often have systolic dysfunction with reduced left ventricular ejection fraction and left ventricular dilatation. Less commonly, left ventricular hypertrophy or diastolic dysfunction may be observed. Some studies suggest that patients with hypertensive disorders of pregnancy (HDP) usually have smaller left ventricular end-diastolic and systolic dimensions due to left ventricular hypertrophy.46,47 However, the difference in left ventricular dimensions and wall thickness between patients with hypertensive disorders of pregnancy and patients with PPCM has not been proven in all studies.48,49 Taking into account the changes in the right ventricle, it should be noted that its dysfunction may occur in patients with both PE and PPCM.50 Similar findings were demonstrated by cardiac MRI.51
Diagnosis and Clinical Presentation
In patients with severe preeclampsia and symptoms of heart failure, peripartum cardiomyopathy should always be listed as a differential diagnosis.52 It is very important to recognize peripartum cardiomyopathy promptly to improve patient outcomes and facilitate earlier intervention. This condition is defined by four criteria: (1) no identifiable cause of heart failure; (2) development of heart failure late in pregnancy or within a few months after delivery (3) no recognized heart disease before the last month of pregnancy; and (4) left ventricular systolic dysfunction confirmed by classical echocardiographic criteria.53 Distinguishing normal findings in late pregnancy from subtle signs of heart failure (foot swelling, exertional dyspnea, fatigue) is a challenge for the medical team.54 It is largely a diagnosis by exclusion. Other causes of heart disease, both congenital and acquired, such as pulmonary hypertension, myocardial infarction causing left ventricular dysfunction, or valvular heart disease should be excluded first.1,11,55
In the last month of pregnancy, many women develop symptoms similar to those of heart failure - palpitations, exertional dyspnea, nocturnal dyspnea, cough, foot edema, fatigue.7,11,56,57 On examination, features of right-sided (edema, elevated jugular venous pressure) and left-sided (pulmonary rales) overload may be found,58 apical beat displacement, murmurs due to tricuspid or mitral regurgitation.56 Heart failure may be manifested by pleural effusions. This may be related to hypoalbuminemia and capillary leakage syndrome occurring in preeclamptic patients.59 Less commonly, PPCM manifests with cardiogenic shock requiring mechanical or inotropic circulatory support or symptomatic or even unstable arrhythmias60,61 and coronary artery thrombosis.62
Preeclampsia is often associated with complaints such as dyspnea, headache, epigastric pain, nausea, vomiting or visual disturbances. These symptoms are registered by the mother and are usually the first indication for diagnosis.63 However, the most alarming symptoms are coexisting proteinuria and hypertension. This does not mean that deviations do not exist. It should be noted that some pregnant women present the absence of proteinuria, which determines the need to expand the diagnostic requirements.64 According to studies, the criterion of headache as diagnostic for severe preeclampsia is also ambiguous and therefore unreliable.63,65 There are also abnormalities in cardiotocographic recordings and abnormal spectrum of flow in the placental-fetal vessels. So, how to correctly diagnose preeclampsia? According to the diagnostic scheme for preeclampsia, blood pressure should be the first concern. In women with a previously normal blood pressure after the 20th week of gestation, a blood pressure of 140/90 mmHg or higher and a proteinuria of ≥ 300 mg in a 24-hour collection of urine (a result of at least +1 in a strip test or a protein/creatinine ratio of ≥ 0.3) are indicative. In contrast, finding at least one of the following: BP ≥ 160/110 mmHg confirmed on two occasions (at least 6 hours apart) in a patient lying in bed, or proteinuria ≥5g/day (result of 3+ on a strip test in two urine samples collected at least 4 hours apart), or signs of organ dysfunction such as acute kidney injury (serum creatinine ≥ 1 mg/dL, 90 μmol/L), hepatic complications (increase in transaminase activity - AspAT or ALT > 40 IU/L), pulmonary edema or cyanosis, neurological complications (eclampsia, blackouts, stroke, clonic spasm, psychiatric disorders, severe headache, and dark circles), right upper quadrant or epigastric pain, presence of thrombocytopenia (platelet count < 150,000/μL, DIC, hemolysis), or signs of fetal distress (abnormal flow in the umbilical artery, IUGR, or intrauterine fetal demise) change the diagnosis to severe preeclampsia. However, this definition varies from country to country.38,66 Nonetheless, the non-specificity of the presenting symptoms calls for individualized attention and careful diagnosis.63,65
Management
Blood tests are necessary in all patients diagnosed with PPCM, but troponins, creatine kinase (CK-MB), and creatinine cannot definitively confirm or exclude the diagnosis of PPCM. If peripartum cardiomyopathy is suspected, chest radiography should be ordered at every stage of pregnancy. This examination is safe because it shows little radiation to the fetus. It enables to visualize radiological signs of heart failure such as pulmonary congestion, cardiomegaly, and pleural effusion.67 Another examination used is the ECG. It is classified as a non-specific test showing arrhythmias (atrial fibrillation and flutter, ventricular tachycardia) or sinus tachycardia.68 We should also mention endomyocardial biopsy, which is considered controversial by many.69 It has a specificity of 99% and a sensitivity of 50%, so its result may also be positive in other conditions such as myocarditis.8 Biopsy is indicated when the cause causing the symptoms is unclear and a disease requiring specific treatment is suspected - infiltrative or storage diseases (amyloidosis, hemochromatosis, sarcoidosis), myocarditis. MRI has not detected a specific variable to help distinguish peripartum cardiomyopathy from other forms of cardiomyopathy but is useful in the differential diagnosis when other methods (echocardiography and coronary angiography) have failed to establish the diagnosis, especially in the diagnosis of pericardial disease and cardiac tumors.70–72 This will allow the medical team to quickly implement appropriate treatment.
