Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
The Protective Role and Mechanism of Mild Therapeutic Hypothermia Protection on Brain Cells
Authors Liang S, Ti Y, Li X, Zhou W
Received 12 March 2023
Accepted for publication 22 June 2023
Published 17 July 2023 Volume 2023:19 Pages 1625—1631
DOI https://doi.org/10.2147/NDT.S412227
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Suixin Liang,1 Yunxing Ti,2 Xiuhong Li,1 Wenjia Zhou1
1Department of CICU, Shenzhen Children’s Hospital, Shenzhen City, Guangdong Province, People’s Republic of China; 2Department of Cardiothoracic Surgery, Shenzhen Children’s Hospital, Shenzhen City, Guangdong Province, People’s Republic of China
Correspondence: Suixin Liang, Department of CICU, Shenzhen Children’s Hospital, 7019#, Yitian Road, Futian District, Shenzhen City, Guangdong Province, 518038, People’s Republic of China, Email [email protected]
Background: Moderate therapeutic hypothermia is protective against several cellular stressors. However, the mechanisms behind this protection are not entirely known. In the current investigation, we investigated that therapeutic hypothermia at 33°C administered following peroxide-induced oxidative stress might protect human oligodendroglioma cells using an in vitro model.
Methods and Results: Tert-butyl peroxide treatment for one hour significantly increased cell apoptosis and suppressed cell viability. In the range of 50– 1000 M tert-butyl peroxide, this cell death was dose-dependent. MTT assay and cell apoptosis assay were applied to analyze cell viability/death at 24 hours after peroxide-induced stress. Therapeutic hypothermia at 33°C delivered for two hours after peroxide exposure significantly increased cell viability and suppressed cell death. Even 15 minutes after peroxide washout when delayed hypothermia was used, this protection was still apparent. Three FDA-approved antioxidants (Tempol, EUK134, and Edaravone at 100 M) were added immediately after tert-butyl peroxide, followed by hypothermia treatment. These three antioxidants greatly increased cell viability and cell apoptosis. RT-qPCR was applied to determine the effects of hypothermia treatment on the expression of caspase-3 and − 8 as well as tumor necrosis factor-alpha (TNF-α). Therapeutic hypothermia significantly downregulated these three factors.
Conclusion: Overall, these findings confirmed that hypothermia and antioxidants quenching reactive oxygen species may lower mitochondrial oxidative stress and/or apoptotic pathways. Further investigation are needed to investigate the role of hypothermia in other cell models.
Keywords: peroxide-induced oxidative stress, moderate therapeutic hypothermia, cell viability
Introduction
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality across all age groups, affecting nearly 50 million individuals worldwide each year.1,2 Hypothermia has long been used as a therapeutic intervention in individuals with severe brain injury, with the goal of reducing body temperature to that of the head or the entire body.3–5 This approach has been shown to improve neuronal survival in the brain and other tissues following ischemia-reperfusion, and its neuroprotective properties have been demonstrated to mitigate further damage caused by brain trauma, stroke, and spinal cord injury.6,7 Mild therapeutic hypothermia also served as a neuroprotective treatment following spinal cord injury, with notable results observed in both preclinical and clinical settings.
The neuroprotective mechanisms of hypothermia are complex and multifaceted, including reduction in brain metabolic rate, alterations in cerebral blood flow, a lowering of the critical oxygen transport threshold, attenuation of oxidative stress-induced processes, calcium antagonistic effects, and others.8–10 Elevated levels of peroxide can result in increased cytosolic and mitochondrial Ca2+ levels, leading to mitochondrial overload and damage to DNA.11,12 Hypothermia treatment has been shown to modulate calcium levels in several animal models.13–15 Therefore, it is reasonable to speculate that therapeutic hypothermia may protect against brain cell injury by regulating Ca2+ levels in cells exposed to peroxide. However, the interplay between therapeutic hypothermia and peroxide is unknown. This study aims to investigate the potential protective effects of therapeutic hypothermia on cells under peroxide treatments.
