Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
The Prognostic Role of HuR Varies Between Different Subtypes of Breast Cancer Patients: Data Mining and Retrospective Analysis
Authors Liao Y , Liao Y, Li J, Li Y, Fan Y
Received 9 November 2022
Accepted for publication 28 January 2023
Published 11 February 2023 Volume 2023:15 Pages 135—146
DOI https://doi.org/10.2147/BCTT.S395984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pranela Rameshwar
Yuqian Liao,1,* Yulu Liao,2,* Jun Li,2 Yong Li,1 Ying Fan3
1Department of Oncology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province, People’s Republic of China; 2Department of Radiation Oncology, Jiangxi Cancer Hospital, Nanchang, Jiangxi Province, People’s Republic of China; 3Department of Medical Oncology, Cancer Institute and Hospital, Peking Union Medical College, Chinese Academy of Medical Science, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ying Fan, Department of Medical Oncology, Cancer institute and hospital, Peking Union Medical college, Chinese Academy of Medical science, No. 17, Nan Li, Panjiayuan, Beijing, 100021, People’s Republic of China, Tel +86 13693656671, Email [email protected] Yong Li, Department of Oncology, The First Affiliated Hospital of Nanchang University, No. 17, Yongwaizhengjie, Donghu, Nanchang, 330006, Jiangxi Province, People’s Republic of China, Tel +86 15879155066, Email [email protected]
Objective: Human-antigen R (HuR) is an RNA-binding protein, which regulates the expression of several oncogenes and tumor suppressor genes through post-transcriptional mechanisms. But the role of HuR in breast cancer remains controversial. The aim of this study was to verify the association between cytoplasmic HuR level and prognosis of breast cancer patients.
Methods: Data mining from the Human Protein Atlas (HPA) and Kaplan–Meier Plotter (KMP) databases was performed. Then, 394 patients with stage I–III primary breast cancer were enrolled between January 2005 and December 2016. We investigated the association between cytoplasmic HuR level and clinicopathological characteristics or survival of these patients. Immunohistochemical analysis was performed to determine HuR expression level. SPSS 21.0 statistical software was used for analysis.
Results: In the HPA and KMP datasets, HuR protein and mRNA expression level were not significantly associated with overall survival of all breast cancer patients enrolled. Results from our 394 patients indicated that higher expression level of cytoplasmic HuR was associated with larger tumor size, lymph node positive, ER negative and triple-negative subtype. For all patients enrolled, the results indicated that compared with HuR negative patients, the DFS (disease-free survival) of HuR 1+ was longer (60.5% vs 78.8, P=0.053, HR=0.616, 95% CI: 0.378– 1.005), the P value was borderline. In the triple-negative breast cancer (TNBC) subgroup, HuR positive patients had significantly longer DFS than HuR negative patients (65.5% vs 30.8%, P=0.001, HR=0.345, 95% CI: 0.180– 0.658). In the HR+HER2– subgroup, HuR low (0~1+) patients had significantly longer OS than HuR high (2+~3+) patients (97.0% vs 89.5%, P=0.033, HR=2.482, 95% CI: 1.074– 5.736).
Conclusion: In conclusion, our results revealed that higher expression level of HuR was related to aggressive biological characteristics which supported the findings from previous researches. In the HR+HER2– subgroup, lower HuR expression level patients had better survival time, while in the TNBC subgroup we got the opposite results. Our work indicated that HuR might play different roles in different breast cancer subtypes.
Keywords: human antigen R, breast cancer, immunohistochemistry, clinicopathological parameters, prognosis
Introduction
Breast cancer is one of the most common cancers and is the leading cause of cancer-related death in women around the world.1 It is a heterogeneous disease. During the last 10 years, molecular subtypes based on different gene expression profiling have been investigated to predict prognosis and guide treatment.2 Since multi-gene molecular assay is expensive and not always available, it is not routinely used in our clinical practice. In 2013, surrogate subtypes based on immunohistochemical markers of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and Ki-67 were proposed at the St Gallen conference.3 According to the recommendation, breast cancer patients are divided into luminal A, luminal B, HER2-enriched and triple negative (TNBC) subtypes. However, patients with the same subtypes might respond differently to treatment and have different prognosis, the underlying mechanisms are uncertain and further exploring of new biomarkers is needed.
