Back to Journals » Journal of Pain Research » Volume 16
The Relationship Between Chronic Pain and Cognitive Impairment in the Elderly: A Review of Current Evidence
Received 3 May 2023
Accepted for publication 23 June 2023
Published 7 July 2023 Volume 2023:16 Pages 2309—2319
DOI https://doi.org/10.2147/JPR.S416253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jonathan Greenberg
Jintao Chen,1 Xinyi Wang,2 Zherong Xu1
1Department of Geriatrics, The First Affiliated Hospital, Zhejiang University, School of Medicine, Hangzhou, People’s Republic of China; 2Department of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Zherong Xu, Department of Geriatrics, The First Affiliated Hospital, Zhejiang University, School of Medicine, Hangzhou, People’s Republic of China, Email [email protected]
Abstract: Chronic pain and cognitive impairment are prevalent geriatric syndromes in the population of older adults, and they are the main cause of disability in people over sixty-five years of age. As the global population continues to age, chronic pain and cognitive impairment will affect an increasing number of older adults. While numerous studies in recent years have shown that chronic pain is associated with cognitive decline, the exact mechanisms linking the two remain unclear. In this review, we aim to present the available evidence on the connection between chronic pain and cognitive impairment and to discuss the potential mechanisms by which chronic pain affects cognitive function. In addition, we review potential therapeutic interventions targeting psychological factors, microglia activation, and altered gut flora that may improve and prevent cognitive decline in people with chronic pain.
Keywords: chronic pain, cognitive impairment, microglia, psychosocial factors, gut flora
Introduction
Cognitive impairment is defined as impairment in one or more functions in multiple domains including executive, memory, attention, numeracy, language, literacy, and orientation, and is classified as mild cognitive impairment and dementia.1 As life expectancy continues to improve, the incidence of cognitive impairment is increasing exponentially. Currently, approximately 50 million people worldwide suffer from dementia, and this number is expected to increase to 152 million by 2050.2 Cognitive impairment is not only the main cause of disability in the elderly but also brings a huge burden to families and society. A multi-center survey of 3098 dementia patients in China reported that the total annual socioeconomic cost per person with dementia was $19,144, and the total annual dementia-related cost is expected to be $1.89 trillion by 2050.3 In view of the fact that there are no effective drugs for cognitive impairment, it is particularly important to prevent and delay the onset of dementia symptoms.4 A growing body of research now demonstrates that improving the management and monitoring of risk factors for cognitive impairment to halt or slow the progression of mild cognitive impairment is key to reducing the overall prevalence of dementia.5,6 For example, The 2020 Lancet Commission report on dementia prevention, intervention, and care identified 12 modifiable risk factors, including low educational attainment, high blood pressure, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, little social interaction, excessive alcohol consumption, head injuries, and air pollution, that could prevent or delay up to 40% of dementia.6 Thus if more risk factors can be identified and effective interventions implemented, the onset of dementia may be delayed in the future, and the number of people with dementia will be reduced even more.
Pain is defined by the International Association for the Study of Pain (IASP) as an unpleasant sensory and emotional experience associated with or similar to actual or potential tissue damage.7 Chronic pain, which lasts for three months or longer, affects over 30% of people worldwide and is prevalent in older populations.8 Unlike acute pain, chronic pain manifests itself in a pathological and inadequate manner, causing distress to the affected individual.9 Chronic pain frequently predicts the onset of adverse outcomes such as disability, loss of daily function, mood disorders, reduced quality of life, and increased health costs.10 According to the author’s clinical experience and analysis of the available literature,11–15 it has been found that the damages observed by chronic pain patients far exceed the pain itself. These patients also exhibit changes in the cognitive and affective domains to varying degrees.16 For example, the presence of pain can cause greater vulnerability to depression and social isolation. These all seem to indicate that chronic pain may be a potential risk factor for cognitive decline in older adults.
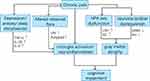
Prevention is more effective than cure, which is why modifiable risk factors are becoming increasingly important. According to analysis of the available literature, in this review, we present current evidence for the relationship between cognitive impairment and chronic pain and for overlapping pathological changes in these two disorders. We also discuss the possibility that chronic pain exacerbates the pathogenesis of cognitive impairment in people with chronic pain through elevated levels of pro-inflammatory cytokines, hormonal imbalances, and neurotransmitter disturbances. (Figure 1) he lack of a systematic search for evidence should be considered.