Materials and Methods
Cell Culture
The HOG cell line, which is derived from human oligodendroglioma, is frequently employed by scientists to investigate the effects of hypothermia on brain cells. The HOG cells (SCC163, Sigma-Aldrich) were cultured in growth medium (GM) composed of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (50 U/mL), and streptomycin (50 g/mL) in a 5% CO2 atmosphere at 37 °C. The cells were grown on Petri plates to allow for cell attachment and growth.
Treatment with Tert-Butyl Peroxide (TbH2O2)
To treat HOG cells, cell culture medium was supplemented with different concentrations of tert-butyl hydroperoxide (TbH2O2), including 50, 100, 200, 500, and 1000 µm, for 60 minutes at 37 °C. TbH2O2 has been commonly used to study the underlying mechanisms of oxidative stress. After washing the cells with fresh medium three times, they were divided into three groups. The first group was continuously cultured at 37 °C for 2 hours, while the second group was exposed to hypothermia treatment at 33 °C immediately after TbH2O2 washout, followed by a 2-hour culture at the same temperature. The third group received the same hypothermia treatment for 2 hours, followed by cell culture at 37 °C. After 24 hours, cell viability and apoptosis were analyzed to determine the effects of the treatments. It is worth noting that the survival rates and apoptotic indices were assessed using multiple approaches.
Combination Therapy
In this study, we examined the effects of mild hypothermia treatment in combination with various antioxidants, namely EUK-134 (100 μM), tempol (100 μM), or edaravone (Edr, 100 μM) purchased from Millipore Sigma. After subjecting the cells to TbH2O2 stress, the antioxidants were added, and mild hypothermia treatment was initiated, followed by a 24-hour incubation in the F-12K media. The first two groups mentioned in the “Treatment with tert-butyl Peroxide (TbH2O2)” section were included in this analysis. The purpose of the investigation was to investigate the potential beneficial effects of the antioxidant treatment in combination with mild hypothermia treatment on the cells under TbH2O2 stress.
MTT Assay
HOG cells were cultivated, and their viability was assessed using the MTT assay, following the manufacturer’s instructions. Specifically, 5000 cells were seeded into each well of a 96-well plate and left to adhere overnight. After that, 20 µL of 5 mg/mL MTT (Sigma-Aldrich, St Louis, MO, USA) was added to each well and the cells were incubated for 4 hours. Following this, the supernatant was discarded and 200 µL of dimethyl sulfoxide was added to each well to dissolve the formazan crystals. The absorbance was measured at 490 nm using an enzyme immunoassay analyzer (Bio-Rad Laboratories) to determine cell viability. To explore the effects of tert-butyl hydroperoxide (TbH2O2) on HOG cells, we introduced varying concentrations of TbH2O2 into the cell culture medium. The concentrations used were 0, 50, 100, 200, 500, and 1000 µM. Subsequently, the cells were incubated at 37 °C for a duration of 60 minutes.
Cell Apoptosis Assay
The HOG cells were collected and washed twice with ice-cold PBS. Next, the cells were stained using the Annexin V-FITC/PI detection kit obtained from BD Biosciences in San Diego, CA, USA. The stained cells were then incubated for 15 minutes at room temperature in the dark. The proportion of apoptotic cells was then measured using a flow cytometer provided by BD Biosciences. To examine the impact of tert-butyl hydroperoxide (TbH2O2) on HOG cells, we added different concentrations of TbH2O2 (0, 50, 100, 200, 500, and 1000 µM) to the cell culture medium. Subsequently, the cells were incubated at 37 °C for 60 minutes.
RNA Extraction and RT-qPCR
To isolate RNA from HOG cultures, cells were washed with 1x PBS prior to RNA extraction. Trizol (Invitrogen) was used to extract RNA from the lysate, which was disrupted by cell resuspension and mixed with 2x denaturing solution. The RNA purification process involved acid-phenol: chloroform extraction, which removes most DNA, followed by the addition of 100% ethanol and filtering through a glass-fiber filter. The filter was then washed three times before RNA was eluted with warm sterile water (Ultrapure DEPC-Treated; Invitrogen) in a volume of 50–100 μL. The extracted RNA samples were used for cDNA synthesis using SSRT IV (ThermoFisher Scientific 4368814), and gene expression assays were quantified by qPCR using TaqMan assays. TNF-α, Caspase-3, and Caspase-8 (Applied BioSystems) were the genes of interest. Data was normalized to GAPDH mRNA as the endogenous reference, and quantitation of data was performed using the normalized cycle threshold method, and the data was converted to fold-change relative to the control group.