There is still a lack of clinical trials comparing the treatment modalities for PPCM and selecting the best therapy for them, so patients should be started on standard therapeutic management dedicated to heart failure to reduce myocardial preload and afterload, increase contractility, and prevent complications and mortality. Angiotensin Converting Enzyme Inhibitors (ACEI) - hydralazine with nitrates or without them (reduction of arterial pressure, antiatherosclerotic effect, inhibition of left ventricular enlargement and fibrosis), β-blockers (do not use atenolol or metoprolol), digoxin (for atrial arrhythmias) and diuretics (reduce pulmonary congestion and alleviate symptoms, reducing preload) should be implemented, with loop diuretics available in the hospital setting, and fluid intake limited to 2 liters per day.7,73 According to American and European guidelines, limiting sodium intake is the primary control. It is worth remembering that excessive diuresis can cause uterine hypoperfusion and hypotension in the mother. More careful observation of the risk of fetal bradycardia and monitoring of fetal growth in women taking beta-blockers may be considered.74 Aldosterone receptor blockers (aldosterone antagonists) - eplerenone and spironolactone should not be used in pregnant women because they cross the placenta.68 Given the safety of cardioversion or defibrillation during pregnancy, they should be performed in emergency situations.75
The medical team must also consider the safety of the neonate; therefore, it is worth remembering that angiotensin-converting enzyme inhibitors and ACEI should not be used in late pregnancy and puerperium, nor should class III (amiodarone) or class IV (verapamil) antiarrhythmics.5 Pregnancy and puerperium increase thromboembolic risk, as do most types of cardiomyopathies, so low molecular weight heparin (LMWH), which does not cross the placental barrier, is recommended to prevent this.76 Its dosing is more frequent, and the dose is determined by weight in early pregnancy. Warfarin, unlike heparin, crosses this barrier and therefore cannot be used in pregnancy.77,78 According to European guidelines, bromocriptine is class IIB, whereas American guidelines consider it a drug still under investigation.79,80 In 2016, a study with the combined use of bromocriptine was conducted in Germany. 96% of patients showed improvement and 47% showed “full recovery”. In 15% of the subjects, no improvement was proven, but their baseline LVEF was ≤ 0.25.47 Full recovery (LVEF ≥ 50%) occurred in 52% of patients in the one-week bromocriptine group and 68% in the eight-week group.81 Based on these two studies, bromocriptine treatment was associated with a high rate of complete LV recovery. It is worth mentioning that levosimendan is preferred as an inotropic drug in Europe, but it is not available in Canada and the United States.82 The authors chose to present the drugs discussed in the text used in PPCM in Table 1.
 |
Table 1 Selected Drugs Used in PPCM Prepared on the Basis of Hilfiker-Kleiner et al. 81 |
The management of pregnant women with PPCM is based on obstetric guidelines and recommendations. Vaginal delivery is chosen instead of cesarean section. It has the advantage of greater hemodynamic stability, low blood loss, and lower risk of postoperative infection. Cesarean section is associated with an increased risk of uteritis and pulmonary embolism.83 However, according to AHA and ESC guidelines, cesarean section should be considered for acute heart failure and obstetric indications.80,84 In patients in whom PPCM is diagnosed before delivery, it is advisable to appoint a team of physicians - obstetricians, anaesthesiologists and cardiologists, who will individually select the management regarding the time and mode of delivery.80,85 The treatment algorithm is outlined by the authors below (Figure 1).