 |
Figure 1 Summarizing the content and highlights of the article. |
The Relationship Between Chronic Pain and Cognitive Impairment
It has been found that chronic pain and cognitive impairment are highly comorbid in older adults. An epidemiological analysis of community-dwell people and pain clinics estimated that more than 50% of pain patients reported cognitive decline.11 Similarly, a recent meta-analysis showed that 45.8% of people with dementia reported having chronic pain.17 Perhaps in the elderly population, the relationship between chronic pain and cognitive impairment is bidirectional. Chronic pain may increase the risk of cognitive impairment, while neurodegenerative pathologies associated with cognitive impairment possibly exacerbate the pain sensation. Numerous cross-sectional studies have reported poorer overall performance in cognition in people with chronic pain. Compared to the general elderly population, people with chronic pain often have memory deficits, reduced attention, disorientation, executive dysfunction, and impaired emotional decision-making.12,18–21 In recent years, an increasing number of longitudinal cohort studies with large samples are also available to support this view.13–15,22,23 For example, a cohort study that followed 10,065 older Americans for 12 years reported that persistent pain was associated with accelerated cognitive decline and an increased probability of dementia.13 Similarly, a prospective longitudinal study recruiting 693 elderly patients in France also reported that the presence of chronic pain was associated with higher cognitive decline in older adults.14 Moreover, as research continues, some studies suggest that it is the persistence of pain interference (defined as the degree to which pain impairs activities of daily living), rather than pain intensity, that is associated with an increased risk of cognitive impairment.24,25
Interestingly, two recent meta-analyses have shown different results. One of these meta-analyses, which included 37 studies, supported our previous point.26 The other meta-analysis of 10 longitudinal cohort studies showed that chronic pain was not associated with the incidence of cognitive impairment.27 We believe that some of the studies that produced negative results may be due to differences in the pain and cognitive assessment methods used. This is because some studies have shown that Mini-Mental State Examination is not as accurate as Montreal cognitive assessment.28 Mini-Mental State Examination is effective only if the cognitive impairment is severe enough. Just as some studies have found no correlation between pain and memory performance,29 others have suggested a link between the two,22 the primary reason for this result was the different choice of assessment scales in the research methods. Choosing the appropriate scale for subjective assessment is particularly important, especially when studying the elderly, as their pain tolerance may be stronger than that of young people, which may affect the accuracy of the data. Overall, although there is some controversy, the majority of research suggests that chronic pain may contribute to cognitive impairment.
Potential Mechanisms for Cognitive Impairment in People with Chronic Pain
The link between chronic pain and cognitive impairment is multifactorial. Multiple direct and indirect mechanisms are involved in chronic pain leading to cognitive impairment. Although the precise mechanisms have not been elucidated, several hypotheses and theories have been proposed. For example, the most common cognitive resource competition hypothesis, in which patients with chronic pain have difficulty adequately diverting attention and memory resources away from pain-related sensations, feelings, and thoughts, leaving fewer cognitive resources for other concurrent cognitive processes, may account for their cognitive decline.30,31 In addition to the cognitive resource competition hypothesis, based on recent research advances, we summarize four mechanisms of cognitive impairment in older adults with chronic pain: (1) altered neural network activity and structural changes in the brain; (2) Overactivation of microglia and neuroinflammation; (3) psychosocial variables; and (4) altered intestinal flora. We will discuss each of these possible mechanisms in the following sections. (Figures 2 and 3).
Altered Activity in the Brain’s Default Mode Network and Structural Changes in Different Cortical Regions May Directly Impair Cognitive Function
With the use of functional magnetic resonance imaging (fMRI), altered activity in the brain’s default mode network (DMN) in patients with chronic pain has been widely reported. The DMN is a largescale cerebral network comprising multiple functionally linked brain regions, consisting primarily of the posterior cingulate/precuneus, the medial prefrontal cortex (mPFC), the medial temporal lobes (including the hippocampus and parahippocampal cortices), and the inferior parietal lobules.32 Many elements of the DMN control cognitive functions such as working memory, decision-making, and attention allocation.33,34 Some studies have suggested that adequate inactivation of DMN might be required for optimal performance on target-directed cognitive tasks.35,36 However, many studies have demonstrated that chronic pain states can lead to a lower degree of inactivation of the DMN.37–39 Thus, the overactivation of DMN in chronic pain patients may account for their poor performance on attention allocation and working memory. We speculate that increased DMN activity may be related to pain handling. As mentioned earlier in the cognitive resource competition hypothesis, people with chronic pain display resting-state mPFC overactivity. This is because individuals with chronic pain probably require resource allocation in the mPFC to regulate and manage negative emotions generated by pain. Thus, individuals who experience higher pain intensity have higher resting-state mPFC activity than those with mild pain, raising the possibility that the former may engage in more resource competition in the mPFC.
