Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
The Relationship Between Bone Metabolism and Peripheral Artery Disease in Patients on Hemodialysis: The Potential Role of Osteocalcin
Authors Chen ZY, Yang J, Tian CY, Jia W
Received 25 August 2023
Accepted for publication 10 October 2023
Published 26 October 2023 Volume 2023:16 Pages 3331—3337
DOI https://doi.org/10.2147/DMSO.S432345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Zi-Ye Chen,1 Jie Yang,1 Chen-Yang Tian,2 Wei Jia2
1Department of Nephrology, Beijing Jishuitan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Vascular Surgery, Beijing Jishuitan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Zi-Ye Chen, Department of Nephrology, Beijing Jishuitan Hospital, Capital Medical University, Xinjiekou No. 31 East Street, Xicheng District, Beijing, 100035, People’s Republic of China, Tel +86-13681058086, Email [email protected]
Introduction: To examine the factors associated with PAD, with a specific focus on bone metabolism factors such as osteocalcin.
Methods: This cross-sectional study examined factors about demographic, clinical, and laboratory parameters including bone metabolism biomarkers in hemodialysis patients. The ankle-brachial index (ABI) was measured in all patients, with PAD diagnosed as an ABI < 0.9.
Results: Out of the 71 patients, PAD was found in 23 individuals. These patients had an average age of 63.5± 13.0 years, with 59.2% being male. Compared to non-PAD patients, those with PAD were older, had a lower proportion of males, and had a higher prevalence of diabetes and coronary artery disease. Among the factors related to bone metabolism, only osteocalcin exhibited a significant increase in the PAD group compared to the non-PAD group.
Conclusion: PAD in patients on hemodialysis was independently linked to high levels of osteocalcin in the bloodstream, indicating the presence of bone metabolism disorders.
Keywords: hemodialysis, osteocalcin, bone metabolism disorder, peripheral artery disease
Introduction
Peripheral artery disease (PAD) is a chronic medical condition that is becoming more common worldwide and is associated with a poor outlook.1–3 Recent study revealed that inflammation was associated with acute and chronic PAD as detected by neutrophils / lymphocyte ratio. Moreover, there were studies showed that inflammatory burden was increased as renal dysfunction progressed and predicted adverse clinical prognosis in hemodialysis patients.4,5 Thus, the development of PAD seems to be related to renal dysfunction. The diagnosis of PAD using the ankle-brachial index (ABI) is more prevalent in individuals undergoing hemodialysis and has been linked to the prediction of cardiovascular disease and overall mortality.2 The Dialysis Outcomes and Practice Patterns Study (DOPPS) collected data indicating that approximately one-third of patients on hemodialysis suffer from PAD, which significantly increases the risks of both illness and death compared to the general population.6 Despite numerous studies investigating potential risk factors for PAD, these factors have not been adequately explored in patients undergoing hemodialysis. The involvement of abnormal bone metabolism in the development of cardiovascular disease, particularly in patients on hemodialysis, has been a well-known fact for a considerable period.7 Due to the distinct combination of biochemical, endocrine, and molecular irregularities in bone metabolism disorders specific to these patients, there is evidence indicating a consistently higher prevalence of systemic atherosclerosis, including PAD.8 A previous study conducted on patients on hemodialysis in Central Nepal revealed a significant and independent association between PAD and levels of intact parathyroid hormone exceeding 300 ng/mL.9 However, a subsequent study yielded conflicting results.10 Although a few studies have examined the predictive role of calcium and phosphate metabolism biomarkers for PAD in patients on hemodialysis,11,12 the available information remains insufficient and contradictory. The objective of this study was to investigate the factors associated with PAD in individuals undergoing hemodialysis and to assess the influence of bone metabolism, specifically osteocalcin serum levels, in these patients.
Materials and Methods
Patients
We included participants in our study who were patients on hemodialysis, aged 18 years or older, and received treatment at Beijing Jishuitan Hospital, Capital Medical University from April 2021 to April 2022. The treatment regimen consisted of three sessions per week, each lasting four hours. All the patients in the study underwent treatment for regulating calcium-phosphorus levels such as Vitamin D Receptor Activators and calcimimetics and were monitored by qualified staff. Patients with a duration of hemodialysis of less than six months, as well as those with severe infections or malignancy, were excluded from the study.
The Institutional Review Board of Beijing Jishuitan Hospital, Capital Medical University granted approval for this study, and the procedures followed in the study adhered to the principles outlined in the Declaration of Helsinki. Prior to their participation in the study, written informed consent was obtained from all patients.