Statistical Analysis
The statistical analyses for this study were performed using GraphPad Prism 6 software. To compare multiple groups, ANOVA followed by post hoc Tukey’s test was employed. For comparison of two groups, an unpaired t-test was utilized. All experiments were performed in triplicate biological replicates to ensure reproducibility of results. Data were expressed as mean ± standard error. A significance level of P<0.05 was considered to indicate statistically significant differences between groups.
Results
The Effects of Tert-Butyl Peroxide Treatment on Cell Viability and Apoptosis
To investigate the effects of tert-butyl hydroperoxide (TbH2O2) on HOG cells, we supplemented the cell culture medium with varying concentrations of TbH2O2, namely 0, 50, 100, 200, 500, and 1000 µM, and incubated the cells at 37 °C for 60 minutes. After the treatment, cells were cultivated for 26h and cell viability and apoptosis were examined using the MTT assay and cell apoptosis assay, respectively. Our results showed that the one-hour treatment of tert-butyl peroxide significantly inhibited cell viability (Figure 1A, p<0.01), while promoting cell apoptosis (Figure 1B, p<0.01). Furthermore, we found that the increase in cell death was dependent on the dose of tert-butyl peroxide within the range of 50–1000 µM. Our experiments were performed in triplicate.
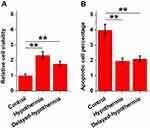
The Effects of Hypothermia on the Survival and Apoptosis of HOG Cells with 100 µm TbH2O2 Treatment for 60 Min
To investigate the effect of hypothermia on the survival and apoptosis of HOG cells treated with TbH2O2, the cells were first cultured in a medium containing 100 µM TbH2O2 at 37 °C for 60 minutes. Then, the cells were divided into three groups: the control group, the hypothermia group, and the delayed-hypothermia group. The control group was continuously cultured at 37 °C for 26 hours, while the hypothermia group was subjected to hypothermia treatment (33 °C) for 2 hours followed by cell culture at 37 °C for another 24 hours. The same hypothermia treatment was also performed on delayed-hypothermia group at 15 min after TbH2O2 (100 µM) treatment. The cell viability and apoptosis were evaluated using the MTT assay and cell apoptosis assay, respectively. Our results demonstrated that, compared to the control group, the hypothermia group exhibited significantly increased cell viability (Figure 2A, p<0.001) and significantly reduced cell apoptosis (Figure 2B, p<0.01).
Combined Therapy with Antioxidants Further Improved Cell Viability and Suppressed Cell Viability
The effects of mild hypothermia treatment in combination with three antioxidants, namely EUK-134 (100 μM), tempol (100 μM), or edaravone (Edr, 100 μM) were analyzed. After subjecting the cells to TbH2O2 stress, the antioxidants were added into one group of cells, and mild hypothermia treatment was initiated, followed by a 24-hour incubation in the F-12K media (antioxidants-hypothermia group). In other group, hypothermia treatment was performed without the addition of antioxidants (hypothermia group). Compared to hypothermia group, the addition of EUK-134, tempol, or edaravone significantly increased cell viability (Figure 3A, p<0.01) and decreased cell apoptosis (Figure 3B, p<0.01).
The Effects of Hypothermia Treatment on the Expression of Caspase-3, Caspase-8 and TNF-α
To examine the effect of hypothermia on the expression of caspase-3, caspase-8, and TNF-α, RT-qPCR analysis was performed. HOG cells were divided into two groups. The cells in the control group were incubated at 37 °C for 2 hours, while cells in the hypothermia group were treated with hypothermia at 33 °C for 2 hours. RNA extraction was carried out, followed by RT-qPCR analysis. The results revealed that hypothermia treatment significantly decreased the expression levels of caspase-3 (Figure 4A, p<0.01), caspase-8 (Figure 4B, p<0.01), and TNF-α (Figure 4C, p<0.01).
