Back to Journals » OncoTargets and Therapy » Volume 12
The prognostic significance of SHP2 and its binding protein Hook1 in non-small cell lung cancer
Authors He L, Li Y, Huang X, Cheng H, Ke Y, Wang L
Received 28 March 2019
Accepted for publication 26 June 2019
Published 22 July 2019 Volume 2019:12 Pages 5897—5906
DOI https://doi.org/10.2147/OTT.S210223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Lingjuan He,1 Yinyan Li,1 Xin Huang,1 Hongqiang Cheng,2 Yuehai Ke,2 Linrun Wang1
1Department of Pharmacy, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou 310000, Zhejiang, People’s Republic of China; 2Department of Pathology and Pathophysiology, Program in Molecular Cell Biology, Zhejiang University School of Medicine, Hangzhou 310058, People’s Republic of China
Background: We previously reported that Hook1 inhibits the phosphatase activity of SHP2 in the regulation of the epithelial-mesenchymal transition (EMT) in lung cancer. In this study, we performed a comprehensive analysis of SHP2 and Hook1 expression and relationships with the prognosis of patients with non-small cell lung cancer (NSCLC).
Materials and methods: A total of 121 patients with NSCLC were included in this study. Expression of SHP2 and Hook1 was assessed by immunohistochemistry and Western blot analysis. The overall survival rate of NSCLC patients was analysed using Cox’s ratio hazard multivariate analysis and the log-rank test.
Results: In tumour tissue specimens, positive expression rates of SHP2 proteins were 58.4% by immunohistochemical analysis. A significant correlation between expression of SHP2 and that of Hook1 was observed. Based on Western blot analysis, we found that Hook1 was downregulated and that SHP2 has a tendency to overexpress without statistical significance in NSCLC tissues compared with their levels in normal lung tissues. The median overall survival (OS) of NSCLC patients who presented low levels of SHP2 expression were better (40 vs 24 months, p=0.004) than those of patients who exhibited high levels of SHP2 expression. The results of multivariate analysis showed that the level of SHP2 expression was an independent prognostic factor for OS.
Conclusion: SHP2 might play an important role in NSCLC and has the potential to serve as a clinical biomarker or NSCLC.
Keywords: SHP2, Hook1, non-small cell lung cancer (NSCLC), protein expression
Introduction
According to a recent estimate from the World Health Organization (WHO), lung cancer is the leading cause of cancer-related death, accounting for an estimated 1.59 million deaths worldwide.1 Although tyrosine kinase inhibitors (TKIs) have exhibited a superior clinical benefit in lung cancer treatment, unfortunately, almost complete drug resistance and tumour recurrence occur in patients receiving TKI-targeted therapy. Recently, Wang et al reported that dioscin overcomes TKI resistance in epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma cells by reducing the expression of SHP2 and its interaction with GAB1.2 Additionally, Chen et al found a selective oral SHP2 allosteric inhibitor (SHP099) and demonstrated its ability to inhibit ERK phosphorylation and cancer growth.3 As the first proto-oncogene encoding a tyrosine phosphatase,4 src homology 2 domain-containing tyrosine phosphatase 2 (SHP2), encoded by the ptpn11 gene, plays an important role in the development of tumours, inflammation, transcriptional regulation and cell migration.4–6 Somatic activating SHP2 mutations has been investigated in various types of cancer, including breast cancer, acute myeloid leukaemia, colorectal cancer and lung cancer.5–7 Nevertheless, SHP2 has been shown to inhibit tumour formation in liver cancer.8,9 Thus, SHP2 plays different roles in different tumours depending on the tissue and disease stage, yet the association between the expression level of SHP2 and patient prognosis in non-small cell lung cancer (NSCLC) remains unclear.
SHP2 contains one protein tyrosine phosphatase (PTP) catalytic domain and two N-terminal Src homology 2 (SH2) domains.10–13 In the inactive state, one of the N-terminal SH2 domains prevents PTP domain binding. However, when the appropriate ligand binds to its receptor, the SH2 domain is recruited to specific phosphotyrosine sites on the ligand receptor or receptor protein associated with the receptor, thereby relieving self-inhibition.12–14 In our previous study, we identified a novel SHP2-interacting protein, Hook1, that directly interacts with the N-SH2 and PTP domains of SHP2.15 Hook1 is an endogenous negative regulator of SHP2 activation.