Demographic and Clinical Characteristics
The medical records of the enrolled patients were examined. We gathered information on various demographic factors such as gender, age, and body mass index (BMI). Additionally, we recorded specific details related to hemodialysis, including primary kidney disease, duration of hemodialysis, dialysis adequacy, and the presence of diabetes mellitus (DM), coronary artery disease or fractures. Dialysis adequacy was assessed using Kt/V, while BMI was calculated using the formula weight/height2 (kg/m2).
Laboratory Parameters
The patients’ laboratory values were regularly assessed on a monthly basis, encompassing various parameters such as serum albumin, potassium, sodium, uric acid, triglyceride, total cholesterol, hemoglobin levels, and C-reactive protein (CRP). Additionally, bone-related parameters, including serum calcium, phosphorus, intact parathyroid hormone, 25-hydroxyvitamin D (25[OH]D), and osteocalcin, were also routinely evaluated each month. These measurements were conducted at the Clinical Laboratory of Beijing Jishuitan Hospital, Capital Medical University, and the data was obtained from the electronic clinical database.
ABI Measurements
The ABI values were determined by the same skilled doctor using a portable handheld bidirectional Doppler device and was conducted within one week after blood test. The patients were positioned on their backs, and monitoring cuffs were attached to appropriate areas. The doctor measured the systolic blood pressure in the brachial artery of the arm without vascular access, as well as in the dorsalis pedis artery and posterior tibial artery of both lower limbs using a Doppler probe. To calculate the ABI values, the systolic pressure at the ankle was divided by the systolic pressure in the arm. The lower ABI value from both legs was considered the leg index. The diagnosis of PAD was based on an ABI value below 0.9, which is in line with the guidelines provided by the American College of Cardiology/American Heart Association and consistent with previous studies.2,12–14
Statistical Analysis
The mean±standard deviation or median value and interquartile range were used to express continuous variables, depending on the distribution of the data. Numbers and percentages were used to express categorical variables. The comparison of continuous variables was done using Student’s t-test and the Mann–Whitney U-test, while the chi-square test was used for comparing categorical variables. To examine the relationship between the variables and PAD, multivariate logistic regression models were created, including only those variables that showed statistical significance in the univariate analyses. The correlation between osteocalcin and these variables was also tested using Spearman’s Rank-Order Correlation. A P value < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS software for Windows (version 25.0; SPSS, Chicago, IL, USA).
Results
In the study, a total of 71 patients were included. Their average age was 63.5±13.0 years, with 40.8% being female and 59.2% male. These patients had been undergoing dialysis for an average duration of 75.2±50.0 months. Among them, 23 patients were identified as having PAD and were assigned to the PAD group, while the remaining 48 patients were classified as the non-PAD group. The baseline characteristics of the patients are provided in Table 1. The most common cause of end-stage renal disease (ESRD) was diabetes mellitus (36.6%), followed by glomerulonephritis (25.4%). When comparing the PAD group with the non-PAD group, it was found that the PAD patients were older (72.2±10.9 vs 59.3±11.9 years, p<0.001), had a lower percentage of males (47.8% vs 64.4%, p<0.001), and a higher prevalence of diabetes mellitus or coronary artery disease (56.5% vs 31.3%, p<0.001; 65.2% vs 27.1%, p<0.001, respectively). Eight patients had history of fracture, three were in the PAD group and five were in the non-PAD group without statistic significant difference (13.0% vs 10.4%, p=0.872). No statistically significant differences were found between patients with or without PAD in terms of the traditional markers related to bone metabolism. These markers include phosphate (1.54±0.44 vs 1.53±0.45, p=0.908), calcium (2.29±0.11 vs 2.24±0.15, p=0.138), intact parathyroid hormone calcium (286.07±35.82 vs 235.39±36.52, p=0.393), and 25(OH)D (11.61±5.44 vs 13.74±6.13, p=0.166). However, there was an increase in osteocalcin levels in the PAD group compared to the non-PAD group (166.07±11.34 vs 118.04±15.33, p=0.017) as shown in Table 1. A single-variable analysis revealed a significant correlation between PAD and various factors such as age, duration of dialysis, diabetes mellitus (DM), coronary artery disease, and osteocalcin level. However, other indicators of bone metabolism did not exhibit any association with PAD. Subsequent multivariable analyses demonstrated that only osteocalcin remained an independent risk factor for the development of PAD. The odds ratio (OR) for osteocalcin was 1.03 with a confidence interval (CI) of 1.00 to 1.05 and a p-value of 0.021, indicating its significant impact (Table 2). No significant relationship was found between osteocalcin and these factors (Table 3).
