Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 12
The Relationship Between Aspartate Aminotransferase To Alanine Aminotransferase Ratio And Metabolic Syndrome In Adolescents In Northeast China
Authors Lin S, Tang L, Jiang R, Chen Y , Yang S, Li L , Li P
Received 25 May 2019
Accepted for publication 26 September 2019
Published 18 November 2019 Volume 2019:12 Pages 2387—2394
DOI https://doi.org/10.2147/DMSO.S217127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Shuang Lin,1,2 Lei Tang,1 Ranhua Jiang,3 Yu Chen,1 Sheng Yang,1 Ling Li,1 Ping Li1
1Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, People’s Republic of China; 2Department of Cardiology, Zhongyi Northeast International Hospital, Shenyang, Liaoning Province, People’s Republic of China; 3Department of Endocrinology, Liaoyang Diabetes Hospital, Liaoyang, Liaoning Province, People’s Republic of China
Correspondence: Ping Li
Department of Endocrinology, Shengjing Hospital of China Medical University, No.39, Huaxiang Road, Tiexi District, Shenyang 110022, Liaoning Province, People’s Republic of China
Tel +86 18940255673
Email [email protected]
Ling Li
Department of Endocrinology, Shengjing Hospital of China Medical University, No.36, Sanhao Street, Heping District, Shenyang 110004, Liaoning Province, People’s Republic of China
Tel +86 18940251181
Fax +86 02425944460
Email [email protected]
Aim: To investigate the relationship of the aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT) and metabolic syndrome (MetS) in adolescents in northeast China.
Methods: A stratified cluster random sample of 935 students 11–16 years of age in a city in the northeast of China were enrolled in 2010–2011. Participants were given a physical examination and a laboratory evaluation, and 93 participants were followed-up after 5 years.
Results: AST/ALT was negatively correlated with waist circumference (WC), waist-to-hip ratio, body mass index (BMI), diastolic blood pressure, triglycerides, low-density lipoprotein, uric acid, fasting insulin, and insulin resistance. It was positively correlated with high-density lipoprotein. Multivariate logistic regression showed that the risk of MetS was 6.02 times greater in adolescents with the lowest, compared with the highest, AST/ALT. Central obesity was the MetS component most closely associated with low AST/ALT [odds ratio (OR) =5.13, 95% CI: 2.83, 9.28]. Five years later, baseline AST/ALT was negatively correlated with WC (r=−0.21, P=0.046), BMI (r=−0.29, P=0.005) and fasting plasma glucose (r=−0.25, P=0.017).
Conclusion: In adolescents, AST/ALT was significantly associated with MetS and its components and predicted overweight/obesity in adulthood.
Keywords: metabolic syndrome, aspartate aminotransferase, alanine aminotransferase, adolescents
Introduction
The annual incidence of metabolic syndrome (MetS) in children and adolescents in China is increasing.1 Early diagnosis of MetS is complicated2 because the syndrome includes diverse components including blood lipids, blood glucose, and blood pressure that must be evaluated at the same time. The availability of easily measurable serum markers that are closely associated with MetS would be of great help for early recognition of MetS. MetS can lead to abnormal hepatocyte metabolism, fat deposition, and necrosis which is related to the necrosis and abnormal metabolism of hepatocytes.3 Changes in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) reflect hepatocyte injury and liver function. Both ALT and AST activity increase with liver injury and disease, but not in parallel. Elevated transaminase activity is indicative of liver disease, but the AST/ALT ratio is correlated with severity and prognosis. Studies in adults4–6 have shown that AST/ALT can be used as a marker of multiple liver diseases, including nonalcoholic fatty liver disease, which is closely related to MetS. Adolescents who are still growing and developing have different metabolic characteristics than adults, and there are no published on the association of AST/ALT and the presence of MetS in adolescents in China. There are also no prospective studies of changes in AST/ALT and its relation to MetS when adolescents enter adulthood. This study enrolled adolescents at 11–16 years of age in Liaoyang, a city in northeast China with medium-level economic development, with follow-up after 5 years. The aim was to analyze the relationship between AST/ALT and MetS, and its related components to add to our understanding of the pathophysiological role of transaminases in metabolic diseases, and to provide a basis for novel serum markers of MetS in adolescents.