Hook1 is a microtubule-binding protein involved in microtubule cytoskeleton dynamics, endocytic trafficking, and cell differentiation.16–18 Experimental data indicate that Hook1 is downregulated in ovarian cancer, breast cancer and hepatocellular carcinoma (HCC) patients.19–21 We have reported that by regulating activation of SHP2, Hook1 negatively modulates the TGFβ1-induced epithelial-mesenchymal transition (EMT) in NSCLC. However, the role of Hook1 and SHP2 in NSCLC remains unclear.15
In this study, we examined expression of SHP2 and Hook1 in NSCLC by immunohistochemistry (IHC) and Western blotting to evaluate associations between the expression levels of SHP2 and Hook1 and the clinical outcome of NSCLC patients. Our results suggest that assessment of SHP2 and Hook1 expression levels is appropriate for use in clinical decision-making.
Materials and methods
Ethical approval
All procedures that were performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University. All participants signed an informed consent form prior to participation in the research study.
Patients
We reviewed NSCLC patients undergoing surgical resection in our hospital between 2008 and 2014. The tumour tissue samples included 20 frozen lung tissue samples and 101 paraffin-embedded tissues. The treatment regimens as follow: first-line treatment with cisplatin at a dose of 75 mg/m2 or carboplatin on day 1 and gemcitabine at a dose of 1,200 mg/m2 on days 1 and 8 or paclitaxel 175 mg/m2 on day 1.Clinical data and information on the follow-up duration for all patients were collected from the First Affiliated Hospital, Zhejiang University database. The final follow-up visit was February 2017 (detailed information is shown in Table 1).
 |
Table 1 Clinical characteristics of 101 patients |
Western blot analysis
Twenty pairs of lung tissue samples (20 normal lung tissues, 20 lung cancer) were homogenized in liquid nitrogen and lysed with radioimmunoprecipitation assay (RIPA) buffer according to the manufacturer’s instructions (Beyotime, Beijing, China). Equal volumes of cell lysates were resolved by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were transferred to 0.45-µm polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% BSA (bovine serum albumin) in TBST (Tris-buffered saline plus Tween-20) and then incubated with an anti-SHP2 primary antibody (Abcam, Cambridge, UK) or anti-Hook1 antibody (Abcam, Cambridge, UK). Afterwards, the membrane was washed three times with TBST and probed with corresponding secondary antibodies (LI-COR Biosciences, Lincoln, NE). The signals were scanned and visualized using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE).
Immunohistochemistry
Briefly, 4-μm sections from paraffin-embedded tumour tissues were deparaffinized in xylene and dehydrated in graded alcohols. For antigen retrieval, the slides were boiled in 10 mM citrate buffer in a microwave for 20 min and then cooled at room temperature for 30 min. Endogenous peroxidase activity was quenched by incubating the slides in 0.3% H2O2 and then washing with phosphate-buffered saline (PBS). The slides were incubated with the anti-SHP2 primary antibody (Abcam, Cambridge, UK) or anti-Hook1 antibody. After washing with PBS, the slides were incubated with the secondary antibody in a humidified chamber for 60 min, followed by 3,3-diaminobenzidine (DAB) chromogen and haematoxylin nuclear counterstaining. Positive control tissue was stained in parallel.
IHC evaluation
The number of positive cells was calculated by counting in the 200x amplified high-power field. The scores for each patient were based on the intensity and distribution of the staining. The distribution was scored as 0 (0%), 1 (1–50%), or 2 (>50%), representing the percentage of positive cells per field. The staining intensity was scored as 0 (no expression), 1 (mild expression), 2 (mediate expression), or 3 (strong expression). The intensity and distribution scores were summed as a total score (0–5), where 0–2 was negative and 3–5 was positive.22
Statistical analysis
All statistical analyses were performed using SPSS version 18. A chi-square test was employed to assess correlations between SHP2 and Hook1 protein expression and clinic pathological characteristics. Kaplan-Meier survival curves and log-rank tests were used to analyse univariate distributions for progression-free survival (PFS) and overall survival (OS). The hazard ratio (HR) and its 95% confidence interval (CI) based on Cox’s ratio hazard multivariate analysis were used to assess the effects of SHP2 and Hook1 protein expression on PFS and OS. p<0.05 was considered statistically significant.