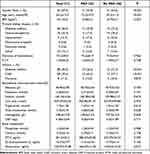 |
Table 1 Baseline Characteristics of Study Population |
 |
Table 2 Univariate and Multivariate Analysis for Peripheral Arterial Disease |
 |
Table 3 Correlation Between Osteocalcin and Other Factors |
Discussion
This research aimed to examine the factors associated with PAD in patients on hemodialysis. Previous study showed that PAD patients undergoing popliteal and infrapopliteal percutaneous peripheral arterial interventions with renal dysfunction (GFR<60 mL/min/1.73 m2) and hemodialysis had increased risk of major amputations and mortality. This study called for novel therapeutic strategies to improve outcomes of this population.15 Specifically, our study revealed that the bone metabolism marker osteocalcin exhibited an independent correlation with PAD. To the best of our knowledge, this is the first study to establish a connection between PAD and bone metabolism in patients on hemodialysis, considering the unique bone metabolism disorder in this population that may contribute to vascular calcification. Numerous studies conducted over the past few decades have endeavored to identify predictive factors for PAD. Age, female gender, body mass index, critical limb ischemia, diabetes mellitus, hypertension, cerebrovascular disease, heart failure, and chronic kidney disease (CKD) have been associated with poor limb salvage, mortality, or readmission following peripheral revascularization in patients with or without hemodialysis.16–20 Recent studies have further demonstrated that patients undergoing hemodialysis are more susceptible to PAD and experience worse outcomes.8,14,21–26 Cross-sectional studies have reported a high prevalence of PAD in patients on hemodialysis and have identified lower serum albumin levels, elevated C-reactive protein levels, Hickman vascular access, and foot deformity as independent predictors of PAD or foot ulceration in these patients.10,12 However, a prospective study involving 450 dialysis patients only found neuropathy and previous ulceration to be significant risk factors for foot ulceration, while serum albumin and C-reactive protein levels showed no significant association with this condition.27 Risk factors also varied between patients with and without prior ulceration. Raikou et al28 conducted an analysis of 150 patients undergoing on-line-predilution hemodiafiltration and discovered that low-grade inflammation, as indicated by elevated monocyte chemoattractant protein-1 serum concentrations was a significant predictor for PAD. This biomarker has long been recognized as a key mediator of osteoclastogenesis in HD patients with PAD, which suggested an association between inflammatory response and bone metabolism. But the relationship between bone metabolism, especially the osteocalcin and PAD was not discussed by the authors. Another study involving peritoneal dialysis patients demonstrated that osteoprotegerin was an independent predictor of PAD, establishing a link between bone metabolism and arterial calcification.13 Collectively, while these previous studies identified both traditional and novel risk factors, the findings were not consistent, and most factors were non-intervenable. In line with previous research, our study focused on a group of hemodialysis patients with a low ABI. These patients tended to be older, more likely to be female, and had a higher prevalence of diabetes mellitus or coronary artery disease compared to those with a normal ABI. We examined bone metabolism and found that commonly used indicators such as phosphate, calcium, intact parathyroid hormone, and 25(OH)D did not show any significant differences between hemodialysis patients with PAD and those without PAD.
Bone metabolism disorders have long been linked to arterial calcification, which can lead to PAD. Previous studies, such as the NEFRONA study,11 have noted lower levels of 25(OH)D in patients with CKD with an ABI ≤0.9. A meta-analysis also revealed reduced 25(OH)D levels in non-hemodialysis patients with PAD, suggesting it could be an independent risk factor for PAD.29 However, a recent study from China found no association between vitamin D deficiency and PAD in middle-aged and elderly diabetic patients. The authors attributed this inconsistency to age differences, as the expression level of the vitamin D receptor decreases linearly with age.30 Notably, our study also observed that patients with PAD were older than those without, consistent with these findings.