In addition to altered activity in the DMN, structural changes are also observed in the gray matter of many cortical regions. It has been reported in kinds of literature that the hippocampus, amygdala, medial prefrontal cortex, insular cortex, thalamus, and dorsolateral prefrontal cortex are all atrophic in individuals with chronic pain.40,41 However, most of these brain regions are involved in different cognitive functions, such as the medial prefrontal cortex, which is involved in decision-making, executive control, and emotional processing; the hippocampus is involved in memory and learning; the dorsolateral prefrontal cortex is involved in attention. Therefore, brain gray matter atrophy may be one of the pathophysiological mechanisms for the development of cognitive dysfunction in patients with chronic pain. It is still unclear what gray matter atrophy in the brain represents in the context of chronic pain. Interestingly, the loss of gray matter volume in patients with chronic pain is reversible, and the volume of atrophied gray matter increases after effective treatment and elimination of chronic pain.42,43 This may indicate that the gray matter atrophy is not caused by neuronal loss, but by other changes in neuronal organization. Studies of magnetic resonance spectral labeling of neuronal integrity in patients with fibromyalgia also support this, confirming that the decrease in gray matter is not due to impaired neuronal integrity.44 According to the current research, there may be two pathways leading to gray matter atrophy in patients with chronic pain. One is the altered neuronal dendritic morphology caused by hypothalamic-pituitary-adrenal (HPA) axis dysfunction.45 Chronic pain puts patients in a constant state of stress. The chronic stress response can affect the function of the HPA axis, which in turn disrupts glucocorticoid (GC) and corticotropin releasing hormone (CRH) homeostasis in the body. Excess GC and CRH eventually flood into the central nervous system, leading to collapse of synaptic spines and contraction of dendrites, which then leads to loss of gray matter volume. The other is due to neurotransmitter dysregulation leading to changes in blood flow or volume in brain regions. Dysregulation of glutamate and GABA (the brain’s main excitatory and inhibitory neurotransmitters) has been observed in various cerebral regions of individuals with chronic pain. These dysregulated neurotransmitters can affect microvasculature through neurovascular signaling and pericytes—contractile cells activation resulting in changes in blood flow or volume in various brain regions that have been proposed to appear as grey matter decreases on T1‐weighted scans.46
Overactivation of Microglia and Neuroinflammation May Directly Impair Cognitive Function
Microglia are a type of macrophage in the central nervous system which regulate homeostasis in the brain and spinal cord.47 Activated microglia have two phenotypes: pro-inflammatory M1 and anti-inflammatory M2. Activated M1 phenotypic microglial lead to synaptic remodeling and changes in brain network function by releasing interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α), inducible nitrous oxide synthase (iNOS), and reactive oxygen species, while activated M2 phenotypic microglial promote phagocytosis, extracellular matrix remodelling, and tissue repair through release of anti-inflammatory cytokines such as IL-4, IL-10, and IL-13.48 It has been shown that different relative ratios of M1 /M2 phenotypes can produce neuroprotective effects or cytotoxicity affecting cognitive performance. Just as microglia play a role in the pathogenesis of Alzheimer’s disease. In the early stage of Alzheimer’s disease, activated microglia exhibit an anti-inflammatory phenotype, and then microglia activation lose their homeostasis and then turn into long-term and pathological neuroinflammation.49
However, it is worth noting that chronic pain is related to the upregulation of inflammatory microglia in different regions of the brain. Numerous preclinical and clinical researches have shown that activated microglia and neuroinflammation play a key role in the occurrence and maintenance of chronic pain.50,51 This also suggests a partial overlap in the pathophysiological mechanisms of chronic pain and cognitive impairment. Chronic pain usually sets in after a musculoskeletal injury. Its persistence is not always caused by the progression of the initial injury, but in some cases by the onset of central sensitization.52 Much scientific data has demonstrated that this central sensitization is caused by multiple complex interactions between the nervous system and immune system, driven by neuroinflammation in the peripheral and central nervous systems. A characteristic feature of neuroinflammation is the activation of microglia cells in the spinal cord and brain, leading to the release of pro-inflammatory cytokines and chemokines.53 Therefore, Overactivation of microglia may be one of the pathophysiological mechanisms of cognitive impairment in people with chronic pain. The increased relative proportion of pro-inflammatory M1 phenotype triggers a pro-inflammatory cascade response to generate neurotoxicity that leads to synaptic remodeling and deposition of amyloid and tau proteins, and then cognitive decline.
Psychosocial Variables May Mediate and/or Further Accelerate Cognitive Impairment in Patients with Chronic Pain
Psychosocial factors play an integral role in developing, maintaining, and treating chronic pain conditions. The biopsychosocial model describes pain and cognitive impairment as a multidimensional, dynamic integration among physiological, social, and psychological factors that reciprocally influence one another.54 It is widely accepted that older adults with chronic pain usually co-exist with psychosocial variables such as depression, anxiety, poor social environment, and sleep disturbances.8 And there is a wealth of evidence that these psychosocial variables contribute strongly to long-term outcomes of chronic pain such as physical disability,55 suicide,56 and mortality.57 We, therefore, consider that psychosocial factors may mediate or further accelerate cognitive impairment in the elderly with chronic pain.