Results
Patient characteristics
From January 2008 to May 2014, 136 NSCLC patients were recruited from First Affiliated Hospital, Zhejiang University. Among them, 15 patients were excluded from the study due to incomplete data; NSCLC tissues and their corresponding adjacent normal lung tissues were obtained for 20 patients to further investigate the relationship between the expression levels of SHP2 and Hook1; data from for 101 patients were extracted at the end of the follow-up period. Among the 101 patients, the median age was 60 years (range, 33–76 years). The majority of patients were male (73%) and presented with histological features of adenocarcinoma (56%). Regarding histopathological staging, 70% patients were at stage I–II, and 30% were at stage III–IV (Table 1). According to the Performance Status (PS), 75% of the patients scored 0, and 25% of the patients scored 1 or 2. All patients were involved in the survival analysis. According to data until February 2017, 77 patients had died, and 23 were still alive. The median OS was 25.0 months (95% CI: 20.5–29.5 months), and the median PFS was 8.0 months (95% CI: 5.9–10.0 months).
SHP2 and Hook1 protein expression in NSCLC
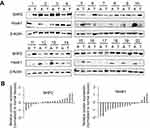
To identify whether Hook1 and SHP2 proteins are key in NSCLC, we examined protein expression in 20 NSCLC tissues and corresponding adjacent normal lung tissues by Western blot analysis. The results showed that SHP2 was overexpressed in tumour tissue samples compared with corresponding normal lung tissue samples but that Hook1 expression was lower in lung tissue samples (Figure 1A). Using densitometry analyses of the relative fold change in either SHP2 or Hook1 expression compared to that of β-actin, we observed that expression of Hook1 in lung tumour samples was significantly lower than that in their corresponding adjacent tissues (Figure 1B, p=0.01); in contrast, we found no statistical significance with regard to SHP2 expression (p=0.202).
The relationship between SHP2 and Hook1 protein expression in NSCLC
In our previous studies, we identified that a novel SHP2-interacting protein, Hook1, a negative regulator of SHP2 activation, negatively modulated TGFβ1-induced EMT in NSCLC.15 To further explore the relationship between expression of SHP2 and Hook1, we performed a correlation study on SHP2 and Hook1 protein expression in 101 NSCLC patients using immunohistochemical analysis (Figure 2). Fifty-nine of the 101 lung tumour tissues showed immunoreactivity with the SHP2 antibody, whereas 46 tumour tissues presented a negative protein expression of Hook1. However, among the 59 patients with positive results for SHP2 expression, 41 patients were also positive for Hook1 expression. Overall, SHP2 expression was significantly correlated with Hook1 expression in lung tumour tissue (r=0.358, p<0.001, Table 2).
 |
Table 2 Correlation between SHP2 and Hook1 protein expression |
Correlation between SHP2 and Hook1 protein expression and clinical characteristics
We further assessed differences between protein expression levels as dichotomous variables (low vs high) across all of the clinical and pathological factors using the chi-square test. We observed that Hook1 expression levels in lung tumours were significantly related to Union for International Cancer Control (UICC) staging (p=0.036), with no significant correlations with other demographic variables, such as age, sex, histology, smoking status, and performance status. Similarly, SHP2 expression showed no correlation with patient age, smoking status, performance stage, or tissue histology (p>0.05, Table 3).
 |
Table 3 Relationship between SHP2 and Hook1 expression and clinical characteristics |
Cox multivariate regression analysis of potential prognostic factors of NSCLC patients
In addition to the correlation between SHP2 and Hook1 protein expression and clinical characteristics, we investigated whether SHP2 and Hook1 protein expression may be used as a prognostic biomarker. Cox multivariate regression analysis was performed to analyse the effect of SHP2 and Hook1 protein expression on PFS and OS in patients. In this study, a total of 75 patients had died at the end of the study. The median OS was 25 months, and the median PFS was 8 months. The median OS of patients with high expression of SHP2 was significantly worse (median OS, 24 months) than those with low expression of SHP2 (median OS, 40 months; p=0.004; HR=2.397; 95% CI=1.373–4.183, Figure 3A). However, there was no statistically significant difference in the median OS (34 vs 25 months; p=0.38, HR=1.251; 95% CI=0.759–2.063, Figure 3B) of patients with low or high expression of Hook1. In addition, the median OS of patients with low expression of SHP2 and Hook1 was longer than that of patients with a high level of expression of at least one of the proteins (48 vs 25 months; p=0.02; HR=1.831; 95% CI=1.007–3.327, Figure 3C). Moreover, there was no significant difference in the median PFS among the various groups (p>0.05, Table 4).