Discussion
In this study, using peroxide-treated human oligodendroglioma cells as an in vitro model, the effects of therapeutic hypothermia on brain cell injury were explored. We found that therapeutic hypothermia treatment for 2h after peroxide treatment can significantly improve the survival of brain cells.
Oxygen-containing compounds including peroxide are known to induce cell apoptosis to cause organ injury.16 In effect, hydrogen peroxide is involved in many human diseases.17 Therefore, protecting cells from peroxide-induced cell apoptosis and improve cell survival is the key for the treatment of many diseases. Oligodendroglioma cells are frequently used as a cell model to study brain injury.18 In this study, hydrogen peroxide treatment significantly reduced the viability of oligodendroglioma cells and increased the apoptosis of those cells (Figure 1). Gene expression analysis showed that hypothermia treatment significantly decreased the expression levels of caspase-3, caspase-8, and TNF-α (Figure 4). Therefore, hypothermia treatment can suppress cell apoptosis pathways to promote cell survival.
The modulation of calcium levels by hypothermia has been observed in several animal models.13–15 Calcium levels can also be affected by peroxide.11,12 However the interplay between therapeutic hypothermia and peroxide is unknown. In this study, hypothermia treatment immediately after hydrogen peroxide treatment significantly improved cell survival. Although, delayed hypothermia treatment also showed protective effects on these cells, the protective effects are reduced (Figure 3). Our study confirmed that hypothermia treatment played protective effects on peroxide-induced brain injury, and this treatment should be carried out timely to prevent potential irreversible brain injuries.
Antioxidant therapies are frequently used in clinical practices to treat traumatic brain injuries.19–21 In addition, the use of antioxidant in newborns has also been extensively investigated.22,23 However, studies on the role of of combined antioxidant therapies and hypothermia treatment in the recovery of traumatic brain injuries are relatively rare. In this study, compared to cells only received hypothermia treatment, the addition of antioxidant EUK-134, tempol, or edaravone significantly increased cell viability and decreased cell apoptosis. Our data suggest that the combined antioxidant and hypothermia therapies may be applied in clinical practices to further promote the recovery of brain injuries and inhibit brain damages.
In conclusion, hypothermia therapy can protect brain cells from hydrogen peroxide-induced damages. Combination with antioxidant is recommended in clinical practices to improve brain injury recovery.
Data Sharing Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of Shenzhen Children’s Hospital. All experimental protocols were approved by Shenzhen Children’s Hospital Ethics committee. The research has been carried out in accordance with the World Medical Association Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
We thank the financial support from Shenzhen Science and Technology Planning Project (JCYJ20190809171015673).
Disclosure
The authors declare that they have no competing interest.
References
1. Risdall JE, Menon DK. Traumatic brain injury. Philosop Transac Royal Soc B. 2011;366(1562):241–250. doi:10.1098/rstb.2010.0230
2. Jiang J-Y, Gao G-Y, Feng J-F, et al. Traumatic brain injury in China. Lancet Neurol. 2019;18(3):286–295. doi:10.1016/S1474-4422(18)30469-1
3. Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury—mechanisms and practical aspects. Nat Rev Neurol. 2012;8(4):214–222. doi:10.1038/nrneurol.2012.21
4. Wu X, Tao Y, Marsons L, et al. The effectiveness of early prophylactic hypothermia in adult patients with traumatic brain injury: a systematic review and meta-analysis. Austr Crit Care. 2021;34(1):83–91. doi:10.1016/j.aucc.2020.05.005
5. Kalisvaart ACJ, Prokop BJ, Colbourne F. Hypothermia: impact on plasticity following brain injury. Brain Circ. 2019;5(4):169–178. doi:10.4103/bc.bc_21_19
6. Wang L, Sun Y, Kong F, et al. Mild hypothermia alleviates complement C5a-induced neuronal autophagy during brain ischemia–reperfusion injury after cardiac arrest. Cell Mol Neurobiol. 2022;9(1):1853.
