Back to Journals » Cancer Management and Research » Volume 11
The prognosis comparison of different molecular subtypes of breast tumors after radiotherapy and the intrinsic reasons for their distinct radiosensitivity
Authors He L , Lv Y, Song Y, Zhang B
Received 27 April 2019
Accepted for publication 25 May 2019
Published 28 June 2019 Volume 2019:11 Pages 5765—5775
DOI https://doi.org/10.2147/CMAR.S213663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Video abstract presented by Lin He.
Views: 328
Lin He,1 Yang Lv,2 Yuhua Song,1 Biyuan Zhang3
1Breast Center B Ward, The Affiliated Hospital of Qingdao University, Qingdao, Shandong Province, People’s Republic of China; 2Department of Oncology, The PLA Navy Anqing Hospital, Anqing, Anhui Province, People’s Republic of China; 3Department of Radiotherapy, The Affiliated Hospital of Qingdao University, Qingdao, Shandong Province, People’s Republic of China
Abstract: Radiotherapy can increase the cell cycle arrest that promotes apoptosis, reduces the risk of tumor recurrence and has become an irreplaceable component of systematic treatment for patients with breast cancer. Substantial advances in precise radiotherapy unequivocally indicate that the benefits of radiotherapy vary depending on intrinsic subtypes of the disease; luminal A breast cancer has the highest benefit whereas human epidermal growth factor receptor 2 (HER2)-positive and triple negative breast cancer (TNBC) are affected to a lesser extent irrespective of the selection of radiotherapy strategies, such as conventional whole-breast irradiation (CWBI), accelerated partial-breast irradiation (APBI), and hypofractionated whole-breast irradiation (HWBI). The benefit disparity correlates with the differential invasiveness, malignance, and radiosensitivity of the subtypes. A combination of a number of molecular mechanisms leads to the strong radioresistant profile of HER2-positive breast cancer, and sensitization to irradiation can be induced by multiple drugs or compounds in luminal disease and TNBC. In this review, we aimed to summarize the prognostic differences between various subtypes of breast tumors after CWBI, APBI, and HWBI, the potential reasons for drug-enhanced radiosensitivity in luminal breast tumors and TNBC, and the robust radioresistance of HER2-positive cancer.
Keywords: radiotherapy, molecular subtype, breast cancer, molecular mechanism, radiosensitivity
Introduction
Adjuvant radiotherapy is one of the essential components in the treatment of breast cancer and has been recommended in combination with breast-conservation surgery (BCS) for early-stage breast cancer (ESBC) patients and with mastectomy for high-risk patients.1 Compared with total mastectomy and lumpectomy alone, 50 Gy breast irradiation following lumpectomy dramatically lowers the rate of local recurrence (LR) by 7.5% and 6.1%, respectively.2 Moreover, the distant metastasis (DM) rate is decreased in mammary cancer population with radiosensitive characteristics after receiving radiotherapy.3,4 Reduction in overall mortality in breast cancer produced by radiotherapy is essentially identical to systemic chemotherapy.5,6
Multiple radiotherapy strategies are used to treat women at different tumor stages. For the majority of ESBC patients who are qualified for organ preservation, preoperative radiotherapy is a widely adopted standard intervention, whereas postmastectomy radiotherapy is suitable for patients with advanced breast cancer. Nevertheless, not all patients undergoing radiotherapy benefit from it; a large cohort of the patients inevitably suffer radiation-related adverse effects, including fatigue, telangiectasia, angiosarcoma, skin erythema, and cosmetic damage.7–9
Historically, the implementation of radiotherapy for breast cancer is mainly determined by the following patient-related factors: age, comorbidity, tumor stage, lymphatic vessel invasion, etc. The progress in biological methods in the past two decades has elucidated the heterogeneity of diverse molecular subtypes used to design individualized treatment. According to the expression levels of Ki-67 protein and the status of estrogen receptor (ER), progesterone receptor and human epidermal growth factor receptor 2 (HER2), breast cancer can be categorized into four subtypes: luminal A, luminal B, HER2-overexpression, and triple negative breast cancer (TNBC),10 which are outlined in Table 1.
 |
Table 1 The classification of four molecular subtypes of breast cancer |
Several studies investigated whether the intrinsic molecular subtype of breast cancer can influence the outcome of radiotherapy11–13 due to differential prognosis and feedback between chemotherapy and endocrinotherapy.14–20 The EORTC 22881-10882 boost vs no boost trial prescribed or did not prescribe a boost radiation dose of 16 Gy to patients with stage Ⅰ and stage Ⅱ breast cancer who underwent BCS plus conventional whole-breast irradiation (CWBI) of 50 Gy and found that certain phenotypes of tumors are radioresistant and rarely benefit from extra irradiation dose21 suggesting the existence of the dose-benefit gradient of radiotherapy in breast cancer. Therefore, a number of radiotherapy paradigms with low toxicity have been advocated in clinical studies, such as accelerated partial-breast irradiation (APBI) and hypofractionated whole-breast irradiation (HWBI); however, the clinical utility of these methods across four phenotypes of the disease using the same treatment modality is significantly different, which is attributed to inherent radiosensitive or radioresistant properties of the phenotypes to an extent.