 |
Table 4 SHP2 and Hook1 protein expression and PFS and OS of NSCLC patients |
We then analysed the prognostic value of the biological association between SHP2 and Hook1 as a possible prognostic factor. The median OS of patients with low SHP2 and low Hook1 (median OS, 48 months) was significantly increased compared with that of patients with high SHP2/high Hook1 (median OS, 24 months; p=0.013; HR=2.158; 95% CI=1.176–3.960), high SHP2/low Hook1 (median OS, 21 months; P=0.04; HR=2.105; 95% CI=1.034–4.286) or low SHP2/high Hook1 expression (median OS, 27 months; p=0.636; HR=1.222; 95% CI=0.533–2.798, Figure 4).
Cox regression analysis was used to determine the HR of relevant prognostic characteristics, including sex, age, smoking status, performance status, pathological stage and the level of Hook1 expression. The level of SHP2 expression was an independent prognostic factor for OS in multivariate analysis (HR=2.401, p=0.003 Table 5). Among the other variables, only tissue histology (squamous vs adenocarcinoma vs others) was confirmed as an independent prognostic factor for OS (HR=1.521, p=0.033, Table 5). These results suggest that the level of SHP2 expression is an important prognostic factor for NSCLC patients.
 |
Table 5 Univariate/Multivariate Cox-regression analysis for overall survival in NSCLC patients |
Discussion
In recent years, the identification of molecular prognostic markers, including in EGFR, ALK, Kras, Ros1, PD-L1, ROR1, in most lung cancer patients has allowed the emergence of personalized targeted therapies and created new expectations for these patients. EGFR mutation can lead to autophosphorylation of intracellular receptors and activation of tyrosine kinase and participate in the proliferation, growth, invasion, metastasis and angiogenesis of tumour cells.23 Zheng et al reported that ROR1 protein expression was significantly higher in lung ADC tissues than in their adjacent non-tumour tissues, and ROR1 expression significantly correlates with the malignant attributes of lung ADC.24 As a widely expressed and important phosphatase, SHP2 plays different roles in various tumours and microenvironments, including in processes of cell proliferation, survival, invasion, metabolism, migration, transformation and morphogenesis.6,7,12–15,25 SHP2 is a crucial oncogene that has been extensively investigated, and recent experimental data show that inhibition of SHP2 activity suppresses EGFRL858R-driven lung adenocarcinoma.26 Wang et al also found that dioscin overcame TKI resistance in EGFR-mutated lung adenocarcinoma cells via decreases in SHP2 expression.2
The recently discovered small molecule SHP2 inhibitor SHP099 inhibits the growth of multiple ALK-inhibitor-resistant patient-derived cells (PDCs) in combination with anaplastic lymphoma kinase (ALK) inhibitor ceritinib.27 According to clinical data for 80 NSCLC patients, expression of SHP2 in tumour tissues is highly specific and sensitive and is closely related to lymph node metastasis.28 Indeed, SHP2 expression may promote the invasion and metastasis of NSCLC via the lymphatic system.29 These findings demonstrate that SHP2 is associated with drug sensitivity and the progression of lung cancer, suggesting that it is a potential target for anticancer therapy.
In the present study, we used 20 pairs of frozen lung tissue samples and 101 paraffin-embedded tissues to investigate expression of SHP2 and its binding partner Hook1 in NSCLC patients. Western blot analysis showed that SHP2 is overexpressed in lung cancer tissue; in contrast, the Hook1 protein is expressed at low levels, indicating that expression of SHP2 and Hook1 may play a vital role in the pathogenesis of NSCLC. IHC analysis of SHP2 protein expression in 101 NSCLC patients revealed SHP2 expression in 59 patients, for an expression rate of 58.5% (59/101). Surprisingly, the rate of Hook1 positivity in 101 NSCLC patients was 54.4% (55/101). It is likely that the number of patients, UICC staging and approaches, such as Western blotting and IHC, might influence the results, and more efforts are needed to confirm these findings. Our chi-square test analysis showed that levels of Hook1 expression were significantly related to UICC staging (P=0.036), suggesting that Hook1 protein expression and the occurrence and development of NSCLC are closely related. Hook1 is a member of the hook protein family that participates in endocytosis and in maintaining cell shape.16,30 In recent decades, Hook1 has been reported to be downregulated in ovarian cancer and breast cancer.19,21 Recently, Sun and colleagues20 reported Hook1 to be downregulated in HCC patients, and its expression level was associated with malignancy.