RNA binding-proteins (RBPs) have been recognized as key regulators of post-transcriptional events.4 RBPs participate in a wide range of biological and pathological processes such as cell adhesion, RNA synthesis and degradation and so on.5,6 Human-antigen R (HuR) is an RNA-binding protein, belonging to the embryonic lethal abnormal vision (ELAV) family. HuR regulates the expression of several oncogenes and tumor suppressor genes through post-transcriptional mechanisms, including messenger RNA (mRNA) trafficking, mRNA decay and protein translation.7 The intracellular location of HuR is predominantly nuclear, it shuttles from the nucleus to the cytoplasm under stimulation conditions.8 Elevated cytoplasmic HuR expression was related to the etiology and prognosis of several kinds of cancers.9,10 But the relationship between the expression of HuR and survival of breast cancer patients is controversial.11–14 Some researches indicated that higher expression of HuR is associated with poor prognosis,11–13 while others showed that higher HuR expression level leads to decreased tumor growth by downregulating VEGF in a mouse model of TNBC.14
The effects of HuR on chemotherapy response were complex. Increased HuR expression was found to be related to increased gemcitabine response10 and doxorubicin-induced apoptosis.15 In contrast, higher expression level of HuR was associated with paclitaxel16 and tamoxifen resistance.17,18 In breast cancer cells, HuR was found to be associated with radioresistance.19,20 HuR is a promising target in cancer drug development, but till now no HuR inhibitors are investigated in clinical trial.21 With the above controversial data, researchers suggested that the combination of HuR inhibitors with established agents should be designed rationally based on the molecular profiling of the cancer.21
For the past 20 years, more than a hundred papers have investigated the role of HuR in cancers using in vitro cell culture methods with fewer in vivo studies.22 The aim of this study was to analyze the association between cytoplasmic HuR level and prognosis in breast cancer patients with different subtypes. In our study, we explored the HuR protein expression profile and prognostic role in breast cancer by analyzing the Human Protein Atlas (HPA) and Kaplan–Meier Plotter (KMP) databases. Then, 394 breast cancer samples from Cancer Hospital of Chinese Academy of Medical Science were tested to verify the prognostic role of HuR.
Materials and Methods
Data Mining and Processing
The nuclear protein level, cytoplasmic protein level and mRNA level of HuR in normal and different tumor tissues were analyzed using data from the Human Protein Atlas (HPA) (http://www.proteinatlas.org/).23 Representative immunohistochemistry images of cytoplasmic HuR protein level were also downloaded from the HPA.
The HPA dataset contains the HuR mRNA level and corresponding overall survival time of breast cancer patients. The HuR protein level and overall survival time were obtained from the KMP database (http://kmplot.com/analysis/).24 We analyzed the prognostic role of HuR by using data from the HPA and KMP datasets.
Experiments with Specimens from 394 Patients
In our study, a total of 394 patients with stage I–III primary breast cancer were enrolled between January 2005 and December 2016. Stage was determined according to American Joint Committee on Cancer 2010 classification.25 ER, PR and HER2 status were defined according to guidelines issued by the American Society of Clinical Oncology (ASCO) and the College of American Pathologists (CAP) in 2010.26,27 This investigation was approved by the Institutional Review Board of the Chinese Academy of Medical Sciences Cancer Hospital and the First Affiliated Hospital of Nanchang University. It was conducted according to the ethical standards of the Declaration of Helsinki and following the national and international guidelines. All patients have consented to their tumor tissue and clinical information being used in this study. We collected clinical and pathological data. Patients were followed until December 2021 to collect data on recurrence and death.
Immunohistochemistry
Immunohistochemistry of HuR was performed according to previous researches.10 A monoclonal mouse antibody directed against HuR (3A2; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for HuR immunostainings on formalin-fixed, paraffin-embedded breast tissue sections. Incubation with primary HuR antibodies was performed for 1 h at room temperature (37 °C), at a dilution of 1:200.10 The standard two-step peroxidase conjugated polymer technique (DAKO Envision kit; DAKO, Carpinteria, CA, USA) was then performed.10 At a next step, immunostainings were visualized with diaminobenzidine tetrahydrochloride solution (DAB; Sigma, Saint Louis, MO, USA).
HuR cytoplasmic expression level was confirmed independently by two observers. HuR staining levels were rated as follows:28 0 points, no staining; 1 point, weak and/or focal staining (<10% of cells); 2 points, moderate or strong staining (10–50% of cells); and 3 points, moderate or strong staining (>50% of cells). Combined scores of 0 or 1 points represented low expression, while combined scores of 2 or 3 points indicated high expression (Figure 1A–D).