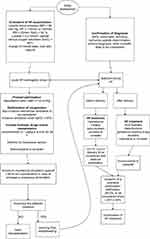 |
Figure 1 Treatment algorithm for PPCM prepared on the basis of Hilfiker-Kleiner et al.81 |
The 2018 Guidelines of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) disagree, citing the possibility of treating patients with mild preeclampsia in the outpatient setting and emphasizing that preeclampsia is an indication for hospitalization, where both maternal and fetal status can be monitored. Urgent hospitalization is recommended for women with systolic blood pressure ≥ 160 mmHg and/or diastolic RR ≥ 110 mmHg in multiple measurements over 15–30 minutes. Efforts should be made to lower blood pressure pharmacologically with labetalol or nicardipine and magnesium sulfate. If there is no improvement, termination of pregnancy may be considered. As with gestational age >37 weeks, significant worsening of liver function markers, renal function, hemolysis, decreased platelet count, intravascular coagulation syndrome (DIC), eclampsia or other neurologic signs with visual disturbances, headache, signs of premature placental separation, fetal life threatening, or intrauterine fetal death. If premature termination of pregnancy is necessary, a 48-hour intramuscular course of steroid therapy with betamethasone or dexamethasone at a total dose of 24 mg in pregnancies < 34 weeks is used to stimulate fetal lung maturity. Treatment for preeclampsia includes hypotensive therapy-until blood pressure values <160/110 mmHg are achieved in women with severe hypertension, followed by implementation of chronic oral drug therapy in the puerperium, treatment with low-molecular-weight heparin (with daily proteinuria > 3.5 g) and intravenous magnesium sulfate, which prevents eclampsia and is responsible for fetal neuroprotection, is continued. In the puerperium, hypotensive treatment and surveillance are continued for at least 48 hours after delivery due to the risk of postpartum eclampsia.5
Complications
It is important to note the importance of monitoring and follow-up of patients in the form of annual echocardiographic evaluation to catch possible complications of PPCM and side effects of treatment more quickly.86 For this condition, the prognosis largely depends on the return of left ventricular function and size within 6 months after delivery.87 Another study concluded that patients with PPCM, should be followed for 6–12 months after diagnosis.88 Demakis et al concluded that left ventricular dysfunction was evident in about half of 27 women, with a 5-year mortality rate of 85%.53 Other authors have concluded that the disease is irreversible if left ventricular systolic function does not return to normal within 6 months after delivery.78 The most common complication of PPCM appears to be thromboembolism, occurring in 6.6% of women with PPCM in the United States;12 a similar incidence (6.8%) was recorded in the EURObservational Research Programme Worldwide Registry study.21 Thrombosis may occur in both the right and left ventricle.89–91 Another noteworthy complication of peripartum cardiomyopathy is cardiogenic shock found in 2.6% of women in the United States between 2004 and 2011. In the face of these complications, mechanical circulatory support was used in 1.5% of cases and heart transplantation was performed in only 0.5% of women.12 It is worth noting that between 2004 and 2011 in the United States, 2.9% of women underwent cardiac implantation and 2.1% suffered cardiac arrest.12 Another analysis of 9841 cases with PPCM found that arrhythmias were present in 18.7% of cases, including ventricular tachycardia in 4.2%.8 The statistics are alarming, as peripartum cardiomyopathy accounts for 5% of cases eligible for heart transplantation among women in the United States.92 Within 5 years of diagnosis, as many as 25% of women die from PPCM in developing countries,93 while neonatal mortality ranges from 0% to 75%.94,95 Return of left ventricular size and function in patients with peripartum cardiomyopathy has been described in relation to hypertensive disorders of pregnancy as a variable predictor. Researchers have demonstrated a protective effect of hypertensive disorders in pregnancy and more significant left ventricular recovery among patients with PPCM.46,96,97 On the other hand, another study demonstrates that concurrent hypertensive disorders in pregnancy and PPCM leads to higher mortality.47 Haghikia et al conducted the largest prospective cohort study that verified the effect of hypertension in pregnancy on outcomes among women with PPCM and did not prove an association between outcomes and HDP.48 A characteristic of preeclampsia and peripartum cardiomyopathy is the recurrence rate, which follows a similar pattern in subsequent pregnancies. Early pre-eclampsia is considered to be the more severe phenotype, with a 34% risk of recurrence,98 and in patients with PPCM these figures are 15–50%.81,99 Despite the full recovery of some women, there is concern about the risk of recurrence of the condition during a subsequent pregnancy.1,100,101 Elkayam et al in their study showed that among women after PPCM in whom left ventricular dysfunction persists, 54% develop cardiac dysfunction and 9% die in subsequent pregnancies.102 Currently, authors have not reached consensus on guidelines for future pregnancy in women who have experienced peripartum cardiomyopathy, but left ventricular function is the most important prognostic factor.103 It is worth remembering that the Mirena and Implanon intrauterine system (progestational contraceptive methods) are considered the safest and most effective methods of contraception in women who have experienced PPCM.78
For preeclampsia, maternal mortality two years after onset ranges from 0% to 9%, with higher rates seen in women of African descent, like PPCM.104 When, at the time of diagnosis, the level of heart failure reaches Class I or II according to the New York Heart Association (NYHA), outcomes are generally better than for Class III and IV.104,105
Conclusions
Linking the pathogenesis of these conditions - preeclampsia and peripartum cardiomyopathy - can provide a wealth of information regarding treatment, risk of recurrence in subsequent pregnancies, and accurate diagnosis. Although PPCM is a rare complication of preeclampsia, the interdisciplinary medical team should be aware of its likelihood in patients with preeclampsia.