Moreover, our previous study showed that Hook1 negatively regulates TGFβ1-induced EMT by blocking SHP2 in lung cancer.15 All of these studies indicate that the Hook1 protein is a protective factor in cancer. EGF and TGFβ1 activate SHP-2 enzyme activity through disassociation of Hook1 and SHP-2 PTP domains. Specifically, TGFβ1 activates SHP2 by downregulating Hook1, whereas EGF activates SHP2 without affecting Hook1 protein levels.15 In the present study, expression of SHP2 was significantly correlated with expression of Hook1 in NSCLC tissues. Surprisingly, among the 59 patients positive for SHP2 expression, 41 were also positive for Hook1 expression. It is likely that overexpression of Hook1 may suppress the enzymatic activity of SHP2 to regulate tumour microenvironments as well as signalling in advanced stages of NSCLC. The enzymatic activity of SHP2 is temporally and spatially regulated through scaffolding proteins, including Gab2, Gab1. Gab2 phosphorylation by RSK inhibits recruitment of the tyrosine phosphatase SHP2 in response to growth factors,31 and interaction of SHP2 with Gab1 mediates activation of EGFR and PI3K.32 In general, the mechanism by which SHP2 is activated has been extensively studied.
In this study, we also showed that patients with low SHP2 expression had a prolonged median OS (40 vs 24 months, p=0.004) compared with those with high SHP2 expression. Multivariate analysis showed that the level of SHP2 expression was an independent prognostic factor for median OS, and the patients with higher SHP2 expression tended to have poor clinical outcomes in survival analyses. However, no such correlation was found for Hook1 expression. In addition, significant differences were observed regarding the median OS between patients with a low SHP2/low Hook1 and a high SHP2/low Hook1 expression level (48 vs 21 months; p=0.04) or a high SHP2/high Hook1 expression level (48 vs 24 months; p=0.013) in tumour tissues. These findings indicate that the level of SHP2 expression may serve as a biomarker for diagnosis and prognosis of patients with NSCLC. Surprisingly, among the patients positive for SHP2 expression, there was no significant difference in the OS of those with a low level of Hook1 expression compared with the OS of those with high expression (p>0.05), suggesting that overexpression of Hook1 is not the only mechanism that regulates SHP2 enzyme activity in lung cancer. Further investigation is needed.
Our study demonstrates that SHP2 is overexpressed and that Hook1 is downregulated in NSCLC patients. In addition, patients with a low level of SHP2 expression showed a better prognosis and longer survival than did those with high expression, whereas no such correlation was found for Hook1 expression. Our findings suggest that SHP2 is a potential therapeutic target and prognostic factor for patients with NSCLC. However, only a few samples could be evaluated in this study, so more prospective random studies, with larger sample sizes, are needed to further evaluate the prognostic and predictive value of SHP2 and Hook1 expression. The detailed mechanism of SHP2 in cancer progression and the role of Hook1 in tumours also require further investigation.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No.81673512) and Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2017KY332).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
2. Wang YC, Wu DW, Wu TC, Wang L, Chen CY, Lee H. Dioscin overcome TKI resistance in EGFR-mutated lung adenocarcinoma cells via down-regulation of tyrosine phosphatase SHP2 expression. Int J Biol Sci. 2018;14(1):47–56. doi:10.7150/ijbs.22209
3. Chen YN, LaMarche MJ, Chan HM, et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535(7610):148–152. doi:10.1038/nature18621
4. Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109(3):862–867. doi:10.1182/blood-2006-07-028829
5. Bentires-Alj M, Paez JG, David FS, et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64(24):8816–8820. doi:10.1158/0008-5472.CAN-04-1923
6. Chan G, Kalaitzidis D, Neel BG. The tyrosine phosphatase SHP2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008;27(2):179–192. doi:10.1007/s10555-008-9126-y
7. Mohi MG, Neel BG. The role of SHP2 (PTPN11) in cancer. Curr Opin Genet Dev. 2007;17(1):23–30. doi:10.1016/j.gde.2006.12.011
8. Bard-Chapeau EA, Li S, Ding J, et al. PTPN11/SHP2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell. 2011;19(5):629–639. doi:10.1016/j.ccr.2011.03.023
9. Yang W, Wang J, Moore DC, et al. PTPN11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature. 2013;499(7459):491–495. doi:10.1038/nature12396
10. Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998;6(3):249–254.