Subjects And Methods
Study Design
Between December 2010 and January 2011, junior and senior high school students in Liaoyang, were selected by stratified cluster sampling. Study questionnaires were sent to 3236 students, and written informed consent was given by a legal guardian. A total of 935 students from 11–16 years of age with complete data were included in the statistical analysis. The participants had no history of anemia, diabetes, hypertension, or drug therapy; 47.5% were girls. This study was conducted in accordance with the Decaration of Helsinki and was approved by the Medical Ethics Committee of Shengjing Hospital, China Medical University.
Venous blood samples were collected at 7:00–9:00 in the morning after a ≥10h fast. Height, weight, waist circumference (WC), and hip circumference were measured by a trained physician before blood sample collection. After sitting quietly for more than 10 mins, blood pressure was measured twice using a desktop mercury sphygmomanometer and a 2 min interval between measurements. The average systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. Blood samples were transported to a central laboratory at Liaoyang Diabetes Hospital. Plasma was obtained by centrifugation within 1 hr of collection. Fasting plasma glucose (FPG) was assayed by the glucose oxidase method (Olympus 400, Olympus Optical Company, Japan) within 2 hrs of centrifugation. Serum low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), AST, ALT and uric acid were determined by standard enzymatic methods. Some plasma was stored at −80ºC for assay of fasting insulin (FINS) and high sensitivity C reactive protein by radioimmunoassay at the China Institute of Atomic Energy, Beijing, China. Body mass index (BMI) was calculated and reported as kg/m2. The homoeostatic model assessment of insulin resistance was calculated as HOMA-IR = fasting blood glucose (mmol/L) × fasting insulin (μU/mL)/22.5. In July 2016, after approximately5 years, the subjects were followed-up. As most of the young students had gone to universities in other cities, only 93, 18–22 years of age, and 46.2% women, were evaluated. The methods and investigators were the same as at baseline. None of the 93 subjects had been diagnosed with a new chronic disease or had begun taking long-term medications use during the 5 years.
Diagnostic Criteria
MetS was diagnosed at baseline in the 11–16-year-old adolescents using the 2007 International Diabetes Federation (IDF) diagnostic criteria.7 Obesity was defined as a WC ≥90th percentile (depending on race) and at least two of the following: (1) FPG ≥5.6 mmol/L or a previous diagnosis of type 2 diabetes; (2) SBP ≥130mmHg or DBP ≥85mmHg; (3) HDL-C <1.03 mmol/L; (4) TG ≥1.70 mmol/L. For subjects >16 years of age, IDF adult diagnostic criteria were used, as described below.
At follow-up 5 years later, all subjects >18 years of age, and MetS was diagnosed with IDF adult criteria.8 Central obesity includes a WC >90 cm in Chinese men and >80 cm in Chinese women. Adult obesity also required two of the following: (1) TG ≥1.7 mmol/L (150 mg/dL), or on a specific treatment for that lipid abnormality; (2) HDL-C <1.03 mmol/L (40 mg/dL) in men and <1.29 mmol/L (50 mg/dL) in women, or on a specific treatment for that lipid abnormality; (3) SBP ≥130mmHg or DBP ≥85mmHg, or treatment of previously diagnosed hypertension; (4) FPG ≥5.6 mmol/L (100 mg/dL), or a previous diagnosis of type 2 diabetes.
At baseline, the subjects were stratified to a normal BMI group and an overweight/obese group following the BMI criteria for Chinese children and adolescents of the 2004 Chinese Working Group on Obesity.9 At the 5-year follow-up, the subjects had entered into early adulthood, and those with a BMI ≥24 were defined as overweight/obese in accord with the recommended standard for Chinese adults.10
Statistical Analysis
Statistical analysis was performed using SPSS17.0 software for Windows (SPSS Inc., Chicago, IL, USA). The values of normally distributed variables were compared by the Kolmogorov–Smirnov test. Variables not normally distributed were logarithmically transformed. Normally distributed continuous variables were expressed as means ± standard deviation. Variables not normally distributed were reported as medians and interquartile range (IQR). Categorical data were expressed as percentages and compared by the chi square test. Analysis of variance was used for multiple comparison of normally distributed data. The Kruskal–Wallis H-test was used for multiple comparisons of non-normally distributed data. One-way generalized linear model analysis was used multiple group comparisons after correcting for confounding variables. Partial correlation was used to analyze the relationships of AST/ALT, MetS and other cardiovascular risk factors at baseline and after 5 years. Multiple logistic regression was used to analyze the association of baseline AST/ALT level and MetS, MetS components, and other cardiovascular risk factors at baseline and after 5 years. Differences with P <0.05 were considered statistically significant.