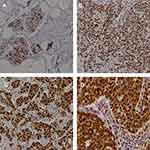 |
Figure 1 Presentative IHC images of cytoplasmic HuR expression levels from 394 breast cancer tissues. (A) Staining: 0 points; (B) staining: 1 point; (C) staining: 2 points; (D) staining: 3 points. |
Statistical Analyses
SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for analysis. Five-year DFS and OS rates of patients with different characteristics were estimated by the Kaplan–Meier product limit method and compared by the Log rank test. Hazard ratios of recurrence/metastasis and death with 95% confidence intervals (CIs) were estimated by Cox regression model. The multivariate analysis was adjusted for age, histological grade, tumor size, lymph node status, subtypes and HuR expression level. All statistical tests were two-sided, and P<0.05 was considered significant.
Results
Protein Expression Profile of HUR in the HPA
From the HPA dataset, we found that the protein level of HUR in normal breast tissue was medium compared with the other 30 human tissue samples (Figure 2A). Most tumor cells displayed weak to moderate nuclear immunoreactivity (Figure 2B) and moderate cytoplasmic positivity (Figure 2C) of HUR protein. Few breast and colorectal cancers exhibited strong immunoreactivity. Several cases of cervical, ovarian, lung, pancreatic, liver, endometrial cancers and lymphomas were weakly stained. In Figure 1C, regarding cytoplasmic expression of HUR protein, breast cancer ranked fifth among the 20 common cancer types. Figure 2D displays representative IHC images of cytoplasmic HuR expression levels, including low, medium and strong.
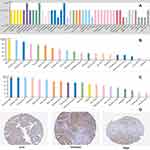 |
Figure 2 HuR protein expression overview in human normal and tumor tissues (data from The Human Protein Atlas; Creative Commons). (A) HuR protein expression overview in human normal tissues. (B) HuR protein nuclear expression overview in human tumor tissues. (C) HuR protein cytoplasmic expression overview in human tumor tissues. (D) Representative IHC images of cytoplasmic HuR expression levels in breast cancer tissues. |
Prognostic Role of HUR Expression Level in Breast Cancer Patients from the HPA and KMP
To explore the association between HuR and OS in breast cancer patients, we used the KMP and HPA datasets. In the KMP, patients with higher protein level of HuR had a worse survival, but the P value was not statistically significant (P=0.12; Figure 3A). In the HPA, the mRNA level of HuR was analyzed. The 5-year OS for patients with low and high HuR mRNA level were not significantly different (83% vs 80%, P=0.12; Figure 3B).
 |
Figure 3 The prognostic role of HuR by using data from the HPA and KMP datasets. (A) Kaplan–Meier curves from the KMP dataset.24 (B) Kaplan–Meier curves from the HPA dataset (data from The Human Protein Atlas; Creative Commons). |
Association Between Clinical Characteristics and Cytoplasmic Expression Level of HuR in 394 Breast Cancer Patients
A total of 394 BC patients were enrolled in this study. The median age at diagnosis is 50 years old (range: 24–85), and 211 (53.6%) patients were ≤50 years old. 174 (44.2%) patients were diagnosed with grade 3 tumors. Most patients were at stage II and III (296 patients, 75.1%). 52.0% of patients were ER negative and 41.1% of patients were TNBC. The association between clinical characteristics and cytoplasmic expression level of HuR are listed in Table 1. The expression level of HuR was significantly associated with tumor size, lymph node status, ER expression and subtypes of breast cancer. Compared with HuR negative patients, patients with HuR 3+ were more likely to be with tumor >2 cm (P=0.028, HR=3.353, 95% CI: 1.142–9.843) and lymph node positive (P=0.028, HR=3.353, 95% CI: 1.142–9.843). In patients with HuR 0, 1+, 2+ and 3+, there were 47.4%, 41.9%, 65.7% and 88.5% ER negative patients. Compared with HuR negative patients, patients with HuR 2+ (P=0.049, HR=2.295, 95% CI: 1.003–5.249) and 3+ (P=0.001, HR=10.615, 95% CI: 2.621–42.992) were more likely to be TNBC.