The objective of this review was to summarize the prognostic distinctions of various subtypes of breast tumors treated with different radiotherapy methods and to explain the intrinsic reasons for differential radiosensitivity of the subtypes. The molecular mechanisms of cell death induced by ionizing radiation in the tumor and in surrounding normal stem cells are also discussed.
The comparison of prognosis between four subtypes under various radiotherapy conditions
Conventional whole-breast irradiation
For the majority of patients with ESBC or ductal carcinoma in situ (DCIS) in the case of intended breast preservation, standard and widely adopted treatment is CWBI at 50.0 Gy irradiation typically administered at the daily dose of 2.0 Gy via 25 fractions over 5 weeks;2,22 this treatment can reduce the risk of LR by 60–70% and 50–60% in invasive and noninvasive breast carcinoma, respectively.2,23–26 Two independent pioneering randomized trials (The British Columbia Randomized Radiation (BCRR) trial27 and The Danish Breast Cancer Group (DBCG) protocol 82b28) demonstrated the benefits of CWBI combined with polychemotherapy in breast cancer. After follow-up of 15 years, the BCRR trial found a reduction in the rate of locoregional recurrence (LRR) and mortality of 33% and 29%, respectively, which was approximately similar to the outcomes of DBCG 82b trial that demonstrated a reduction in the LRR rate by 23% and 9%, respectively, after 10-years follow-up. These findings have a far-reaching impact on the clinical application of CWBI.
The median time of disease relapse in breast cancer after systemic adjuvant therapy may be 2–4 years or can be significantly prolonged to 5–8 years;24,29,30 this delay is linked to tumor biology and molecular subtypes. Compared to luminal breast cancer, TNBC, and HER2-amplified breast carcinomas commonly have strong invasiveness, shortened survival31 and a 2–3-fold increase in the tumor relapse rate.32,33 Moreover, the risk of DM in TNBC during the initial 2–3 years is higher than that in other subtypes of the disease thus emphasizing unfavorable prognosis.34 Multiple studies have corroborated that the prognosis varies depending on the subtype of breast tumor receiving CWBI.12,35–37 A significantly lengthened overall survival (OS) is observed in luminal A and TNBC but not in other tumor phenotypes. In breast cancer patients treated with BCS combined with CWBI, the 5 years and 10 years LR risk in TNBC and HER2-positive subtypes (without anti-HER2 targeted therapy) is up to twofold higher than that in luminal A subset and the relapse-free survival in luminal B molecular phenotype is lower than that in other intrinsic subtypes; however, the 10 years ipsilateral breast tumor relapse (IBTR) among different subtypes is not significantly different (Table 2). In recent years, alongside with introduction of trastuzumab, the LRR rate of HER2-positive breast cancer has been significantly decreased;38 however, this high LRR rate remains a major threat in TNBC due to the lack of suitable targeted therapy.
 |
Table 2 The prognostic comparison of four molecular subtypes of breast cancer under three types of radiotherapy strategies |
Accelerated partial-breast irradiation
Currently, APBI is gradually becoming a surrogate to CWBI due to its discernible advantages including curtailed curative time, superior local control,39 low toxicity, and favorable cosmetic outcomes.40 The American Brachytherapy Society has published a consensus statement on APBI treatment for breast cancer by taking the following factors into consideration and enacted appropriate criteria for patient selection: age ≥45 years, tumor size ≤3 cm, negative lymph nodes, no invasion of lymph-vascular space, all invasive histology and DCIS, positive/negative ER status, and no infiltration of surgical margins.41
Recently, the correlations of molecular subtypes with the prognosis of breast cancer patients who were treated with APBI have been extensively investigated. In the study of Wadasadawala et al, who evaluated the treatment outcomes of ESBC patients after receiving APBI,42 it was shown that the 3 years LR and LRR across different molecular subtypes were not significantly different whereas the 3 years DM-free survival, OS, and disease-free survival (DFS) of HER2-positive subtype were significantly lower than those of luminal A and B phenotypes. Moreover, in 2016, Dr. Wilkinson introduced a 5-year follow-up clinical results of APBI treatment in 278 ESBC patients,43 which indicated no significant difference in the incidence rates of IBTR, DM, DFS, and OS between four phenotypes of breast tumors (Table 2). In contrast, Pashtan and colleagues evaluated 98 ESBC patients who underwent three-dimensional conformal external beam APBI and discovered partial inconsistencies.44 The multivariate analysis indicated that TNBC was the only predictor for the inferior outcome of 5 years IBTR with a high risk of 33% compared to that of 2% in other pooled subtypes. There may be some connotative explanations for different conclusion in both trails; for example, the majority of TNBC patients in the latter study receives chemotherapy prior to APBI, thereby delaying the initiation of radiotherapy.