Depression, sleep disturbances, and little social interaction are known risk factors for cognitive impairment.6 For example, a meta-analysis including 27 observational studies revealed that individuals with sleep problems had a 1.68 (95% CI: 1.51–1.87) times higher risk for the combined outcome of cognitive impairment and/or Alzheimer’s disease. Similarly, a prospective study of 4922 initially cognitively healthy men, aged 71–89 years, found depression symptoms were associated with 1.5 (95% CI 1.2–2.0) times the incidence of dementia.58 These results may be attributed to pro-inflammatory cytokines. Studies have found elevated levels of circulating pro-inflammatory cytokines (eg, IL-6, IL-1, and TNF-α) in people with symptoms of insomnia, anxiety, and depression.59,60 In addition, circulating pro-inflammatory cytokine levels are closely linked to blood-brain barrier (BBB) permeability, and Bowman et al found that individuals with higher levels of serum pro-inflammatory biomarkers (vascular endothelial growth factor (VEGF) and IL-16) had more severely damaged blood-brain barrier, while individuals with more severe blood-brain barrier impairment had higher levels of inflammatory biomarkers (VEGF and IL-8) in the brain-spinal fluid.61 Thus high levels of pro-inflammatory cytokines entering the central system elicit microglia activation and neuroinflammation, which in turn leads to severe neurodegeneration and cognitive impairment. What’s more, depressive symptoms stimulate glucocorticoid production or beta-amyloid deposition through activation of the HPA axis, which in turn damages the hippocampus and results in cognitive impairment.62
Gut Flora May Mediate and/or Further Accelerate Cognitive Impairment in Patients with Chronic Pain
The gut flora plays a critical role in human health and disease. In recent years, evidence has been accumulating that the composition of the gut microbiome changes in individuals with various types of chronic pain, such as osteoarthritis-related pain, fibromyalgia, chronic pelvic pain, irritable bowel syndrome, and so on.63–66 For example, A systematic review showed that osteoarthritis-related pain is associated with altered gut microbes, and hypothesized that gut microbial products and their metabolites contribute to local or systemic inflammatory responses, thus inducing or exacerbating pain.66 In clinical research, compared with the healthy control group, the relative abundance of the genera Bifidobacterium and Faecalibacterium and the species Faecalibacterium prausnitzii in the feces of patients with irritable bowel syndrome decreased, while the relative abundance of Lactobacillaceae, Bacteroides, and Enterobacteriaceae increased.67 Minerbi et al found that the relative abundance of F. prausnitzii and B. uniformis was lower in stool samples from fibromyalgia patients compared to age-matched healthy individuals.64 Similarly, Shoskes et al found that individuals with chronic pelvic pain syndrome had significantly reduced gut microbiota diversity, particularly the abundance of Prevotella species, compared to controls.63 Notably, the gut microbiota has recently emerged as important contributors to homeostasis and dysfunction within the central nervous system.68 Therefore, the role of altered gut flora in cognitive decline cannot be ignored. Altered gut microbiome may mediate and/or further accelerate cognitive impairment in patients with chronic pain.
Studies have shown that altered gut flora composition can increase the permeability of the intestinal and blood-brain barriers.69 These changes in permeability may be useful for promoting brain accumulation of intestinal microbial-derived molecules and metabolites (eg lipopolysaccharides (LPS), amyloid), which in turn alter the homeostasis of pro-inflammatory conditions, thus laying the foundation for the pathogenesis of neurodegenerative diseases.70 For example, LPS could activate a variety of immune cells, including neutrophils, dendritic cells, and macrophages.71 These activated cells would produce abundant pro-inflammatory cytokines (eg TNF-α, IL-1β, IL-2, and IL-6), and then cross the blood-brain barrier into the brain via diffusion and cytokine transporters.71 The accumulation of bacterial-born amyloid in the brain would trigger many other downstream events that activate the NF-kB signaling pathway to trigger microglia inflammatory responses, leading to neurodegeneration.72
Therapeutic Implications of These Underlying Mechanisms for Comorbidity Chronic Pain and Cognitive Impairment
Psychological Interventions
Psychological interventions may be useful in the prevention and treatment of cognitive impairment. The most common psychologically based intervention for chronic pain is cognitive behavioral therapy, which involves restructuring maladaptive beliefs, attitudes, and behaviors that contribute to disease burden.8 Studies have shown that cognitive behavioral therapy is likely to work by reducing stress in patients with chronic pain, thereby reducing activation of the HPA axis and downstream negative effects of this activation.73 For example, cognitive behavioral therapy can be effective in improving symptoms of depression and anxiety in chronic pain patients.74,75 In addition, cognitive behavioral therapy can normalize various aberrant neural patterns and also increase gray matter volume in prefrontal and somatosensory brain regions in individuals with chronic pain.43,76 Therefore, psychological interventions may help prevent and treat cognitive impairment in people with chronic pain.