Further extensive research into effective, causal treatments, such as the use of anti-SFLT1, is needed. Future publications should also provide more evidence on the importance of the role of prolactin in PPCM. Then perhaps bromocriptine will be included in the ESC guidelines as an indispensable part of the treatment of patients. It is also important to answer the question of why some patients who have relapsed experience a recurrence of heart failure after a subsequent pregnancy, despite the initiation of effective treatment.
Our analysis highlights the need to better understand the comorbidity and common etiology of PE and PPCM, and the need to include patients with these conditions in equivalent clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: national heart, lung, and blood institute and office of rare diseases (national institutes of health) workshop recommendations and review. JAMA. 2000;283(9):1183–1188. doi:10.1001/jama.283.9.1183
2. Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999;94:311–316. doi:10.1016/s0029-7844(99)00293-8
3. Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Heart failure association of the European society of cardiology working group on peripartum cardiomyopathy. current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the heart failure association of the European society of cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12:767–778. doi:10.1093/eurjhf/hfq120
4. Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111(16):2050–2055. doi:10.1161/01.CIR.0000162478.36652.7E
5. Polskie Towarzystwo Kardiologiczne. Guidelines of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH); 2018. Available from: https://ptkardio.pl/wytyczne/1-wytyczne_escesh_dotyczace_postepowania_w_nadcisnieniu_tetniczym.
6. Hull E, Hidden E. Postpartal heart failure. South Med J. 1938;31:265–270. doi:10.1097/00007611-193803000-00010
7. Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964–968. doi:10.1161/01.CIR.44.5.964
8. Gunderson EP, Croen LA, Chiang V, et al. Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol. 2011;118:583–591. doi:10.1097/AOG.0b013e318229e6de
9. Bauer ST, Cleary KL. Cardiopulmonary complications of pre-eclampsia. Semin Perinatol. 2009;33(3):158–165. doi:10.1053/j.semperi.2009.02.008
10. Parikh P, Blauwet L. Peripartum cardiomyopathy and preeclampsia: overlapping diseases of pregnancy. Curr Hypertens Rep. 2018;20(8):69. doi:10.1007/s11906-018-0868-9
11. Brar SS, Khan SS, Sandhu GK, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100(2):302–304. doi:10.1016/j.amjcard.2007.02.092
12. Kolte D, Khera S, Aronow WS, et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. 2014;3(3):e001056. doi:10.1161/JAHA.114.001056
13. Fett JD, Carraway RD, Dowell DL, et al. Peripartum cardiomyopathy in the Hospital Albert Schweitzer District of Haiti. Am J Obstet Gynecol. 2002;186:1005–1010. doi:10.1067/mob.2002.122423
14. Mielniczuk LM, Williams K, Davis DR. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006;97(12):1765–1768. doi:10.1016/j.amjcard.2006.01.039
15. Krishnamoorthy P, Garg J, Palaniswamy C, et al. and outcomes of peripartum cardiomyopathy in the United States: findings from the Nationwide Inpatient Sample. J Cardiovasc Med. 2016;17(10):756–761. doi:10.2459/JCM.0000000000000222
16. Wu VC, Chen TH, Yeh JK, et al. Clinical outcomes of peripartum cardiomyopathy: a 15-year nationwide population-based study in Asia. Medicine. 2017;96(43):e8374. doi:10.1097/MD.0000000000008374
17. Barasa A, Rosengren A, Sandström TZ, et al. Heart failure in late pregnancy and postpartum: incidence and long-term mortality in Sweden from 1997 to 2010. J Card Fail. 2017;23(5):370–378. doi:10.1016/j.cardfail.2016.12.011
18. Lee S, Cho GJ, Park GU, et al. Incidence, risk factors, and clinical characteristics of peripartum cardiomyopathy in South Korea. Circ Heart Fail. 2018;11(4):e004134. doi:10.1161/CIRCHEARTFAILURE.117.004134