It should be noted that for breast cancer patients >50 years of age undergoing APBI, HER2-enriched subtype has a significantly higher risk of 5 years IBTR and 5 years regional nodal recurrence (RNR) than that in all other subtypes, whereas luminal A subtype has the lowest risk of all subtypes.45 A similar conclusion was reached in some clinical trials following multi-catheter APBI (mAPBI)42 and single-entry catheter APBI (sAPBI).46 In the mAPBI trial, HER2-positive status was associated with the shortened DM-free survival, DFS, and OS and the 5 years IBTR of HER2-enriched breast tumors and 5 years RNR of TNBC were significantly higher than those in luminal A disease in the sAPBI trial (Table 2).
Hypofractionated whole-breast irradiation
Radiobiological models indicate that an alternative regimen, known as HWBI, with a larger daily dose per fraction within a shorter duration may achieve efficacy similar to that of CWBI47 and has distinct advantages including higher convenience, lower resource expenditure, and decreased LR rate and radiation-related morbidity.48–50 In 2002, a randomized trial investigated a 5-year follow-up outcomes with reference to BCS followed by CWBI or HWBI at the 42.5 Gy dose divided into 16 fractions over a period of 22 days in the treatment of breast cancer patients with negative status of axillary lymph nodes.51 The two cohorts had identical LR rate of 3% and similar cosmetic outcomes reflecting irradiation-associated complications. Considering possible magnification of the radiation-related toxicity at an extended time,52 women with breast tumors may be inclined to receive HWBI instead of CWBI.
HWBI has become the standard surrogate of CWBI for a large proportion of breast cancer patients;53 however, it is less effective in high-grade tumors regardless of positive or negative lymph nodes leading to curtailed DFS and deterioration of DM.54,55 The highest incidence of LR is observed in HER2-enriched breast cancer patients with lymph node negativity;56 however, no substantial differences in IBTR rate are detected across four intrinsic subtypes.57,58 A study in 752 elderly breast cancer patients (age ≥65 years) who were categorized as having grade 3 primary tumors with positive surgical margins administered a tumor bed boost (n=190).59 The 5 years DFS of TNBC was significantly lower than that in other subtypes of the tumors (p<0.01) without a significant difference in 5 years LR rate (p=0.83); the univariate and multivariate analysis indicated that HER2-positive breast cancer and TNBC were positively correlated with the unfavorable DFS (p<0.05). A total number of 989 node-negative breast cancer women who underwent HWBI following BCS were finally enrolled in the trial of Dr. Bane and colleagues,56 demonstrating that the HER2-positive breast tumor was associated with significantly higher 10 years LR than that of Luminal A breast cancer and TNBC (p<0.01) (Table 2). Collectively, the results from these studies demonstrated variable benefit of different phenotypes of breast tumors from CWBI, APBI, and HWBI treatments, which may be attributed to disparate radiosensitivity of the subtypes.
The reasons for distinct radiosensitivity in individual subtypes
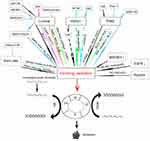
Reparation of DNA double-strand breaks (DDSB) is mainly accomplished through two pathways: non-homologous end joining (NHEJ), the paramount modality of DDSB repair mechanism in terminally differentiated cells that is the dominant pathway in the pre-replicative G1 phase of the cell cycle, and homologous recombination repair pathway (HR) that requires an additional homologous sister chromosome and is the only repair mechanism in the post-replicative S or G2/M phases of the cell cycle. The purpose of radiotherapy is to incite DDSB in the tumor cells to suppress the reparation. Irreversible DNA damage triggers the corresponding cellular mechanisms including cell cycle arrest, apoptosis, and senescence (Figure 1).60,61
In irradiation-induced cell-killing in the tumor, normal stem cells are concomitantly damaged due to their high radiosensitivity.62 Multiple mechanisms predispose stem cells to radiosensitivity. First, the constitutive expression of PP2A in stem cells antagonizes the DDSB reparation. Second, deacetylation and consequent trimethylation of histone 3 lysine-9 (H3K9) increase the radioresistance in the cells; however, stem cells are prone to acetylation and methylation of H3K9 at different residues. Third, a constitutive level of H3K56 acetylation in the stem cells results in chromatin restraining. Finally, the access of S139 site can be sterically dampened by the close proximity of persistent H2AX-pY142 (Figure 1). These mechanisms collectively reinforce the radiosensitivity, interfere with the repair of DDSB and induce apoptosis in normal stem cells, which may partially explain the irreversible damage to X-ray radiation to the tissues adjacent to the tumor.