Another study by Hsu et al31 reported that parathyroidectomy reduced the risk of PAD in patients on hemodialysis with severe secondary hyperparathyroidism, possibly by lowering parathyroid hormone levels. However, subsequent studies in patients on hemodialysis with PAD found no significant differences in intact parathyroid hormone and 25(OH)D levels, as well as phosphate and calcium levels.10,27,32,33 These results align with our study’s findings. The lack of variation in traditional bone metabolism markers may be attributed to severe mineral metabolism abnormalities or the increased use of phosphate binders and vitamin D supplements in patients on hemodialysis, which might have masked the differences. The current study made an important discovery regarding the relationship between high levels of osteocalcin and PAD in patients on hemodialysis. Osteocalcin, a factor produced by osteoblasts primarily involved in bone mineralization, was found to have additional functions beyond bone regulation, including effects on the pancreas, liver, muscle, adipose tissue, testes, vascular system, as well as the central and peripheral nervous system. This indicates that osteocalcin has an endocrine role outside of the skeletal system.34,35
A study conducted by Wu et al36 demonstrated that osteocalcin can protect against neuronal loss and improve outcomes in patients with acute ischemic stroke. This effect is attributed partly to the inhibition of proline hydroxylase 1 and prevention of gasdermin D degradation. Another clinical study on PAD in patients revealed that bone-like structures containing osteocalcin were present in heavily calcified blood vessels.37
Furthermore, both laboratory experiments and studies in living organisms have shown that osteocalcin is involved in the regulation of vascular calcification through the Wnt/β-catenin signaling pathway and glucose metabolism.38,39 Therefore, the increased levels of osteocalcin observed in this study may indicate an interaction between bone metabolism and vascular calcification, or it could suggest that osteocalcin itself plays a significant role in this process. However, further research is needed to fully understand the underlying mechanism involved. The current investigation had certain constraints. To begin with, it employed a cross-sectional approach and involved a restricted number of patients that might resulted in a weak relationship of osteocalcin with PAD. Secondly, bone turnover markers collected in this study was limited because other markers were not routinely tested in our hemodialysis center. Despite these limitations, our study provides significant understanding regarding the connection between bone metabolism and PAD in patients on hemodialysis. Additional studies with a larger sample size, collected more biomarkers and long-term follow-up are necessary within this population to draw definitive conclusions.
Conclusion
To summarize, the study concluded that there is a significant association between serum osteocalcin levels and PAD in patients undergoing hemodialysis. Traditional markers for bone metabolism did not demonstrate enough sensitivity. These findings suggest that osteocalcin may play a role in the development of PAD in patients on hemodialysis, although the exact mechanism requires further investigation.
Abbreviations
PAD, Peripheral artery disease; ABI, ankle-brachial index; DM, diabetes mellitus; CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D; ESRD, end stage renal disease; CKD, chronic kidney disease.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the Institutional Review Board of Beijing Jishuitan Hospital (202104-24). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgment
We thank all the stuff of the Blood Purification Center and the Department of Vascular Surgery of Beijing Jishuitan Hospital for their assistance in the study. The authors would like to express their gratitude to EditSprings for the expert linguistic services provided.
Funding
This research was supported by Beijing JST Research Funding (Code: QN-202111).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi:10.1016/S0140-6736(13)61249-0
2. Writing Committee M, Gerhard-Herman MD, Gornik HL, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med. 2017;22(3):NP1–NP43. doi:10.1177/1358863X17701592