Inhibition of Microglia Overactivation
Inhibition of microglia hyperactivation and excessive neuroinflammation may also prevent and alleviate cognitive impairment. While there are no currently approved drugs that specifically target microglia, some clinically available drugs exhibit a degree of microglial modulation and are being explored as potential analgesics.77 For example, in clinical trials, minocycline (Minocycline is a semi-synthetic tetracycline derivative that is widely used to inhibit microglia activation.)78 can effectively improve peripheral and autonomic neuropathy in diabetes mellitus type 2.79 Low-dose naltrexone (Naltrexone is an opioid antagonist that is introduced into clinical use as a microglial modulator.)80 has been shown to significantly reduce baseline pain and improve life satisfaction and mood.81 In terms of improving cognitive function, microglia inhibitors have now been validated in preclinical experiments, and we believe there will be plenty of clinical data to support this in the future as well.
Improvement of Intestinal Flora
Another possible approach to preventing and alleviating cognitive impairment is to improve intestinal flora. Short-chain fatty acids (SCFAs) are well-established to possess anti-inflammatory, neuromodulatory, and immune regulatory properties. Therefore, improving the gut microbiota to promote the growth of beneficial bacteria that produce short-chain fatty acids may aid in pain relief and improve cognitive function. For instance, animal models have shown that improving gut microbiota through antibiotic, probiotic, or fecal microbiome transplantation can have a positive effect on the incidence of postoperative delirium and pain.67 Similarly, clinical research has suggested that probiotics may have potential benefits for central nervous system disorders such as depression and anxiety.82 Furthermore, it is worth noting that a recent systematic review has indicated that lifestyle interventions such as diet, exercise, and sleep may improve the composition of the gut microbiota, thereby alleviating various chronic widespread pain conditions and improving quality of life.83 Therefore, we recommend that patients with chronic pain can make appropriate lifestyle changes such as increasing their intake of prebiotics and probiotics and engaging in regular exercise to relieve pain and prevent cognitive decline.
Summary and Future Directions
This review provides current evidence of the relationship between cognitive impairment and chronic pain and the overlapping pathological changes in the two disorders. To show that chronic pain may be a controllable factor leading to cognitive impairment in the elderly. Doctors and nurses should pay more attention to the evaluation of chronic pain in the elderly in clinical work, and actively take reasonable intervention measures.
Ultimately, we know that chronic pain may contribute to cognitive impairment, but it remains uncertain whether lifestyle changes such as regular exercise, diet, and sleep, as well as pharmacological and cognitive-behavioral interventions, can significantly reduce the occurrence of cognitive dysfunction in older adults with early intervention and management of chronic pain. Future research is needed not only to integrate knowledge from different research fields, such as neuroscience, psychology, biology, and molecular biology, to more fully understand the relationship between chronic pain and cognitive impairment, but also to conduct large-scale prospective clinical studies to determine whether effective interventions and management of chronic pain can help prevent and delay the onset of cognitive impairment in the elderly.
Abbreviations
Fmri, Functional magnetic resonance imaging; DMN, Default mode network; mPFC, The medial prefrontal cortex; HPA, Hypothalamic-pituitary-adrenal; IL, Interleukin; TNF-α, Tumor necrosis factor-α; iNOS, Inducible nitrous oxide synthase; BBB, Blood-brain barrier; VEGF, Vascular endothelial growth factor; CNS, The central nervous system; CRH, Adrenocorticotropin-releasing hormone; GC, Glucocorticoid; Glu, Glutamate; GABA, Y-aminobutyric acid; LPS, Lipopolysaccharides; SCFAs, Short-chain fatty acids.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, and drawing, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. doi:10.1212/WNL.0000000000004826