19. Melamed N, Ray JG, Hladunewich M, et al. Gestational hypertension and preeclampsia: are they the same disease? J Obstet Gynaecol Can. 2014;36(7):642–647. doi:10.1016/S1701-2163(15)30545-4
20. Bello N, Rendon ISH, Arany Z. The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:1715–1723. doi:10.1016/j.jacc.2013.08.717
21. Sliwa K, Mebazaa A, Hilfiker-Kleiner D, et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): eurobservational research programme in conjunction with the heart failure association of the European society of cardiology study group on PPCM. Eur J Heart Fail. 2017;19(9):1131–1141. doi:10.1002/ejhf.780
22. Reuwer AQ, Reuwer PJ, van der Post JA, et al. Twickler Prolactin fragmentation by trophoblastic matrix metalloproteinases as a possible contributor to peripartum cardiomyopathy and pre-eclampsia. Med Hypotheses. 2010;74:348–352. doi:10.1016/j.mehy.2009.08.029
23. Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5(1):173–192. doi:10.1146/annurev-pathol-121808-102149
24. Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–338. doi:10.1038/nature11040
25. Fett JD. Peripartum cardiomyopathy: a puzzle closer to solution. World J Cardiol. 2014;6(3):87–99. doi:10.4330/wjc.v6.i3.87
26. Nguyen KA, Gniady K, Lelonek M. Peripartum cardiomyopathy — still unknown Current State of Knowledge. Folia Cardiologica. 2019;14:305–314. doi:10.5603/FC.2019.0064
27. Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352. doi:10.1161/HYPERTENSIONAHA.113.01787
28. Patel PA, Hernandez AF. Targeting anti-beta-1-adrenergic receptor antibodies for dilated cardiomyopathy. Eur J Heart Fail. 2013;15:724–729. doi:10.1093/eurjhf/hft065
29. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658.
30. Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–891. doi:10.1161/01.RES.0000147365.86159.f5
31. Rana S, Hacker MR, Modest AM, et al. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension. 2012;60(2):451–458. doi:10.1161/HYPERTENSIONAHA.112.195065
32. Taylor BD, Ness RB, Klebanoff MA, et al. First and second trimester immune biomarkers in pre-eclamptic and normotensive women. Pregnancy Hypertens. 2016;6(4):388–393. doi:10.1016/j.preghy.2016.09.002
33. Charkiewicz K, Jasinska E, Goscik J, et al. Angiogenic factor screening in women with mild preeclampsia—new and significant proteins in plasma. Cytokine. 2017;106:125–130. doi:10.1016/j.cyto.2017.10.020
34. Kaitu’u-Lino TJ, Brownfoot FC, Hastie R, et al. Activating transcription factor 3 is reduced in preeclamptic placentas and negatively regulates sFlt-1 (soluble fms-like tyrosine kinase 1), soluble endoglin, and proinflammatory cytokines in placenta. Hypertension. 2017;70(5):1014–1024. doi:10.1161/HYPERTENSIONAHA.117.09548
35. Agachan B, Attar R, Isbilen E, et al. Association of monocyte chemotactic protein-1 and CC chemokine receptor 2 gene variants with preeclampsia. J Interf Cytokine Res. 2010;30(9):673–676. doi:10.1089/jir.2010.0008
36. Ricke-Hoch M, Bultmann I, Stapel B, et al. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc Res. 2014;101(4):587–596. doi:10.1093/cvr/cvu010
37. American College of Obstetricians, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi:10.1097/01.AOG.0000437382.03963.88
38. Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600. doi:10.1016/j.cell.2006.12.036
39. Blauwet LA, Cooper LT. Diagnosis and management of peripartum cardiomyopathy. Heart. 2011;97(23):1970–1981. doi:10.1136/heartjnl-2011-300349
40. Visser W, Wallenburg HC. Central hemodynamic observations in untreated preeclamptic patients. Hypertension. 1991;17(6 Pt 2):1072–1077. doi:10.1161/01.HYP.17.6.1072
41. Bosio PM, McKenna PJ, Conroy R, et al. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94(6):978–984. doi:10.1016/s0029-7844(99)00430-5
42. Sibai BM, Mabie WC. Hemodynamics of preeclampsia. Clin Perinatol. 1991;18(4):727–747. doi:10.1016/S0095-5108(18)30493-7