Results
The Cross-Sectional Study
As shown in Figure 1, the mean AST/ALT ratio was significantly higher in girls than boys (1.93 ± 0.04 vs. 1.61 ± 0.03, P<0.001) (Figure 1). The AST/ALT ratio was higher in girls than in boys regardless of the number of MetS components that were present, and it decreased with an increase in the number of MetS components (Figure 2). Table 1 shows the clinical characteristics of study subjects stratified into three AST/ALT tertiles from low to high, where T3 had the highest value. As AST/ALT decreased, the prevalence of MetS increased, and the percentage of boys with MetS increased (both P<0.001). After adjusting for age and gender, TG, SBP, waist-to-hip ratio (WHR), BMI, LDL-C, ALT, and uric acid all increased (P<0.01) with the decrease of AST/ALT, and FINS and HOMA-IR were increased (P<0.001). After adjusting for age and gender the AST/ALT ratio was negatively correlated with WC, WHR, BMI, DBP, TG, LDL-C, FINS, HOMA-IR and uric acid, and positively correlated with HDL-C (r=−0.44 to 0.07, P<0.05). The correlation of AST/ALT and BMI (r=−0.44, P<0.001) was the strongest, and that with HDL-C (r=0.07, P=0.026) was the weakest (Table 2).
 |
Table 1 Clinical Characteristics Of The Study Subjects By AST/ALT Tertiles |
 |
Table 2 Relation Of AST/ALT And MetS Risk Factors |
 |
Figure 1 AST/ALT in boys and girls. |
 |
Figure 2 Mean AST/ALT in girls and boys stratified by the number of MetS components. |
Logistic regression analysis (Table 3) using group T3 as the reference revealed that group T1 patients, who had the lowest AST/ALT ratio, had a significantly increased risk of MetS [odds ratio (OR) =5.65, 95% confidence interval (CI): 2.70, 11.82], which persisted after adjusting for age, gender, family history of diabetes, HOMA-IR, and high sensitivity C reactive protein (OR=6.02, 95% CI: 1.93, 18.76). The most closely related MetS component was central obesity (OR=5.13, 95% CI: 2.83, 9.28), followed by hypertension (OR=1.70, 95% CI: 1.07, 2.72) and hypertriglyceridemia (OR=2.45, 95% CI: 1.08, 5.52). Decreased AST/ALT ratio was associated with increased risk of central obesity (OR=5.13, P<0.001), hypertension (OR=1.70, P=0.008), hypertriglyceridemia (OR=2.45, P=0.018), and overweight/obesity (OR=9.18, P<0.001) (Figure 3).
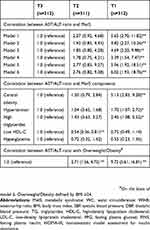 |
Table 3 Association Of AST/ALT With MetS, MetS Components And Overweight/obesity |
 |
Figure 3 Odds ratios (ORs) of MetS components and overweight/obesity at baseline. |
Follow-Up Study
Of the 93 subjects who participated in the follow-up study, the 27 who were in group T1 at baseline and renamed group T1-F at follow-up. Similarly, group T2-F included 32 subjects, and group T3-F included 35. There were no significant differences in the clinical characteristics and baseline serum laboratory test values in the 93 follow-up subjects and those enrolled in the original investigation, but did not participate in the follow-up evaluation (Supplemental Table 1). The follow-up subjects were thus representative of the entire study population. The decrease in baseline AST/ALT from T3-F to T1-F, was associated with increase of WC, TG, and BMI at 5 years (all P<0.01) (Table 4). The results of partial correlation analysis of AST/ALT and MetS risk factor values after 5 years are shown in Table 5. After adjusting for age and gender, baseline AST/ALT was found to have significant negative linear correlations with WC (r=−0.21, P=0.046), BMI (r=−0.29, P=0.005) and FPG (r=–0.25, P=0.017). Using the T3-Ftertile, which had the highest AST/ALT ratio, as the reference, logistic regression analysis (Table 6) showed that after adjusting for age and gender, the T1-F group, which had the lowest baseline AST/ALT ratio, was at increased risk of MetS and the presence of MetS components at 5 years, but the increase was not significant (Figure 4). As only one of the 93 patients had high blood glucose, hyperglycemia was not included in the logistic regression analysis. Figure 4 shows that baseline AST/ALT was significantly correlated with the risk of overweight/obesity after 5 years. The T1-F group had a significantly higher risk of overweight/obesity compared with the T3-F group (OR=4.86, 95% CI: 1.54, 15.30, P<0.01).