The MAP3K4 gene was shown to be a potential target to regulate radiosensitivity63, and molecular oxygen is considered as a highly efficient radiosensitizer64 for breast tumors. The oxygen-dependent radiosensitivity is sharply increased when the partial pressure of oxygen (pO2) elevates from 0 to 10 mmHg and attains its half-maximum value when pO2=3 mmHg;65 moreover, radiosensitivity slowly increases once the pO2 rises above 30 mmHg and up to 100% pure oxygen.64,66 One possible explanation of this fact is that a selective accumulation of TAT-ODD-p53 occurs in hypoxic environment inhibiting mitophagy that plays a key role in maintaining hypoxia-induced radioresistance thereby significantly enhancing the radiosensitivity of tumor cells (Figure 1).67
Luminal breast cancer
The intrinsic mechanisms of radiosensitivity in luminal breast cancer are well recognized and well documented. A significantly elevated radiosensitivity and a series of favorable anticancer outcomes with slight side effects have been attained by treatment targeted against tumor-related epidermal growth factor receptor (EGFR) in preclinical and clinical studies.68,69 The cytotoxic effect of irradiation is increased when the EGFR activity and its downstream signals, such as PI3K-AKT and RAS-MAPK pathways, are downregulated; these pathways can induce cell cycle arrest and apoptosis, suppress cell proliferation and tumor angiogenesis, and reduce the DM incidence.70,71 Luminal subtype of breast cancer can be effectively radiosensitized by treatment with nimotuzumab,72 a humanized monoclonal IgG1 antibody, which can block the function of DNA-PKcs and the binding of EGF, TGF-α, and other ligands to EGFR.73 This benefit may be attributed to the decreased level of phosphorylated EGFR, induction of cell apoptosis and generation of γ-H2AX, a vital indicator of radiation-induced DDSB (Figure 1).72,74,75
Exogenous miR195 can downregulate BCL-276 and ubiquitin-conjugating enzyme E2D3 curtails the accumulation of human telomerase reverse transcriptase and cyclin D1;77 the upregulation of expression of these proteins significantly improves the radiosensitivity in luminal breast cancer (Figure 1). In tumor cells, histone deacetylase inhibitors (HDACis) inhibit the reparation of DDSB via downregulation of the activities of DNA repair proteins, for instance, Rad51, Ku80, and BRCA1.78,79 Based on this theory, Jiang et al, treated patients with luminal advanced breast cancer, who did not benefit from endocrine therapy, with a combination of chidamide, an oral subtype-selective HDACis with multiple functions related to repression of tumor growth and modulation of microenvironment by epigenetic reprogramming, with exemestane and found that the median PFS was significantly prolonged in comparison with that in a cohort treated with placebo plus exemestane (7.4 months vs 3.8 months, respectively; p=0.03).80 Moreover, valproic acid, which is one of the typical HDACis, at a safe dose of 0.5 mM, can substantially elevate radiosensitivity in the luminal tumor cells by interrupting the molecular mechanism of BRCA1-Rad51-mediated HR and Ku80-mediated NHEJ pathways (Figure 1).81
The thioredoxin (Trx) system is the core enzyme family that controls the redox regulation in the cells and is associated with the irradiation effects in the cancer cells.82 Metformin can suppress the Trx expression via the AMPK-FOXO3 pathway that increases the level of intracellular reactive oxygen species (ROS) in the primary humanity aortic endothelial cells to influence redox regulation; metformin can radiosensitize the luminal cancer cells by activating AMPK and suppressing mTOR.83 Moreover, metformin can significantly prolong the breast cancer-specific survival in diabetic women with luminal breast cancer;84 however, metformin is ineffective in TNBC patients85 apparently due to differential metformin-induced radiosensitivity; the effects of the drug are substantial in luminal tumors and are small in TNBC. Several poorly understood reasons may explain unique metformin-induced radiosensitivity; one of the explanations is that changes in ROS levels and attenuation of Trx expression take place in luminal tumors, but these factors remain unchanged in TNBC (Figure 1).86
TNBC
Histamine participates in the regulation of growth and differentiation of mammary cells during development, pregnancy, and lactation of females and regulates the proliferation of malignant cells.87,88 In luminal breast cancer cells, histamine and histamine H4 receptor (H4R) agonist-based magnification of radiosensitivity is attained by induction of the DDSB proteins, such as 8-OHdG, γH2AX, and p53; in TNBC cells, histamine and an H1R agonist also induce the formation of the DDSB proteins, including 8-OHdG and γH2AX but excluding p53, elevate ROS levels and upregulate the LCN-2 expression to sensitize the cells to the X-ray effects.89 An ex vivo study demonstrated that radiosensitivity was amplified in TNBC by Piper longumine, which upregulated the expression of apoptosis-related proteins, BCL2 and BAX, and increases the levels of intracellular ROS (Figure 1).90
Chemokine receptor 4 (CXCR4) promotes trafficking and invasiveness of non-small cell lung cancer cells after ionizing radiation and knockdown of CXCR4 can ameliorate the efficacy of radiotherapy.91 A number of in vivo and in vitro studies demonstrated that combination of AMD3100, a small-molecule CXCR4 inhibitor, with radiotherapy increases radiosensitivity in prostate cancer,92 glioma,93 ovarian carcinoma94, and TNBC.95 In TNBC cells, the ADM3100-induced radiosensitivity is elevated due to the accumulation of BAX and caspase-3 and downregulation of BCL-2, thereby arresting the cell cycle in the G2/M phase and eliciting apoptosis (Figure 1).