43. Dennis AT, Castro J, Carr C, et al. Haemodynamics in women with untreated pre-eclampsia. Anaesthesia. 2012;67(10):1105–1118. doi:10.1111/j.1365-2044.2012.07193.x
44. Timpka S, Macdonald-Wallis C, Hughes AD, et al. Hypertensive disorders of pregnancy and offspring cardiac structure and function in adolescence. J Am Heart Assoc. 2016;5(11):e003906. doi:10.1161/JAHA.116.003906
45. Mutlu K, Karadas U, Yozgat Y, et al. Echocardiographic evaluation of cardiac functions in newborns of mildly preeclamptic pregnant women within postnatal 24–48 hours. J Obstet Gynaecol. 2018;38(1):16–21. doi:10.1080/01443615.2017.1322564
46. Ntusi NB, Badri M, Gumedze F, et al. Pregnancy-associated heart failure: a comparison of clinical presentation and outcome between hypertensive heart failure of pregnancy and idiopathic peripartum cardiomyopathy. PLoS One. 2015;10(8):e0133466. doi:10.1371/journal.pone.0133466
47. Lindley KJ, Conner SN, Cahill AG, et al. Impact of preeclampsia on clinical and functional outcomes in women with peripartum cardiomyopathy. Circ Heart Fail. 2017;10(6):e003797. doi:10.1161/CIRCHEARTFAILURE.116.003797
48. Haghikia A, Podewski E, Libhaber E, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol. 2013;108(4):366. doi:10.1007/s00395-013-0366-9
49. Li W, Li H, Long Y. Clinical characteristics and long-term predictors of persistent left ventricular systolic dysfunction in peripartum cardiomyopathy. Can J Cardiol. 2016;32(3):362–368. doi:10.1016/j.cjca.2015.07.733
50. Blauwet LA, Delgado-Montero A, Ryo K, et al. Right ventricular function in peripartum cardiomyopathy at presentation is associated with subsequent left ventricular recovery and clinical outcomes. Circ Heart Fail. 2016;9(5):e002756. doi:10.1161/CIRCHEARTFAILURE.115.002756
51. Haghikia A, Rontgen P, Vogel-Claussen J, et al. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: a cardiovascular magnetic resonance study. ESC Heart Fail. 2015;2(4):139–149. doi:10.1002/ehf2.12059
52. Bhattacharyya A, Basra SS, Sen P, et al. Peripartum cardiomyopathy: a review. Tex Heart Inst J. 2012;39:8–16.
53. Demakis JG, Rahimtoola SH, Sutton GC, et al. Natural course of peripartum cardiomyopathy. Circulation. 1971;44:1053–1061. doi:10.1161/01.cir.44.6.1053
54. Hsu YC, Huang ST, Ho ST, et al. An unusual case of peripartum cardiomyopathy in a parturient with preeclampsia. Acta Anaesthesiol Taiwan. 2010;48(1):33–36. doi:10.1016/S1875-4597(10)60007-0
55. Lampert MB, Lang RM. Peripartum cardiomyopathy. Am Heart J. 1995;130:860–870. doi:10.1016/0002-8703(95)90089-6
56. Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol. 2011;58(7):659–670. doi:10.1016/j.jacc.2011.03.047
57. Sliwa K, Fett JD, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368:687–693. doi:10.1016/S0140-6736(06)69253-2
58. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;30(364):k5287. doi:10.1136/bmj.k5287
59. Vázquez-Rodríguez JG, Veloz-Martínez MG. Pleural effusion and ascites in severe preeclampsia: frequency and correlation with plasma colloid osmotic pressure and renal filtration function. Cir Cir. 2011;79:299–305.
60. Puri A, Sethi R, Singh B, et al. Peripartum cardiomyopathy presenting with ventricular tachycardia: a rare presentation. Indian Pacing Electrophysiol J. 2009;9:186–189.
61. Gemici G, Tezcan H, Fak AS, et al. Peripartum cardiomyopathy presenting with repetitive monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2004;27:557–558. doi:10.1111/j.1540-8159.2004.00483.x
62. Manikkan A, Sanati M. Peripartum cardiomyopathy presenting as splenic infarct. J Hosp Med. 2008;3(3):274–276. doi:10.1002/jhm.281
63. Thangaratinam S, Gallos ID, Meah N, et al. TIPPS (tests in prediction of pre-eclampsia’s severity) review group how accurate are maternal symptoms in predicting impending complications in women with preeclampsia? a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90(6):564–573.