 |
Table 4 Clinical Characteristics Of Subjects At 5-Year Follow-Up By AST/ALT Tertiles |
 |
Table 5 Relation Of AST/ALT And MetS Risk Factors After 5 Years |
 |
Table 6 Association Of AST/ALT, MetS, And MetS Components After 5 Years |
 |
Figure 4 Odds ratios (ORs) of MetS components and overweight/obesity after 5 years. |
Discussion
This cross-sectional study found that in this group of 935 adolescents from Liaoyang China those with decreased AST/ALT ratios were at increased risk of MetS. The value of the AST/ALT ratio was negatively correlated with levels of cardiovascular risk factors as BMI, LDL-C, systolic BP and HOMA-IR. Decreased AST/ALT ratio was an independent risk factor of MetS and MetS components, and central obesity, hypertension, and hypertriglyceridemia were the most closely associated with the AST/ALT value. The prospective study showed that the baseline AST/ALT value was negatively correlated with BMI and some MetS componentsat5 years, and that the AST/ALT at adolescence was predictive of overweight/obesity in early adulthood.
AST/ALT is an indicator of damage of liver cells, and is useful in evaluating the severity of liver fibrosis and cirrhosis,4,11,12 and the clinical significance of the AST/ALT ratio has been well described,13 and previous studies in Asia14-16 reported that the AST/ALT ratio was more accurate in predicting the risk of MetS or MetS components than AST or ALT alone. However, few data are available on the relationship of AST/ALT and MetS in children and adolescents,17,18 and there have no studies have been conducted in Chinese teenagers. This is the first study to analyze the relationship between AST/ALT and MetS components in Chinese adolescents, and to investigate the ability of AST/ALT at adolescence to predict adult overweight/obesity and other risk factors for cardiovascular disease. There was a significant gender difference in the level of AST/ALT ratio, and regardless of the number of MetS components, the AST/ALT ratio was higher in female than in male subjects. That result differed from the findings of a previous report in Mexican children.18 In this study, baseline data in adolescents was predictive of obesity and metabolic abnormalities in adulthood. However, an association of adolescent the AST/ALT ratio and MetS in adulthood was not observed at the 5-year follow-up, which was probably the result of different MetS diagnosis criteria in adolescence and adulthood. Nevertheless, the close relationship of the baseline AST/ALT value and BMI after 5 years supports the ability of AST/ALT to predict abnormal metabolism in adulthood.
In both adolescence and adulthood, WC, TG and BMI were higher in subjects with a low baseline AST/ALT than in those with a high baseline AST/ALT, which suggests that the correlation of AST/ALT and MetS may be mediated by abdominal obesity and high blood TG. AST/ALT was also negatively correlated with insulin resistance (r=−0.032, P<0.001), which is consistent with the involvement of abdominal obesity and insulin resistance in the pathogenesis of MetS.19 Liver enzymes are markers of liver fat content, a fatty liver is a sign of increased visceral fat, and increased visceral fat content is related to insulin resistance. It is thus plausible that the AST/ALT ratio is indicative of visceral fat inflammation, insulin resistance, and predictive of MetS and overweight/obesity.
The study has limitations. First, because there was no screening of hepatitis viruses or ultrasound examination of the liver, gall bladder, and spleen, elevation of liver enzyme levels caused by other reasons cannot be completely ruled out. Second, the relatively small number of subjects who were evaluated at followed-up was relatively small, which may have introduced study bias. Expanding the follow-up sample size and duration will help further clarify the relationship between AST/ALT and metabolic diseases during the development from adolescence to adulthood. The prevalence of obesity, type 2 diabetes, and other metabolic diseases in Chinese adolescents is increasing.20 The AST/ALT ratio would be useful for epidemiological screening and as a simple and effective serum marker of MetS. It also has practical value in adolescence to help predict metabolic status in adulthood.