HER2 positive breast cancer
Ionizing radiation can directly activate the EGFR family in tumor cells and reduplicative irradiation at 2 Gy contributes to upregulation of EGFR expression in HER2-enriched breast cancer. These phenomena indicate that HER2-positive status has a potential biological function impacting the radiation response.96 Radioresistant HER2-overexpressing breast cancer patients treated by mastectomy in combination with radiotherapy universally experience a high LRR rate, poor prognosis, and minor treatment benefits.33,36,97–99 However, in a retrospective trial conducted on women with lymph node-negative, HER2-positive breast cancer who received BCS and CWBI, the authors represented that the 3 years LRR rate was 1% for the trastuzumab cohort and 9% for the no-trastuzumab cohort,100 suggesting that the characteristic of HER2-positive breast tumor resisting to irradiation may have little impact on the prognosis of lymph node-negative patients. The molecular mechanisms of the reason for robust radioresistance of HER2-positive breast tumors have been successfully investigated. The transactivation of the NF-κB-mediated HER2 promoter induces HER2 overexpression which is responsible for radioresistance.101 Furthermore, increased radioresistance is associated with breast cancer stem cells and may be induced by epithelial-to-mesenchymal transition through a key molecular substance named as β-catenin that can be detected in invasive and metastatic HER2-positive tumors .102–106 Importantly, in vivo studies have confirmed that substantial clinical benefits can be achieved by inhibiting the Fak-mediated pathway107–109 that plays a crucial role in upregulation of the radioresistance of HER2-enriched breast cancer (Figure 1).110
Conclusion
Radiotherapy significantly ameliorates the prognosis and decreases the incidence rate of life loss in breast cancer patients and has been an indispensable element in the systematic treatment of the disease. Regardless of the use of radiotherapy, luminal A breast cancer has the most favorable clinical outcomes after ionizing irradiation compared to that in HER2-positive cancer and TNBC. Differences in outcomes between these subtypes of the disease are mainly determined by differential radioresistivity, aggressiveness, and malignance of the subtypes. X-rays eliminate tumor cells through increased cell cycle arrest, which concomitantly induces an unavoidable severe side effect in normal stem cells in the adjacent tissues. The intensification of radioresistance in HER2-positive breast cancer is ascribed to multiple molecular mechanisms; in contrast, several drugs or compounds sensitize the cells to radiation and increase irradiation efficacy in luminal cancer and TNBC via specific pathways.
Highlights
- Irrespective of the selection of radiotherapy paradigm, luminal A breast cancer has an overall favorable prognosis relative to HER2-positive and TNBC subtypes partially due to individual radiosensitivity of these subtypes.
- Ionizing irradiation induces ablation of the tumor mainly through increasing the cell cycle arrest to promote apoptosis and senescence; however, ionizing radiation induces serious adverse effects in the normal stem cells in the adjacent tissues.
- HER2-positive breast cancer has high radioresistance that is correlated to the transactivation of the NF-κB-mediated HER2 promoter inducing HER2 overexpression, β-catenin expression during EMT and the Fak-mediated pathway.
- Medications or compounds reinforce radiosensitivity in luminal breast cancer and TNBC largely due to an increase in the ROS level and modulation of DNA double-strand break- and/or apoptosis-related proteins, such as 8-OHdG, γH2AX, and p53.