3. Abola MTB, Golledge J, Miyata T, et al. Asia-Pacific consensus statement on the management of peripheral artery disease: a report from the Asian Pacific Society of Atherosclerosis and Vascular Disease Asia-Pacific Peripheral Artery Disease Consensus Statement Project Committee. J Atheroscler Thromb. 2020;27(8):809–907. doi:10.5551/jat.53660
4. Aktas G, Yilmaz S, Kantarci DB, et al. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgrad Med. 2023;135(5):519–523. doi:10.1080/00325481.2023.2214058
5. Tekce H, Kin tekce B, Aktas G, Tanrisev M, Sit M. The evaluation of red cell distribution width in chronic hemodialysis patients. Int J Nephrol. 2014;2014:754370. doi:10.1155/2014/754370
6. Rajagopalan S, Dellegrottaglie S, Furniss AL, et al. Peripheral arterial disease in patients with end-stage renal disease: observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation. 2006;114(18):1914–1922. doi:10.1161/CIRCULATIONAHA.105.607390
7. Villain C, Ecochard R, Bouchet JL, et al. Relative prognostic impact of nutrition, anaemia, bone metabolism and cardiovascular comorbidities in elderly haemodialysis patients. Nephrol Dial Transplant. 2019;34(5):848–858. doi:10.1093/ndt/gfy272
8. Ambur V, Park P, Gaughan JP, et al. The impact of chronic kidney disease on lower extremity bypass outcomes in patients with critical limb ischemia. J Vasc Surg. 2019;69(2):491–496. doi:10.1016/j.jvs.2018.05.229
9. Ghimire M, Pahari B, Das G, Sharma SK, Das GC. Prevalence of Peripheral Arterial Disease (PAD) in End Stage Renal Disease (ESRD) patients on hemodialysis: a study from central Nepal. Kathmandu Univ Med J. 2014;12(47):181–184. doi:10.3126/kumj.v12i3.13714
10. Kaminski MR, Raspovic A, McMahon LP, et al. Factors associated with foot ulceration and amputation in adults on dialysis: a cross-sectional observational study. BMC Nephrol. 2017;18(1):293. doi:10.1186/s12882-017-0711-6
11. Arroyo D, Betriu A, Valls J, et al. Factors influencing pathological ankle-brachial index values along the chronic kidney disease spectrum: the NEFRONA study. Nephrol Dial Transplant. 2017;32(3):513–520. doi:10.1093/ndt/gfw039
12. Asceric RR, Dimkovic NB, Trajkovic GZ, et al. Prevalence, clinical characteristics, and predictors of peripheral arterial disease in hemodialysis patients: a cross-sectional study. BMC Nephrol. 2019;20(1):281. doi:10.1186/s12882-019-1468-x
13. Lin WC, Tsai JP, Lai YH, et al. Serum osteoprotegerin level is positively associated with peripheral artery disease in patients with peritoneal dialysis. Ren Fail. 2020;42(1):131–136. doi:10.1080/0886022X.2020.1714654
14. Tian SL, Zhang K, Xu PC. Increased prevalence of peripheral arterial disease in patients with obese sarcopenia undergoing hemodialysis. Exp Ther Med. 2018;15(6):5148–5152. doi:10.3892/etm.2018.6002
15. Vuruşkan E, Saraçoğlu E, Küçükosmanoğlu M, Yavuz F, Kuzu Z, Sincer İ. Emergency endovascular treatment of peripheral arterial injuries occurring during the Syrian civil war: gaziantep Dr. Ersin Arslan Education and Research Hospital Experience. Anatol J Cardiol. 2016;16(4):298–304.
16. Rivero M, Nader ND, Blochle R, Harris LM, Dryjski ML, Dosluoglu HH. Poorer limb salvage in African American men with chronic limb ischemia is due to advanced clinical stage and higher anatomic complexity at presentation. J Vasc Surg. 2016;63(5):1318–1324. doi:10.1016/j.jvs.2015.11.052
17. Higashitani M, Uemura Y, Mizuno A, et al. Cardiovascular outcome and mortality in patients undergoing endovascular treatment for symptomatic peripheral artery disease- short-term results of the Toma-Code Registry. Circ J. 2018;82(7):1917–1925. doi:10.1253/circj.CJ-18-0105
18. Norvell DC, Thompson ML, Boyko EJ, et al. Mortality prediction following non-traumatic amputation of the lower extremity. Br J Surg. 2019;106(7):879–888. doi:10.1002/bjs.11124
19. Smith SL, Matthews EO, Moxon JV, Golledge J. A systematic review and meta-analysis of risk factors for and incidence of 30-day readmission after revascularization for peripheral artery disease. J Vasc Surg. 2019;70(3):996–1006 e7. doi:10.1016/j.jvs.2019.01.079
20. Ko T, Higashitani M, Uemura Y, et al. Clinical outcome and diverse risk factors for different therapeutic target locations of peripheral artery disease. J Atheroscler Thromb. 2020;27(8):769–779. doi:10.5551/jat.52647