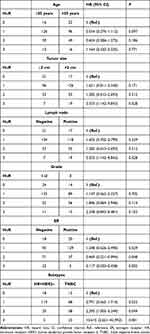 |
Table 1 Clinical Characteristics and Cytoplasmic Expression Level of HUR in 394 Breast Cancer Patients |
Clinical Characteristics and Survival in 394 Breast Cancer Patients
The 5-year OS and DFS rate were 87.7% and 75.1%, respectively. The survival rates for patients with different clinicopathological characteristics are listed in Table 2. The Kaplan–Meier analysis revealed a significantly higher DFS and OS for patients with tumor size ≤2 cm, negative lymph node metastasis. Grade I–II and ER positive patients had significantly higher OS than grade III and ER negative patients. Stage III patients had significantly lower DFS (P<0.001, HR=2.828, 95% CI: 1.798–4.450) and OS (P<0.001, HR=4.903, 95% CI: 2.497–9.626) rates compared to stage I patients. The DFS (P=0.001, HR=1.789, 95% CI: 1.266–2.527) and OS (P<0.001, HR=3.427, 95% CI: 2.089–5.622) of TNBC patients were much worse compared to HR+HER2– patients.
 |
Table 2 Clinicopathological Characteristics and Survival of 394 Breast Cancer Patients |
Cytoplasmic Expression Level of HuR and Survival in 394 Breast Cancer Patients
No patient in the HER2+ subgroup was HuR 3+ and only 3 patients in the HR+HER2– subgroup were HuR 3+, so we only compared the survival time of patients with HuR 0, 1+, 2+ and 3+ in all patients enrolled and in the TNBC subgroup (Table 3). For all patients enrolled, the results indicated that compared with HuR 0 patients, the DFS of HuR 1+ was longer (60.5% vs 78.8, P=0.053, HR=0.616, 95% CI: 0.378–1.005), the P value was borderline. In the TNBC subgroup, patients with HuR 1+, 2+ and 3+ had significantly favorable DFS than patients with HuR 0. After multivariate analysis, lymph node status and surrogate subtypes were proved to be independent prognostic factors for both DFS and OS. HuR expression level was an independent prognostic factor for DFS (P=0.022, HR=0.762, 95% CI: 0.604–0.962) but not OS.
 |
Table 3 Cytoplasmic Expression Level of HUR and Survival of 394 Breast Cancer Patients |
To explore the best cut-off value that predicts prognosis, we also compared survival time between HuR negative (0) and positive (1+~3+) or between HuR low (0~1+) and high (2+~3+) patients (Table 3). For all patients enrolled, the OS for patients with low and high cytoplasmic HuR expression was not significantly different (Figure 4A), which was consistent with the results from the HPA and KMP datasets. In the TNBC subgroup, HuR positive patients had significantly longer DFS than HuR negative patients (65.5% vs 30.8%, P=0.001, HR=0.345, 95% CI: 0.180–0.658; Figure 4B). In the HR+HER2– subgroup, HuR low patients had significantly longer OS than HuR high patients (97.0% vs 89.5%, P=0.033, HR=2.482, 95% CI: 1.074–5.736; Figure 4C).
Discussion
The Hu protein family comprises four vertebrate members, the primarily neuronal proteins HuB (Hel-N1), HuC (PLE21), HuD, and the ubiquitously expressed HuR.29 HuR localizes in the nucleus of healthy cells, and increased cytoplasmic HuR levels have been reported to be associated with several kinds of malignancies.30 By analyzing the HPA dataset, we found that most tumor cells displayed moderate cytoplasmic positivity of HuR protein, and breast cancer ranked fifth among the 20 common cancer types, which suggested that HuR might play an important role in breast cancer. Both analysis of the HPA and KMP datasets and of 394 breast cancer patients showed that protein or mRNA level of HuR was not significantly associated with prognosis. But the subgroup analysis of 394 breast cancer patients indicated that the prognostic value of HuR varied between different subtypes. We also found that higher expression level of HuR was related to aggressive biological characteristics which was consistent with results from previous studies.
The HuR gene encodes a 32 kDa protein containing three highly conserved RNA-binding domains belonging to the RNA recognition motif (RRM) superfamily.31 RRM-1 and RRM-2 are responsible for AU-rich elements (ARE) binding, whereas RRM-3 binds simultaneously to the mRNA poly(A) tail.32 Due to its ubiquitous expression, HuR regulates the stability, translation and intracellular trafficking of thousands of target mRNAs by binding to their 3′ untranslated regions (UTRs) containing AU-rich elements (AREs).33 The regulation of HuR can be either positive or negative.