7. Ransom SC, Brown NJ, Pennington ZA, et al. Hypothermia therapy for traumatic spinal cord injury: an updated review. J Clin Med. 2022;11(6):1585. doi:10.3390/jcm11061585
8. Seitz M, Köster C, Dzietko M, et al. Hypothermia modulates myeloid cell polarization in neonatal hypoxic–ischemic brain injury. J Neuroinflam. 2021;18(1):266. doi:10.1186/s12974-021-02314-9
9. Wang J, Xu C, Li X, et al. Mechanism of mild hypothermia promoting nerve regeneration after traumatic brain injury in rats. Chin J Trauma. 2019;2019:274–281.
10. Thabet F, Tabarki B. Therapeutic hypothermia in children: which indications remain in 2018? Archives de Pédiatrie. 2019;26(5):308–311. doi:10.1016/j.arcped.2019.05.010
11. Wu Y, Song J, Wang Y, Wang X, Culmsee C, Zhu C. The potential role of ferroptosis in neonatal brain injury. Front Neurosci. 2019;13:115. doi:10.3389/fnins.2019.00115
12. Chan PH. The role of oxygen radicals in brain injury and edema. Cell Antioxidant Def Mech. 2019;2019:89–109.
13. Averin AS, Zakharova NM, Ignatiev DA. The effect of the extracellular Ca2+ concentration on the force–frequency dependence in the myocardium of the guinea pig: potentiation by a pause under pronounced hypothermia. Biophysics. 2021;66(6):1011–1015. doi:10.1134/S0006350921060026
14. Nilsen JH, Schanche T, Kondratiev TV, Hevrøy O, Sieck GC, Tveita T. Maintaining intravenous volume mitigates hypothermia-induced myocardial dysfunction and accumulation of intracellular Ca2+. Exp Physiol. 2021;106(5):1196–1207. doi:10.1113/EP089397
15. Chen Q, Xu J, Jin X, Wu C, Li Z, Wang M. Effects of hypothermia on Ca2+∕ calmodulin-dependent protein kinaseII and cell autophagy in brain tissues after cardiac arrest and cardiopulmonary resuscitation in swine. Chin J Anesthesiol. 2019;2019:490–493.
16. L-J S, Zhang J-H, Gomez H, et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. 2019;2019:5080843. doi:10.1155/2019/5080843
17. Pravda J. Hydrogen peroxide and disease: towards a unified system of pathogenesis and therapeutics. Mol Med. 2020;26(1):41. doi:10.1186/s10020-020-00165-3
18. Volpe JJ. Brain injury in the premature infant: neuropathology, clinical aspects, pathogenesis, and prevention. Clin Perinatol. 1997;24(3):567–587. doi:10.1016/S0095-5108(18)30159-3
19. Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7(1):51–61. doi:10.1016/j.nurt.2009.10.021
20. Di Pietro V, Yakoub KM, Caruso G, et al. Antioxidant Therapies in Traumatic Brain Injury. Antioxidants. 2020;9(3):260. doi:10.3390/antiox9030260
21. Shen Q, Hiebert JB, Hartwell J, Thimmesch AR, Pierce JD. Systematic Review of Traumatic Brain Injury and the Impact of Antioxidant Therapy on Clinical Outcomes. Worldviews Evid Based Nurs. 2016;13(5):380–389. doi:10.1111/wvn.12167
22. Perrone S, Negro S, Tataranno ML, Buonocore G. Oxidative stress and antioxidant strategies in newborns. J Matern Fetal Neonatal Med. 2010;23 Suppl 3(sup3):63–65. doi:10.3109/14767058.2010.509940
23. Poggi C, Dani C. Antioxidant strategies and respiratory disease of the preterm newborn: an update. Oxid Med Cell Longev. 2014;2014:721043. doi:10.1155/2014/721043
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.