Abbreviation list
BCS, breast-conservation surgery; ESBC, early-stage breast cancer; LR, local recurrence; DM, distant metastasis; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple negative breast cancer; CWBI, conventional whole-breast irradiation; APBI, accelerated partial-breast irradiation; HWBI, hypofractionated whole-breast irradiation; DCIS, ductal carcinoma in situ; LRR, locoregional recurrence; IBTR, ipsilateral breast tumor relapse; DFS, disease-free survival; OS, overall survival; DDSB, DNA double-strand breakage; NHEJ, non-homologous end joining; HR, homologous recombination repair pathway; H3K9, histone 3 lysine-9; pO2, partial pressure of oxygen; EGFR, epidermal growth factor receptor; HDACis, histone deacetylase inhibitors; Trx, thioredoxin; ROS, reactive oxygen species; H4R, histamine 4 receptor; CXCR 4, chemokine receptor 4.
Ethics statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi:10.1093/annonc/mdv221
2. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi:10.1056/NEJMoa022152
3. Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys. 2009;75(2):489–496. doi:10.1016/j.ijrobp.2009.06.014
4. Eschrich S, Zhang H, Zhao H, et al. Systems biology modeling of the radiation sensitivity network: a biomarker discovery platform. Int J Radiat Oncol Biol Phys. 2009;75(2):497–505. doi:10.1016/j.ijrobp.2009.05.056
5. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi:10.1016/S0140-6736(05)67887-7
6. Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi:10.1016/S0140-6736(11)61629-2
7. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260–2266.
8. Holli K, Saaristo R, Isola J, Joensuu H, Hakama M. Lumpectomy with or without postoperative radiotherapy for breast cancer with favourable prognostic features: results of a randomized study. Br J Cancer. 2001;84(2):164–169. doi:10.1054/bjoc.2000.1571
9. Lilla C, Ambrosone CB, Kropp S, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;106(1):143–150. doi:10.1007/s10549-006-9480-9
10. Britten A, Rossier C, Taright N, Ezra P, Bourgier C. Genomic classifications and radiotherapy for breast cancer. Eur J Pharmacol. 2013;717(1–3):67–70. doi:10.1016/j.ejphar.2012.11.069
11. Liu FF, Shi W, Done SJ, et al. Identification of a low-risk luminal A breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol. 2015;33(18):2035–2040. doi:10.1200/JCO.2014.57.7999
12. Sjostrom M, Lundstedt D, Hartman L, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the swedish breast cancer group 91 radiotherapy randomized clinical trial. J Clin Oncol. 2017;35(28):3222–3229. doi:10.1200/JCO.2017.72.7263
13. Tramm T, Kyndi M, Myhre S, et al. Relationship between the prognostic and predictive value of the intrinsic subtypes and a validated gene profile predictive of loco-regional control and benefit from post-mastectomy radiotherapy in patients with high-risk breast cancer. Acta Oncol. 2014;53(10):1337–1346. doi:10.3109/0284186X.2014.925580
14. Nielsen TO, Parker JS, Leung S, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16(21):5222–5232. doi:10.1158/1078-0432.CCR-10-1282
15. Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105(19):1504–1511. doi:10.1093/jnci/djt244
16. Prat A, Bianchini G, Thomas M, et al. Research-based PAM50 subtype predictor identifies higher responses and improved survival outcomes in HER2-positive breast cancer in the NOAH study. Clin Cancer Res. 2014;20(2):511–521. doi:10.1158/1078-0432.CCR-13-0239
17. Cheang MC, Voduc KD, Tu D, et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin Cancer Res. 2012;18(8):2402–2412. doi:10.1158/1078-0432.CCR-11-2956
18. Chia SK, Bramwell VH, Tu D, et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin Cancer Res. 2012;18(16):4465–4472. doi:10.1158/1078-0432.CCR-12-0286
19. Nielsen TO, Jensen MB, Burugu S, et al. High-risk premenopausal luminal A breast cancer patients derive no benefit from adjuvant cyclophosphamide-based Chemotherapy: results from the DBCG77B clinical trial. Clin Cancer Res. 2017;23(4):946–953. doi:10.1158/1078-0432.CCR-16-1278
20. Jorgensen CL, Nielsen TO, Bjerre KD, et al. PAM50 breast cancer intrinsic subtypes and effect of gemcitabine in advanced breast cancer patients. Acta Oncol. 2014;53(6):776–787. doi:10.3109/0284186X.2013.865076
21. Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25(22):3259–3265. doi:10.1200/JCO.2007.11.4991
22. Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328(22):1587–1591. doi:10.1056/NEJM199306033282202
23. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi:10.1056/NEJMoa020989
24. van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–1150. doi:10.1093/jnci/92.14.1143
25. Bijker N, Meijnen P, Peterse JL, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853 – a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24(21):3381–3387. doi:10.1200/JCO.2006.06.1366
26. Houghton J, George WD, Cuzick J, Duggan C, Fentiman IS, Spittle M. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003;362(9378):95–102.
27. Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956–962. doi:10.1056/NEJM199710023371402
28. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b trial. N Engl J Med. 1997;337(14):949–955. doi:10.1056/NEJM199710023371401
29. Touboul E, Buffat L, Belkacemi Y, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys. 1999;43(1):25–38.
30. Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 1999;44(5):1005–1015.
31. Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi:10.1073/pnas.191367098
32. Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26(14):2373–2378. doi:10.1200/JCO.2007.14.4287
33. Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi:10.1200/JCO.2009.24.9284
34. Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24(36):5652–5657. doi:10.1200/JCO.2006.06.5664
35. Mao JH, Diest PJV, Perez-Losada J, Snijders AM. Revisiting the impact of age and molecular subtype on overall survival after radiotherapy in breast cancer patients. Sci Rep. 2017;7(1):12587. doi:10.1038/s41598-017-12949-5
36. Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–841. doi:10.1007/s10549-011-1891-6
37. Garvin S, Vikhe Patil E, Arnesson LG, et al.. Differences in intra-tumoral macrophage infiltration and radiotherapy response among intrinsic subtypes in pT1-T2 breast cancers treated with breast-conserving surgery. Virchows Arch. 2019. doi:10.1007/s00428-019-02563-3
38. Tseng YD, Uno H, Hughes ME, et al. Biological subtype predicts risk of locoregional recurrence after mastectomy and impact of postmastectomy radiation in a large national database. Int J Radiat Oncol Biol Phys. 2015;93(3):622–630. doi:10.1016/j.ijrobp.2015.07.006
39. Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet. 2016;387(10015):229–238. doi:10.1016/S0140-6736(15)00471-7
40. Bitter SM, Heffron-Cartwright P, Wennerstrom C, Weatherford J, Einstein D, Keiler LC. WBRT vs. APBI: an interim report of patient satisfaction and outcomes. J Contemp Brachyther. 2016;8(1):17–22. doi:10.5114/jcb.2016.57816
41. Shah C, Vicini F, Shaitelman SF, et al. The American Brachytherapy Society consensus statement for accelerated partial-breast irradiation. Brachytherapy. 2018;17(1):154–170. doi:10.1016/j.brachy.2017.09.004
42. Wadasadawala T, Mondal M, Paul SN, et al. Should molecular subtype be recommended as one of the selection criteria for accelerated partial breast irradiation? Preliminary results from an Asian cohort. J Contemp Brachytherapy. 2018;10(1):47–57. doi:10.5114/jcb.2018.74137
43. Wilkinson JB, Shah C, Amin M, et al. Outcomes according to breast cancer subtype in patients treated with accelerated partial breast irradiation. Clin Breast Cancer. 2017;17(1):55–60. doi:10.1016/j.clbc.2016.07.010
44. Pashtan IM, Recht A, Ancukiewicz M, et al. External beam accelerated partial-breast irradiation using 32 gy in 8 twice-daily fractions: 5-year results of a prospective study. Int J Radiat Oncol Biol Phys. 2012;84(3):e271–e277. doi:10.1016/j.ijrobp.2012.04.019
45. Anderson BM, Kamrava M, Wang PC, et al. Locoregional recurrence by molecular subtype after multicatheter interstitial accelerated partial breast irradiation: results from the Pooled Registry Of Multicatheter Interstitial Sites research group. Brachytherapy. 2016;15(6):788–795. doi:10.1016/j.brachy.2016.08.012
46. Saini A, Kuske R, Quiet C, Pantoja C, Reed D, Zannis V. Outcomes by molecular subtype after accelerated partial breast irradiation using single-entry catheters. Brachytherapy. 2018;17(2):415–424. doi:10.1016/j.brachy.2017.10.009
47. Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–694. doi:10.1259/0007-1285-62-740-679
48. Ash DV, Benson EA, Sainsbury JR, Round C, Head C. Seven-year follow-up on 334 patients treated by breast conserving surgery and short course radical postoperative radiotherapy: a report of the Yorkshire Breast Cancer Group. Clin Oncol (R Coll Radiol). 1995;7(2):93–96.
49. Olivotto IA, Weir LM, Kim-Sing C, et al. Late cosmetic results of short fractionation for breast conservation. Radiother Oncol. 1996;41(1):7–13.
50. Shelley W, Brundage M, Hayter C, Paszat L, Zhou S, Mackillop W. A shorter fractionation schedule for postlumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;47(5):1219–1228.
51. Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94(15):1143–1150. doi:10.1093/jnci/94.15.1143
52. Curran D, van Dongen JP, Aaronson NK, et al. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC trial 10801. The European Organization for Research and Treatment of Cancer (EORTC), Breast Cancer Co-operative Group (BCCG). Eur J Cancer. 1998;34(3):307–314.