HuR has been demonstrated to promote expression of many breast cancer-related genes including CCL2,34 Cyclin D1,35 MMP-935 and VEGF.12 But the role of HuR in breast cancer remains controversial. Giaginis et al found that high HuR expression was positively associated with larger tumor size and advanced disease stage (P=0.0234 and P=0.0361, respectively), being more frequently observed in ER negative cases (P=0.0208).11 These results were consistent with ours. And in Giaginis et al’s study, high HuR expression was associated with poor overall and disease-free survival.11 Another four researches also demonstrated that higher expression level of HuR was associated with poor prognosis in 208 breast cancer patients,12 in 125 invasive breast cancer patients,13 in 139 breast cancer patients receiving paclitaxel and anthracycline containing neoadjuvant chemotherapy,16 and in 525 familial non-BRCA1/2 breast cancer cases.36 However, there were studies that derived opposite results. Ortega et al37 investigated the HuR mRNA expression level in 89 patients, and they found that a low tumor expression of HuR predicts a higher risk of disease recurrence in early-stage breast cancer patients. In Yuan et al’s research,38 the patients with poor prognosis, including those with metastasis and those who died of breast cancer, had a significantly lower level of HuR transcripts compared to the disease-free patients.
One explanation for the discordance of these results is that the previous five studies investigated the HuR expression at protein level by immunohistochemistry, while the latter two researches quantified the mRNA level of HuR by reverse transcription polymerase chain reaction technique. Some investigators assumed that post-transcriptional modifications of HuR protein may affect its clinical and prognostic impact,11 but the underlying mechanisms still need to be clarified. Different antibodies used may also contribute to this controversy. Besides, the different characteristics of patients enrolled in studies may be responsible for this controversy. We found that studies that demonstrated positive correlation of HuR and survival enrolled more HR positive patients (more than 60%). While in Yuan et al’s research that HuR expression level was negatively related to survival, 68% patients were ER negative. Similarly, we enrolled 52.0% ER negative patients in our study, and HuR positive patients had better DFS than HuR negative patients (76.6% vs 60.5%, P=0.051, HR=0.625 95% CI: 0.390–1.002). The P value was borderline.
Actually, some basic researches had demonstrated that HuR plays different roles in different subtypes of breast cancer. Yuan et al35 found that HuR knockdown dramatically reduced cell growth in MCF-7. In MDA-MB-231 cells, HuR knockdown reduced cell invasiveness but not cell growth. Reduced growth of MCF-7 cells was associated with reduction of cyclin D1. While reduced invasiveness seemed to be linked with decreased MMP-9 levels in MDA-MB-231 cells.35 In Gubin et al’s research,14 HuR overexpression results in significantly reduced tumor growth and mass. They revealed that HuR regulates a cluster of genes involved in blood vessel formation. Overexpression of HuR impairs tumor growth in triple negative breast cancer associated with deficient angiogenesis.14
Till now, no research has comprehensively investigated the prognostic role of HuR in different subtypes of breast cancer patients. Our study found that higher expression level of HuR was associated with ER negative and TNBC subtype. In the TNBC subgroup, HuR positive patients had significantly longer DFS compared to HuR negative patients. While in the HR+HER2– subgroup, HuR low patients had significantly longer OS than HuR high patients. Our data are consistent with the results from previous clinical and basic researches that HuR plays different roles in different subtypes.
However, there are some limitations of our study. Firstly, this is a retrospective study, 41.1% patients are TNBC. While in the Chinese BC population, the incidence of TNBC is usually 10–15%. Secondly, patients in our study received varies therapies which might influence the results, since it has been reported that HuR expression associated with the efficacy of tamoxifen,17,18 taxanes,16 gemcitabine10 and so on. Thirdly, HuR Antibody (3A2) is the most commonly used antibody for recognizing HuR, but the exact epitope has not been very well defined. According to the literature,39 the 3A2 epitope is located in RRM 1 of HuR, which is highly conserved among all ELAV proteins. Although HuB, HuC and HuD were primarily neuronal proteins and were weakly stained or mostly negative in breast cancer tissue, we cannot totally exclude their influence of the staining. Fourthly, the sample size of our research is limited, and we are now collecting more samples to verify our results and searching the underlying mechanisms of HUR.