64. Gestational Hypertension and. Preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135(6):e237–e260. doi:10.1097/AOG.0000000000003891
65. Sperling JD, Dahlke JD, Huber WJ, et al. The role of headache in the classification and management of hypertensive disorders in pregnancy. Obstet Gynecol. 2015;126:297–302. doi:10.1097/AOG.0000000000000966
66. Butalia S, Audibert F, Côté A-M, et al. Hypertension Canada’s 2018 guidelines for the management of hypertension in pregnancy. Can J Cardiol. 2018;34(5):3–526. doi:10.1016/j.cjca.2018.02.021
67. Toppenberg KS, Hill DA, Miller DP. Safety of radiographic imaging during pregnancy. Am Fam Physician. 1999;59:1813–1818.
68. Murali S, Baldisseri MR. Peripartum cardiomyopathy. Crit Care Med. 2005;33:S340–S346.
69. Zimmermann O, Kochs M, Zwaka TP, et al. Myocardial biopsy based classification and treatment in patients with dilated cardiomyopathy. Int J Cardiol. 2005;104(1):92–100. doi:10.1016/j.ijcard.2005.02.052
70. Surdacki A, Bednarek J, Kruszelnicka O, et al. Przewlekła niewydolność serca (PNS). Medycyna Praktyczna; 2021. Available from: https://www.mp.pl/interna/chapter/B16.II.2.19.1.#74967.
71. Marmursztejn J, Vignaux O, Goffinet F, et al. Delayed-enhanced cardiac magnetic resonance imaging features in peripartum cardiomyopathy. Int J Cardiol. 2009;137(3):e63–4. doi:10.1016/j.ijcard.2009.04.028
72. Mouquet F, Lions C, de Groote P, et al. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol. 2008;18(12):2765–2769. doi:10.1007/s00330-008-1067-x
73. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961–967. doi:10.1161/CIRCEP.114.001517
74. Ommen SR, Mital S, Burke MA, et al. American Heart Association. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;76(25):212.
75. Jeejeebhoy FM, Zelop CM, Lipman S, et al. American heart association emergency cardiovascular care committee, council on cardiopulmonary, critical care, perioperative and resuscitation, council on cardiovascular diseases in the young, and council on clinical cardiology cardiac arrest in pregnancy: a scientific statement from the American heart association. Circulation. 2015;132(18):1747–1773.
76. McNamara DM, Holubkov R, Starling RC, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103(18):2254–2259. doi:10.1161/01.CIR.103.18.2254
77. James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194(5):1311–1315. doi:10.1016/j.ajog.2005.11.008
78. Pyatt JR, Dubey G. Peripartum cardiomyopathy: current understanding, comprehensive management review and new developments. Postgrad Med J. 2011;87(1023):34–39. doi:10.1136/pgmj.2009.096594
79. Bozkurt B, Colvin M, Cook J, et al. American heart association committee on heart failure and transplantation of the council on clinical cardiology; council on cardiovascular disease in the young; council on cardiovascular and stroke nursing; council on epidemiology and prevention; and council on quality of care and outcomes research current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American heart association. Circulation. 2016;134(23):e579–e646.
80. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi:10.1093/eurheartj/ehy340
81. Hilfiker-Kleiner D, Haghikia A, Berliner D, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J. 2017;38(35):2671–2679. doi:10.1093/eurheartj/ehx355
82. Bauersachs J, Arrigo M, Hilfiker-Kleiner D, et al. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the heart failure association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2016;18(9):1096–1105. doi:10.1002/ejhf.586
83. Velickovic IA, Leicht CH. Peripartum cardiomyopathy and cesarean section: report of two cases and literature review. Arch Gynecol Obstet. 2004;270(4):307–310. doi:10.1007/s00404-003-0568-8
84. Canobbio MM, Warnes CA, Aboulhosn J, et al. American heart association council on cardiovascular and stroke nursing; council on clinical cardiology; council on cardiovascular disease in the young; council on functional genomics and translational biology; and council on quality of care and outcomes research management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American heart association. Circulation. 2017;135(8):e50–e87.