21. Huang HL, Jimmy Juang JM, Chou HH, et al. Immediate results and long-term cardiovascular outcomes of endovascular therapy in octogenarians and nonoctogenarians with peripheral arterial diseases. Clin Interv Aging. 2016;11:535–543. doi:10.2147/CIA.S106119
22. Huang HL, Tzeng IS, Chou HH, et al. Contemporary cardiovascular outcomes in Taiwanese patients undergoing endovascular therapy for symptomatic lower extremity peripheral arterial disease. J Formos Med Assoc. 2020;119(6):1052–1060. doi:10.1016/j.jfma.2019.10.011
23. Otte J, van Netten JJ, Woittiez AJ. The association of chronic kidney disease and dialysis treatment with foot ulceration and major amputation. J Vasc Surg. 2015;62(2):406–411. doi:10.1016/j.jvs.2015.02.051
24. Bourrier M, Ferguson TW, Embil JM, Rigatto C, Komenda P, Tangri N. Peripheral artery disease: its adverse consequences with and without CKD. Am J Kidney Dis. 2020;75(5):705–712. doi:10.1053/j.ajkd.2019.08.028
25. Moussa Pacha H, Al-Khadra Y, Darmoch F, et al. In-hospital outcome of peripheral vascular intervention in dialysis-dependent end-stage renal disease patients. Catheter Cardiovasc Interv. 2020;95(3):E84–E95. doi:10.1002/ccd.28522
26. Anantha-Narayanan M, Sheikh AB, Nagpal S, et al. Systematic review and meta analysis of outcomes of lower extremity peripheral arterial interventions in patients with and without chronic kidney disease or end stage renal disease. J Vasc Surg. 2020;73(1):331–340.e4. doi:10.1016/j.jvs.2020.08.032
27. Kaminski MR, Lambert KA, Raspovic A, et al. Risk factors for foot ulceration in adults with end-stage renal disease on dialysis: a prospective observational cohort study. BMC Nephrol. 2019;20(1):423. doi:10.1186/s12882-019-1594-5
28. Raikou VD, Kyriaki D. Factors related to peripheral arterial disease in patients undergoing hemodialysis: the potential role of monocyte chemoattractant protein-1. Hypertens Res. 2019;42(10):1528–1535. doi:10.1038/s41440-019-0259-x
29. Iannuzzo G, Forte F, Lupoli R, Di Minno MND. Association of Vitamin D deficiency with peripheral arterial disease: a meta-analysis of literature studies. J Clin Endocrinol Metab. 2018;103(6):2107–2115.
30. Wang Y, Feng T, Zhou H, Lu K, Bai Y, Zhang P. Vitamin D deficiency may not be an independent risk factor for peripheral arterial disease in middle-aged and elderly patients with type 2 diabetes in China. Dis Markers. 2020;2020:8854717. doi:10.1155/2020/8854717
31. Hsu YH, Yu HY, Chen HJ, Li TC, Hsu CC, Kao CH. The risk of peripheral arterial disease after parathyroidectomy in patients with end-stage renal disease. PLoS One. 2016;11(6):e0156863. doi:10.1371/journal.pone.0156863
32. Laucyte-Cibulskiene A, Petraviciute M, Gudynaite M, et al. Mismatch between stiffness in elastic and muscular arteries as a predictor of vascular calcification in dialysis patients. Aging Clin Exp Res. 2018;30(4):375–382. doi:10.1007/s40520-017-0787-7
33. Ishii T, Takabe S, Yanagawa Y, et al. Laser Doppler blood flowmeter as a useful instrument for the early detection of lower extremity peripheral arterial disease in hemodialysis patients: an observational study. BMC Nephrol. 2019;20(1):470. doi:10.1186/s12882-019-1653-y
34. Wang JS, Mazur CM, Wein MN. Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol. 2021;12:584147. doi:10.3389/fendo.2021.584147
35. Berger JM, Karsenty G. Osteocalcin and the physiology of danger. FEBS Lett. 2022;596(5):665–680. doi:10.1002/1873-3468.14259
36. Wu J, Dou Y, Liu W, Zhao Y, Liu X. Osteocalcin improves outcome after acute ischemic stroke. Aging. 2020;12(1):387–396. doi:10.18632/aging.102629
37. Han KH, Hennigar RA, O’Neill WC. The association of bone and osteoclasts with vascular calcification. Vasc Med. 2015;20(6):527–533. doi:10.1177/1358863X15597076
38. Rashdan NA, Sim AM, Cui L, et al. Osteocalcin regulates arterial calcification via altered wnt signaling and glucose metabolism. J Bone Miner Res. 2020;35(2):357–367. doi:10.1002/jbmr.3888
39. Xia LM, Zhang AP, Zheng Q, et al. Quercetin‑3‑O‑β‑D‑glucuronide inhibits mitochondria pathway‑mediated platelet apoptosis via the phosphatidylinositol‑3‑kinase/AKT pathway in immunological bone marrow failure. World J Tradit Chin Med. 2022;8(1):115–122. doi:10.4103/wjtcm.wjtcm_44_21
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