Conclusion
In conclusion, our results from 394 breast cancer patients revealed that higher expression level of HuR was related to aggressive biological characteristics which supported the findings from previous researches. In the HR+HER2– subgroup, cytoplasmic HuR expression level was negatively associated with survival, while in TNBC patients we got the opposite results. Our work indicated that HuR might play different roles in different subtypes.
Ethical Standards
This investigation was approved by the Institutional Review Board of the Chinese Academy of Medical Sciences Cancer Hospital and the First Affiliated Hospital of Nanchang University. It was conducted according to the ethical standards of the Declaration of Helsinki and following the national and international guidelines. All patients have consented to their tumor tissue and clinical information being used in this study. We collected clinical and pathological data. Patients were followed until December 2021 to collect data on recurrence and death.
Acknowledgment
This study was funded by the Science and technology plan from Health Commission of Jiangxi Province in China (No. SKJP220203930) and Science and technology plan from Administration of Traditional Chinese Medicine of Jiangxi Province in China (No. SZYYB20205193).
Disclosure
The authors declare no competing interests in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–2943.
3. Goldhirsch A, Winer EP, Coates AS, et al. Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi:10.1093/annonc/mdt303
4. Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018;19(5):327–341. doi:10.1038/nrm.2017.130
5. Marchese D, de Groot NS, Lorenzo Gotor N, Livi CM, Tartaglia GG. Advances in the characterization of RNA-binding proteins. Wiley Interdiscip Rev RNA. 2016;7(6):793–810. doi:10.1002/wrna.1378
6. Pereira B, Billaud M, Almeida R. RNA-binding proteins in cancer: old players and new actors. Trends Cancer. 2017;3(7):506–528. doi:10.1016/j.trecan.2017.05.003
7. Wang J, Guo Y, Chu H, Guan Y, Bi J, Wang B. Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int J Mol Sci. 2013;14(5):10015–10041. doi:10.3390/ijms140510015
8. Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65(20):3168–3181. doi:10.1007/s00018-008-8252-68
9. Cai H, Zheng D, Yao Y, Yang L, Huang X, Wang L. Roles of embryonic lethal abnormal vision-like RNA binding proteins in cancer and beyond. Front Cell Dev Biol. 2022;10:847761. doi:10.3389/fcell.2022.847761
10. Toyota K, Murakami Y, Kondo N, et al. Cytoplasmic hu-antigen R (HuR) expression is associated with poor survival in patients with surgically resected cholangiocarcinoma treated with adjuvant gemcitabine-based chemotherapy. Ann Surg Oncol. 2018;25(5):1202–1210. doi:10.1245/s10434-018-6392-y
11. Giaginis C, Sampani A, Kotta-Loizou I, et al. Elevated Hu-antigen receptor (hur) expression is associated with tumor aggressiveness and poor prognosis but not with COX-2 expression in invasive breast carcinoma patients. Pathol Oncol Res. 2018;24(3):631–640. doi:10.1007/s12253-017-0288-1
12. Denkert C, Weichert W, Winzer KJ, et al. Expression of the ELAV-like protein HuR is associated with higher tumor grade and increased cyclooxygenase-2 expression in human breast carcinoma. Clin Cancer Res. 2004;10(16):5580–5586. doi:10.1158/1078-0432
13. Heinonen M, Bono P, Narko K, et al. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. 2005;65(6):2157–2161. doi:10.1158/0008-5472
14. Gubin MM, Calaluce R, Davis JW, et al. Overexpression of the RNA binding protein HuR impairs tumor growth in triple negative breast cancer associated with deficient angiogenesis. Cell Cycle. 2010;9(16):3337–3346. doi:10.4161/cc.9.16.12711
15. Latorre E, Tebaldi T, Viero G, Spartà AM, Quattrone A, Provenzani A. Downregulation of HuR as a new mechanism of doxorubicin resistance in breast cancer cells. Mol Cancer. 2012;11:13. doi:10.1186/1476-4598-11-13
16. Wang J, Li D, Wang B, Wu Y. Predictive and prognostic significance of cytoplasmic expression of ELAV-like protein HuR in invasive breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;141(2):213–224. doi:10.1007/s10549-013-2679-7