85. Stergiopoulos K, Shiang E, Bench T. Pregnancy in patients with pre-existing cardiomyopathies. J Am Coll Cardiol. 2011;58(4):337–350. doi:10.1016/j.jacc.2011.04.014
86. Felker GM, Jaeger CJ, Klodas E, et al. Myocarditis and long-term survival in peripartum cardiomyopathy. Am Heart J. 2000;140(5):785–791. doi:10.1067/mhj.2000.110091
87. Fett JD, Sannon H, Thélisma E, et al. Recovery from severe heart failure following peripartum cardiomyopathy. Int J Gynaecol Obstet. 2009;104(2):125–127. doi:10.1016/j.ijgo.2008.09.017
88. Yaméogo NV, Kaboré E, Seghda A, et al. Embolie pulmonaire grave et ischémie aiguë de membre inférieur compliquant une cardiomyopathie du péripartum traitées avec succès par thrombolyse à la streptokinase [Severe pulmonary embolism and acute lower limb ischemia complicating peripartum cardiomyopathy successfully treated by streptokinase]. Ann Cardiol Angeio. 2016;65(1):38–41. French.
89. Abdulbaki A, Kocherla C, Modi K. Aspiration thrombectomy in a case of acute myocardial infarction due to coronary emboli in a patient with peripartum cardiomyopathy and mural thrombus. Heart Int. 2016;10:e25–7. doi:10.5301/heartint.5000225
90. Kharwar RB, Chandra S, Dwivedi SK, et al. A pedunculated left ventricular thrombus in a women with peripartum cardiomyopathy: evaluation by three dimensional echocardiography. J Cardiovasc Ultrasound. 2014;22(3):139–143. doi:10.4250/jcu.2014.22.3.139
91. US Department of health and human services. Available from: http://optn.transplant.hrsa.gov/latestData/rptData.asp.
92. Sliwa K, Skudicky D, Bergemann A, et al. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol. 2000;35(3):701–705. doi:10.1016/S0735-1097(99)00624-5
93. Clark SJ, Kahn K, Houle B, et al. Young children’s probability of dying before and after their mother’s death: a rural South African population-based surveillance study. PLoS Med. 2013;10(3):e1001409. doi:10.1371/journal.pmed.1001409
94. Fett JD, Murphy JG. Infant survival in Haiti after maternal death from peripartum cardiomyopathy. Int J Gynaecol Obstet. 2006;94(2):135–136. doi:10.1016/j.ijgo.2006.05.009
95. Kamiya CA, Kitakaze M, Ishibashi-Ueda H, et al. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders. -Results from the Japanese Nationwide survey of peripartum cardiomyopathy. Circ J. 2011;75(8):1975–1981. doi:10.1253/circj.CJ-10-1214
96. Barasa A, Goloskokova V, Ladfors L, et al. Symptomatic recovery and pharmacological management in a clinical cohort with peripartum cardiomyopathy. J Matern Fetal Neonatal Med. 2018;31(10):1342–1349. doi:10.1080/14767058.2017.1317341
97. Seeho SK, Algert CS, Roberts CL, et al. Early-onset preeclampsia appears to discourage subsequent pregnancy but the risks may be overestimated. Am J Obstet Gynecol. 2016;215(6):
98. Codsi E, Rose CH, Blauwet LA. Subsequent pregnancy outcomes in patients with peripartum cardiomyopathy. Obstet Gynecol. 2018;131(2):322–327. doi:10.1097/AOG.0000000000002439
99. Guldbrandt Hauge M, Johansen M, Vejlstrup N, et al. Subsequent reproductive outcome among women with peripartum cardiomyopathy: a nationwide study. BJOG. 2018;125(8):1018–1025. doi:10.1111/1471-0528.15046
100. Heider AL, Kuller JA, Strauss RA, et al. Peripartum cardiomyopathy: a review of the literature. Obstet Gynecol Surv. 1999;54(8):526–531. doi:10.1097/00006254-199908000-00023
101. Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344(21):1567–1571. doi:10.1056/NEJM200105243442101
102. Avila WS, de Carvalho ME, Tschaen CK, et al. Pregnancy and peripartum cardiomyopathy. A comparative and prospective study. Arq Bras Cardiol. 2002;79(5):484–493. doi:10.1590/S0066-782X2002001400006
103. Sliwa K, Petrie MC, Hilfiker-Kleiner D, et al. Long-term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: practical guidance paper from the heart failure association of the European society of cardiology study group on peripartum cardiomyopathy. Eur J Heart Fail. 2018;20(6):951–962. doi:10.1002/ejhf.1178
104. American Heart Association (AHA). Classes of heart failure; 2017. Available from: https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure.
105. Sliwa K, Förster O, Libhaber E, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27(4):441–446. doi:10.1093/eurheartj/ehi481
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