2. World Alzheimer Report 2018 - the state of the art of dementia research: new frontiers. New Frontiers. 48.
3. Jia J, Wei C, Chen S, et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018;14(4):483–491. doi:10.1016/j.jalz.2017.12.006
4. Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer’s disease in an older population: updated incidence and life expectancy with and without dementia. Am J Public Health. 2015;105(2):408–413. doi:10.2105/AJPH.2014.301935
5. Pickett J, Brayne C. The scale and profile of global dementia research funding. Lancet. 2019;394(10212):1888–1889. doi:10.1016/S0140-6736(19)32599-1
6. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi:10.1016/S0140-6736(20)30367-6
7. Raja SN, Carr DB, Cohen M, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
8. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi:10.1016/S0140-6736(21)00393-7
9. Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci. 2017;18(2):113. doi:10.1038/nrn.2017.5
10. Enkvist A, Ekström H, Elmståhl S. Life satisfaction (LS) and symptoms among the oldest-old: results from the longitudinal population study called Good Aging in Skåne (GÅS). Arch Gerontol Geriatr. 2012;54(1):146–150. doi:10.1016/j.archger.2011.05.001
11. Baker KS, Gibson S, Georgiou-Karistianis N, Roth RM, Giummarra MJ. Everyday executive functioning in chronic pain: specific deficits in working memory and emotion control, predicted by mood, medications, and pain interference. Clin J Pain. 2016;32(8):673–680. doi:10.1097/AJP.0000000000000313
12. van der Leeuw G, Leveille SG, Dong Z, et al. Chronic pain and attention in older community-dwelling adults. J Am Geriatr Soc. 2018;66(7):1318–1324. doi:10.1111/jgs.15413
13. Whitlock EL, Diaz-Ramirez LG, Glymour MM, et al. Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med. 2017;177(8):1146–1153. doi:10.1001/jamainternmed.2017.1622
14. Rouch I, Edjolo A, Laurent B, et al. Association between chronic pain and long-term cognitive decline in a population-based cohort of elderly participants. Pain. 2021;162(2):552–560. doi:10.1097/j.pain.0000000000002047
15. Kao P-H, Fong-Lin J, Chung-Han H, et al. Chronic pain increases the risk of dementia: a nationwide population-based cohort study. Pain Physician. 2021;24:E849–E856.
16. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511. doi:10.1038/nrn3516
17. van Kooten J, Binnekade TT, van der Wouden JC, et al. A review of pain prevalence in Alzheimer’s, vascular, frontotemporal and lewy body dementias. Dement Geriatr Cogn Disord. 2016;41(3–4):220–232. doi:10.1159/000444791
18. Abeare CA, Cohen JL, Axelrod BN, et al. Pain, executive functioning, and affect in patients with rheumatoid arthritis. Clin J Pain. 2010;26(8):683–689. doi:10.1097/AJP.0b013e3181ed1762
19. Oosterman JM, Derksen LC, van Wijck AJM, Veldhuijzen DS, Kessels RPC. Memory functions in chronic pain: examining contributions of attention and age to test performance. Clin J Pain. 2011;27(1):70–75. doi:10.1097/AJP.0b013e3181f15cf5
20. Innes KE, Sambamoorthi U. The association of perceived memory loss with osteoarthritis and related joint pain in a large appalachian population. Pain Med. 2018;19(7):1340–1356. doi:10.1093/pm/pnx107
21. Cardoso J, Apagueno B, Lysne P, et al. Pain and the Montreal Cognitive Assessment (MoCA) in aging. Pain Med. 2021;22(8):1776–1783. doi:10.1093/pm/pnab003
22. Bell TR, Sprague BN, Ross LA. Longitudinal associations of pain and cognitive decline in community-dwelling older adults. Psychol Aging. 2022;37(6):715–730. doi:10.1037/pag0000699
23. van der Leeuw G, Ayers E, Leveille SG, et al. The effect of pain on major cognitive impairment in older adults. J Pain. 2018;19(12):1435–1444. doi:10.1016/j.jpain.2018.06.009
24. Ikram M, Innes K, Sambamoorthi U. Association of osteoarthritis and pain with Alzheimer’s Diseases and Related Dementias among older adults in the United States. Osteoarthritis Cartilage. 2019;27(10):1470–1480. doi:10.1016/j.joca.2019.05.021
25. Bell T, Franz CE, Kremen WS. Persistence of pain and cognitive impairment in older adults. J Am Geriatr Soc. 2022;70(2):449–458. doi:10.1111/jgs.17542
26. Zhang X, Gao R, Zhang C, et al. Evidence for cognitive decline in chronic pain: a systematic review and meta-analysis. Front Neurosci. 2021;15:737874. doi:10.3389/fnins.2021.737874
27. de Aguiar GPCG, Saraiva MD, Khazaal EJB, et al. Persistent pain and cognitive decline in older adults: a systematic review and meta-analysis from longitudinal studies. Pain. 2020;161(10):2236–2247. doi:10.1097/j.pain.0000000000001932
28. Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr. 2019;31(04):491–504. doi:10.1017/S1041610218001370
29. Oosterman JM, de Vries K, Dijkerman HC, de Haan EHF, Scherder EJA. Exploring the relationship between cognition and self-reported pain in residents of homes for the elderly. Int Psychogeriatr. 2009;21(01):157–163. doi:10.1017/S1041610208007941
30. Landrø NI, Fors EA, Våpenstad LL, et al. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? Pain. 2013;154(7):972–977. doi:10.1016/j.pain.2013.01.013
31. Samartin-Veiga N, González-Villar AJ, Carrillo-de-la-peña MT. Neural correlates of cognitive dysfunction in fibromyalgia patients: reduced brain electrical activity during the execution of a cognitive control task. Neuroimage Clin. 2019;23:101817. doi:10.1016/j.nicl.2019.101817
32. Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316(1):29–52. doi:10.1111/nyas.12360
33. Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25(34):7709–7717. doi:10.1523/JNEUROSCI.2177-05.2005
34. Peterson BS, Potenza MN, Wang Z, et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166(11):1286–1294. doi:10.1176/appi.ajp.2009.08050724
35. Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49(3):2638–2648. doi:10.1016/j.neuroimage.2009.11.008
36. White TP, Jansen M, Doege K, et al. Theta power during encoding predicts subsequent-memory performance and default mode network deactivation. Hum Brain Mapp. 2013;34(11):2929–2943. doi:10.1002/hbm.22114
37. Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi:10.1523/JNEUROSCI.4123-07.2008
38. Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci U S A. 2013;110(46):18692–18697. doi:10.1073/pnas.1312902110
39. Hemington KS, Rogachov A, Cheng JC, et al. Patients with chronic pain exhibit a complex relationship triad between pain, resilience, and within- and cross-network functional connectivity of the default mode network. Pain. 2018;159(8):1621–1630. doi:10.1097/j.pain.0000000000001252
40. Shi H, Yuan C, Dai Z, Ma H, Sheng L. Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin Arthritis Rheum. 2016;46(3):330–337. doi:10.1016/j.semarthrit.2016.06.002
41. Zhou Z, Hui ES, Kranz GS, et al. Potential mechanisms underlying the accelerated cognitive decline in people with chronic low back pain: a scoping review. Ageing Res Rev. 2022;82:101767. doi:10.1016/j.arr.2022.101767
42. Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi:10.1523/JNEUROSCI.3687-09.2009
43. Seminowicz DA, Shpaner M, Keaser ML, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. 2013;14(12):1573–1584. doi:10.1016/j.jpain.2013.07.020
44. Pomares FB, Funck T, Feier NA, et al. Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. J Neurosci. 2017;37(5):1090–1101. doi:10.1523/JNEUROSCI.2619-16.2016
45. Kang D, McAuley JH, Kassem MS, Gatt JM, Gustin SM. What does the grey matter decrease in the medial prefrontal cortex reflect in people with chronic pain? Eur J Pain. 2019;23(2):203–219. doi:10.1002/ejp.1304
46. Franklin TR, Wang Z, Shin J, et al. A VBM study demonstrating ‘apparent’ effects of a single dose of medication on T1-weighted MRIs. Brain Struct Funct. 2013;218(1):97–104. doi:10.1007/s00429-012-0385-6
47. Chen G, Zhang Y-Q, Qadri YJ, Serhan CN, Ji -R-R. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–1311. doi:10.1016/j.neuron.2018.11.009
48. Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173(4):649–665. doi:10.1111/bph.13139
49. Smirnov D, Galasko D. Dynamics of neuroinflammation in AlzheimerAlzheimer’s disease. Lancet Neurol. 2022;21(4):297–298. doi:10.1016/S1474-4422(22)00087-4
50. Loggia ML, Chonde DB, Akeju O, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138(3):604–615. doi:10.1093/brain/awu377
51. Sideris-Lampretsas G, Malcangio M. Microglial heterogeneity in chronic pain. Brain Behav Immun. 2021;96:279–289. doi:10.1016/j.bbi.2021.06.005
52. Vergne-Salle P, Bertin P. Chronic pain and neuroinflammation. Joint Bone Spine. 2021;88(6):105222. doi:10.1016/j.jbspin.2021.105222
53. Ji R-R, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 2018;129(2):343–366. doi:10.1097/ALN.0000000000002130
54. Meints SM, Edwards RR. Evaluating psychosocial contributions to chronic pain outcomes. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt B):168–182. doi:10.1016/j.pnpbp.2018.01.017
55. Hall AM, Kamper SJ, Maher CG, et al. Symptoms of depression and stress mediate the effect of pain on disability. Pain. 2011;152(5):1044–1051. doi:10.1016/j.pain.2011.01.014