17. Hostetter C, Licata LA, Witkiewicz A, et al. Cytoplasmic accumulation of the RNA binding protein HuR is central to tamoxifen resistance in estrogen receptor positive breast cancer cells. Cancer Biol Ther. 2008;7(9):1496–1506. doi:10.4161/cbt.7.9.6490
18. Tan S, Ding K, Chong QY, et al. Post-transcriptional regulation of ERBB2 by miR26a/b and HuR confers resistance to tamoxifen in estrogen receptor-positive breast cancer cells. J Biol Chem. 2017;292(33):13551–13564. doi:10.1074/jbc.M117.780973
19. Mehta M, Basalingappa K, Griffith JN, et al. HuR silencing elicits oxidative stress and DNA damage and sensitizes human triple-negative breast cancer cells to radiotherapy. Oncotarget. 2016;7(40):64820–64835. doi:10.18632/oncotarget.11706
20. Andrade D, Mehta M, Griffith J, et al. HuR reduces radiation-induced DNA damage by enhancing expression of ARID1A. Cancers. 2019;11(12):2014. doi:10.3390/cancers11122014
21. Wu X, The XL. RNA-binding protein HuR in human cancer: a friend or foe? Adv Drug Deliv Rev. 2022;184:114179. doi:10.1016/j.addr.2022.114179
22. Majumder M, Chakraborty P, Mohan S, Mehrotra S, Palanisamy V. HuR as a molecular target for cancer therapeutics and immune-related disorders. Adv Drug Deliv Rev. 2022;188:114442. doi:10.1016/j.addr.2022.114442
23. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi:10.1126/science.1260419
24. Lánczky A and Győrffy B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J Med Internet Res. 2021;23(7):e27633. doi:10.2196/27633
25. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
26. Wolff AC, Hammond ME, Hicks DG, et al. American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
27. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134(6):907–922. doi:10.5858/134.6.907
28. Costantino CL, Witkiewicz AK, Kuwano Y, et al. The role of HuR in gemcitabine efficacy in pancreatic cancer: huR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009;69(11):4567–4572. doi:10.1158/0008-5472
29. Ma WJ, Furneaux H. Localization of the human HuR gene to chromosome 19p13.2. Hum Genet. 1997;99(1):32–33. doi:10.1007/s004390050305
30. Kotta-Loizou I, Giaginis C, Theocharis S. Clinical significance of HuR expression in human malignancy. Med Oncol. 2014;31(9):161. doi:10.1007/s12032-014-0161-y
31. Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265(5172):615–621. doi:10.1126/science.8036511
32. Ma WJ, Chung S, Furneaux H. The Elav-like proteins bind to AU-rich elements and to the poly(A) tail of mRNA. Nucleic Acids Res. 1997;25(18):3564–3569. doi:10.1093/nar/25.18.3564
33. Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008;20(12):2165–2173. doi:10.1016/j.cellsig.2008.05.007
34. Lee SK, Park KK, Kim HJ, et al. Human antigen R-regulated CCL20 contributes to osteolytic breast cancer bone metastasis. Sci Rep. 2017;7(1):9610. doi:10.1038/s41598-017-09040-4
35. Yuan Z, Sanders AJ, Ye L, Wang Y, Jiang WG. Knockdown of human antigen R reduces the growth and invasion of breast cancer cells in vitro and affects expression of cyclin D1 and MMP-9. Oncol Rep. 2011;26(1):237–245. doi:10.3892/or.2011.1271
36. Heinonen M, Fagerholm R, Aaltonen K, et al. Prognostic role of HuR in hereditary breast cancer. Clin Cancer Res. 2007;13(23):6959–6963. doi:10.1158/1078-0432
37. Ortega AD, Sala S, Espinosa E, González-Barón M, Cuezva JM. HuR and the bioenergetic signature of breast cancer: a low tumor expression of the RNA-binding protein predicts a higher risk of disease recurrence. Carcinogenesis. 2008;29(11):2053–2061. doi:10.1093/carcin/bgn185
38. Yuan Z, Sanders AJ, Ye L, Wang Y, Jiang WG. Prognostic value of the human antigen R (HuR) in human breast cancer: high level predicts a favourable prognosis. Anticancer Res. 2011;31(1):303–310.
39. Gallouzi IE, Brennan CM, Stenberg MG, et al. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc Natl Acad Sci U S A. 2000;97(7):3073–3078. doi:10.1073/pnas.97.7.3073
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