11. Li H, Tao C, Cai Z, et al. Frs2alpha and SHP2 signal independently of Gab to mediate FGF signaling in lens development. J Cell Sci. 2014;127(Pt 3):571–582. doi:10.1242/jcs.134478
12. Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6(4):307–320. doi:10.1038/nrc1837
13. Perrinjaquet M, Vilar M, Ibanez CF. Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase. J Biol Chem. 2010;285(41):31867–31875. doi:10.1074/jbc.M110.144923
14. Qu CK. The SHP-2 tyrosine phosphatase: signaling mechanisms and biological functions. Cell Res. 2000;10(4):279–288. doi:10.1038/sj.cr.7290055
15. Li S, Wang L, Zhao Q, et al. SHP2 positively regulates TGFbeta1-induced epithelial-mesenchymal transition modulated by its novel interacting protein Hook1. J Biol Chem. 2014;289(49):34152–34160. doi:10.1074/jbc.M113.546077
16. Kramer H, Phistry M. Mutations in the Drosophila hook gene inhibit endocytosis of the boss transmembrane ligand into multivesicular bodies. J Cell Biol. 1996;133(6):1205–1215. doi:10.1083/jcb.133.6.1205
17. Kramer H, Phistry M. Genetic analysis of hook, a gene required for endocytic trafficking in drosophila. Genetics. 1999;151(2):675–684.
18. Maldonado-Baez L, Cole NB, Kramer H, Donaldson JG. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J Cell Biol. 2013;201(2):233–247. doi:10.1083/jcb.201208172
19. Kurrey NK, Jalgaonkar SP, Joglekar AV, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27(9):2059–2068. doi:10.1002/stem.154
20. Sun X, Zhang Q, Chen W, et al. Hook1 inhibits malignancy and epithelial-mesenchymal transition in hepatocellular carcinoma. Tumor Biol. 2017;39(7):1010428317711098. doi:10.1177/1010428317711098
21. Taube JH, Herschkowitz JI, Komurov K, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A. 2010;107(35):15449–15454. doi:10.1073/pnas.1004900107
22. Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31(4):490–498. doi:10.1200/JCO.2012.45.2052
23. Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer. Lancet Oncol. 2015;16(9):e447–e459. doi:10.1016/S1470-2045(15)00246-6
24. Zheng YZ, Ma R, Zhou JK, et al. ROR1 is a novel prognostic biomarker in patients with lung adenocarcinoma. Sci Rep. 2016;6:36447. doi:10.1038/srep36447
25. Zhang J, Zhang F, Niu R. Functions of SHP2 in cancer. J Cell Mol Med. 2015;19(9):2075–2083. doi:10.1111/jcmm.12618
26. Schneeberger VE, Ren Y, Luetteke N, et al. Inhibition of SHP2 suppresses mutant EGFR-induced lung tumors in transgenic mouse model of lung adenocarcinoma. Oncotarget. 2015;6(8):6191–6202. doi:10.18632/oncotarget.3356
27. Dardaei L, Wang HQ, Singh M, et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018. doi:10.1038/nm.4497
28. Tang C, Luo D, Yang H, et al. Expression of SHP2 and related markers in non-small cell lung cancer: a tissue microarray study of 80 cases. AIMM. 2013;21(5):386–394. doi:10.1097/PAI.0b013e31827da3f9
29. Tang C, Zhou X, Yang H, Wang Q, Zhang R. [Expression and its clinical significance of SHP2 in non-small cell lung cancer]. Zhongguo Fei Ai Za Zhi. 2010;13(2):98–101. doi:10.3779/j.issn.1009-3419.2010.02.03
30. Xu L, Sowa ME, Chen J, Li X, Gygi SP, Harper JW. An FTS/Hook/p107(FHIP) complex interacts with and promotes endosomal clustering by the homotypic vacuolar protein sorting complex. Mol Biol Cell. 2008;19(12):5059–5071. doi:10.1091/mbc.e08-05-0473
31. Zhang X, Lavoie G, Fort L, et al. Gab2 phosphorylation by RSK inhibits SHP2 recruitment and cell motility. Mol Cell Biol. 2013;33(8):1657–1670. doi:10.1128/MCB.01353-12
32. Diaz ME, Gonzalez L, Miquet JG, et al. Growth hormone modulation of EGF-induced PI3K-Akt pathway in mice liver. Cell Signal. 2012;24(2):514–523. doi:10.1016/j.cellsig.2011.10.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.