56. Ilgen MA, Kleinberg F, Ignacio RV, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70(7):692–697. doi:10.1001/jamapsychiatry.2013.908
57. Macfarlane GJ, McBeth J, Silman AJ, Crombie IK. Widespread body pain and mortality: prospective population based study. BMJ. 2001;323(7314):662–665. doi:10.1136/bmj.323.7314.662
58. Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Depression as a modifiable factor to decrease the risk of dementia. Transl Psychiatry. 2017;7(5):e1117. doi:10.1038/tp.2017.90
59. Krogh J, Benros ME, Jørgensen MB, et al. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav Immun. 2014;35:70–76. doi:10.1016/j.bbi.2013.08.014
60. Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702–715. doi:10.1038/s41577-019-0190-z
61. Bowman GL, Dayon L, Kirkland R, et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14(12):1640–1650. doi:10.1016/j.jalz.2018.06.2857
62. Colla M, Kronenberg G, Deuschle M, et al. Hippocampal volume reduction and HPA-system activity in major depression. J Psychiatr Res. 2007;41(7):553–560. doi:10.1016/j.jpsychires.2006.06.011
63. Shoskes DA, Wang H, Polackwich AS, et al. Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. J Urol. 2016;196(2):435–441. doi:10.1016/j.juro.2016.02.2959
64. Minerbi A, Gonzalez E, Brereton NJB, et al. Altered microbiome composition in individuals with fibromyalgia. Pain. 2019;160(11):2589–2602. doi:10.1097/j.pain.0000000000001640
65. Freidin MB, Stalteri MA, Wells PM, et al. An association between chronic widespread pain and the gut microbiome. Rheumatology. 2021;60(8):3727–3737. doi:10.1093/rheumatology/keaa847
66. Sánchez Romero EA, Meléndez Oliva E, Alonso Pérez JL, et al. Relationship between the gut microbiome and osteoarthritis pain: review of the literature. Nutrients. 2021;13(3):716. doi:10.3390/nu13030716
67. Minerbi A, Shen S. Gut microbiome in anesthesiology and pain medicine. Anesthesiology. 2022;137(1):93–108. doi:10.1097/ALN.0000000000004204
68. Zhu S, Jiang Y, Xu K, et al. The progress of gut microbiome research related to brain disorders. J Neuroinflammation. 2020;17(1):25. doi:10.1186/s12974-020-1705-z
69. Sun M-F, Shen Y-Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res Rev. 2018;45:53–61. doi:10.1016/j.arr.2018.04.004
70. Bairamian D, Sha S, Rolhion N, et al. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol Neurodegener. 2022;17(1):19. doi:10.1186/s13024-022-00522-2
71. Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun. 2010;24(1):102–109. doi:10.1016/j.bbi.2009.09.001
72. Wang C, Fan L, Khawaja RR, et al. Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat Commun. 2022;13(1):1969. doi:10.1038/s41467-022-29552-6
73. Eller-Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci. 2018;12:35. doi:10.3389/fncel.2018.00035
74. Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69(4):621–632. doi:10.4088/JCP.v69n0415
75. Spector A, Charlesworth G, King M, et al. Cognitive-behavioural therapy for anxiety in dementia: pilot randomised controlled trial. Br J Psychiatry. 2015;206(6):509–516. doi:10.1192/bjp.bp.113.140087
76. Yoshino A, Okamoto Y, Okada G, et al. Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychol Med. 2018;48(7):1148–1156. doi:10.1017/S0033291717002598
77. Haight ES, Forman TE, Cordonnier SA, James ML, Tawfik VL. Microglial modulation as a target for chronic pain: from the bench to the bedside and back. Anesth Analg. 2019;128(4):737–746. doi:10.1213/ANE.0000000000004033
78. Zhou Y-Q, Liu D-Q, Chen S-P, et al. Minocycline as a promising therapeutic strategy for chronic pain. Pharmacol Res. 2018;134:305–310. doi:10.1016/j.phrs.2018.07.002
79. Syngle A, Verma I, Krishan P, Garg N, Syngle V. Minocycline improves peripheral and autonomic neuropathy in type 2 diabetes: MIND study. Neurol Sci. 2014;35(7):1067–1073. doi:10.1007/s10072-014-1647-2
80. Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33(4):451–459. doi:10.1007/s10067-014-2517-2
81. Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009;10(4):663–672. doi:10.1111/j.1526-4637.2009.00613.x
82. Snigdha S, Ha K, Tsai P, et al. Probiotics: potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol Ther. 2022;231:107978. doi:10.1016/j.pharmthera.2021.107978
83. Gonzalez-Alvarez ME, Sanchez-Romero EA, Turroni S, Fernandez-Carnero J, Villafañe JH. Correlation between the altered gut microbiome and lifestyle interventions in chronic widespread pain patients: a systematic review. Medicina. 2023;59(2):256. doi:10.3390/medicina59020256
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.