Acknowledgments
The authors are grateful to all children and their parents for participating in this study. This work was supported by Fund for young scientists of the National Natural Science Foundation of China (grant number 81600644).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu D, Zhao L, Ma G, et al. Prevalence of metabolic syndrome among 7−17 year-old overweight and obese children and adolescents. Wei Sheng Yan Jiu. 2012;41:410–413.
2. Cooke AA, Connaughton RM, Lyons CL, McMorrow AM, Roche HM. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur J Pharmacol. 2016;785:207–214. doi:10.1016/j.ejphar.2016.04.021
3. Najjar SM. Non-alcoholic fatty liver disease and the metabolic syndrome. In: Ahima RS, editor. Metabolic Basis of Obesity. New York: Springer; 2011:219–227.
4. Chowdhury SD, Ramakrishna B, Eapen CE, et al. Fibrosis in non-alcoholic fatty liver disease: correlation with simple blood indices and association with tumor necrosis factor-alpha polymorphisms. Trop Gastroenterol. 2013;34:31–35.
5. Homsanit M, Sanguankeo A, Upala S, Udol K. Abnormal liver enzymes in Thai patients with metabolic syndromes. J Med Assoc Thai. 2012;95:444–451.
6. Tzima N, Pitsavos C, Panagiotakos D, et al. Adherence to the Mediterranean diet moderates the association of aminotransferases with the prevalence of the metabolic syndrome; the ATTICA study. Nutr Metab. 2009;6:30. doi:10.1186/1743-7075-6-30
7. Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–2061. doi:10.1016/S0140-6736(07)60958-1
8. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi:10.1111/j.1464-5491.2006.01858.x
9. Working Group on Obesity in China. Body mass index reference norm for screening overweight and obesity in Chinese children and adolescents. Chin J Epidemiol. 2004;25:97–102.
10. Obesity Task Force Data Collaborative Analysis Group in China. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin J Epidemiol. 2002;23:5–10.
11. Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. doi:10.1023/a:1026609231094
12. Fouad SA, Esmat S, Omran D, Rashid L, Kobaisi MH. Noninvasive assessment of hepatic fibrosis in Egyptian patients with chronic hepatitis C virus infection. World J Gastroenterol. 2012;18:2988–2994. doi:10.3748/wjg.v18.i23.2988
13. Mohammadi F, Qorbani M, Kelishadi R, et al. Association of cardiometabolic risk factors and hepatic enzymes in a national sample of Iranian children and adolescents: the CASPIAN-III study. J Pediatr Gastroenterol. 2014;58:463–468. doi:10.1097/MPG.0000000000000246
14. Yadav D, Choi E, Song VA, et al. Incremental predictive value of serum AST-to-ALT ratio for incident metabolic syndrome: the ARIRANG Study. PLoS One. 2016;11(8):e0161304.
15. Kawamoto R, Kohara K, Kusunoki T, et al. Alanine aminotransferase/aspartate aminotransferase ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Cardiovasc Diabetol. 2012;11:1–8. doi:10.1186/1475-2840-11-117
16. Lu Q, Liu X, Liu S, et al. The relationship between AST/ALT ratio and metabolic syndrome in Han young adults - AST/ALT ratio and metabolic syndrome, Mehnaz A, recent advances in cardiovascular risk factors. InTech. 2012;247–254.
17. Lee K, Yang JH. Which liver enzymes are better indicators of metabolic syndrome in adolescents: the fifth Korea national health and nutrition examination survey, 2010. Metab Syndr Relat Disord. 2013;11:229–235. doi:10.1089/met.2012.0153
18. Elizondo ML, Ugalde PA, Lam L, et al. Association of ALT and the metabolic syndrome among Mexican children. Obes Res Clin Pract. 2014;8:79–87. doi:10.1016/j.orcp.2012.08.191
19. Hanley AJ, Wagenknecht LE, Festa A, D’Agostino RB, Haffner SM. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: the insulin resistance atherosclerosis study. Diabetes Care. 2007;30:1819–1827. doi:10.2337/dc07-0086
20. Viner R, White B, Christie D. Type 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burden. Lancet. 2017;389:2252. doi:10.1016/S0140-6736(17)31371-5
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