53. Castaneda SA, Strasser J. Updates in the treatment of breast cancer with radiotherapy. Surg Oncol Clin N Am. 2017;26(3):371–382. doi:10.1016/j.soc.2017.01.013
54. Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–520. doi:10.1056/NEJMoa0906260
55. Bellefqih S, Elmajjaoui S, Aarab J, et al. Hypofractionated regional nodal irradiation for women with node-positive breast cancer. Int J Radiat Oncol Biol Phys. 2017;97(3):563–570.
56. Bane AL, Whelan TJ, Pond GR, et al. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol. 2014;25(5):992–998. doi:10.1093/annonc/mdu090
57. Hattangadi-Gluth JA, Wo JY, Nguyen PL, et al. Basal subtype of invasive breast cancer is associated with a higher risk of true recurrence after conventional breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2012;82(3):1185–1191. doi:10.1016/j.ijrobp.2011.02.061
58. Pinnaro P, Giordano C, Farneti A, et al. Short course hypofractionated whole breast irradiation after conservative surgery: a single institution phase II study. J Exp Clin Res. 2017;36(1):191. doi:10.1186/s13046-017-0640-z
59. De Santis MC, Bonfantini F, Di Salvo F, et al. Hypofractionated whole-breast irradiation with or without boost in elderly patients: clinical evaluation of an Italian experience. Clin Breast Cancer. 2018;18:e1059–e1066. doi:10.1016/j.clbc.2018.04.003
60. Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi:10.1038/nrm2233
61. Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16(6):663–669. doi:10.1016/j.ceb.2004.09.011
62. Fabbrizi MR, Warshowsky KE, Zobel CL, Hallahan DE, Sharma GG. Molecular and epigenetic regulatory mechanisms of normal stem cell radiosensitivity. Cell Death Discovery. 2018;4:117.
63. Tanic M, Krivokuca A, Cavic M, et al. Molecular signature of response to preoperative radiotherapy in locally advanced breast cancer. Radiat Oncol. 2018;13(1):193. doi:10.1186/s13014-018-1129-4
64. Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med. 2009;9(4):442–458.
65. Hockel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991;51(22):6098–6102.
66. Kirkpatrick JP, Cardenas-Navia LI, Dewhirst MW. Predicting the effect of temporal variations in PO2 on tumor radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;59(3):822–833. doi:10.1016/j.ijrobp.2004.02.015
67. Zheng R, Yao Q, Xie G, et al. TAT-ODD-p53 enhances the radiosensitivity of hypoxic breast cancer cells by inhibiting Parkin-mediated mitophagy. Oncotarget. 2015;6(19):17417–17429. doi:10.18632/oncotarget.4002
68. Verheij M, Vens C, van Triest B. Novel therapeutics in combination with radiotherapy to improve cancer treatment: rationale, mechanisms of action and clinical perspective. Drug Resist Updates. 2010;13(1–2):29–43. doi:10.1016/j.drup.2010.01.002
69. Raben D, Helfrich B, Chan DC, et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res. 2005;11(2 Pt 1):795–805.
70. Chen DJ, Nirodi CS. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 2007;13(22 Pt 1):6555–6560. doi:10.1158/1078-0432.CCR-07-1610
71. Zhuang HQ, Sun J, Yuan ZY, et al. Radiosensitizing effects of gefitinib at different administration times in vitro. Cancer Sci. 2009;100(8):1520–1525. doi:10.1111/j.1349-7006.2009.01190.x
72. Qu YY, Hu SL, Xu XY, et al. Nimotuzumab enhances the radiosensitivity of cancer cells in vitro by inhibiting radiation-induced DNA damage repair. PLoS One. 2013;8(8):e70727. doi:10.1371/journal.pone.0070727
73. Boland W, Bebb G. The emerging role of nimotuzumab in the treatment of non-small cell lung cancer. Biol Targets Ther. 2010;4:289–298.
74. Tanaka T, Munshi A, Brooks C, Liu J, Hobbs ML, Meyn RE. Gefitinib radiosensitizes non-small cell lung cancer cells by suppressing cellular DNA repair capacity. Clin Cancer Res. 2008;14(4):1266–1273. doi:10.1158/1078-0432.CCR-07-1606
75. Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65(8):3328–3335. doi:10.1158/0008-5472.CAN-04-3547
76. Zhu J, Ye Q, Chang L, Xiong W, He Q, Li W. Upregulation of miR-195 enhances the radiosensitivity of breast cancer cells through the inhibition of BCL-2. Int J Clin Exp Med. 2015;8(6):9142–9148.
77. Wang W, Yang L, Hu L, et al. Inhibition of UBE2D3 expression attenuates radiosensitivity of MCF-7 human breast cancer cells by increasing hTERT expression and activity. PLoS One. 2013;8(5):e64660. doi:10.1371/journal.pone.0064660
78. Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat Rev Mol Cell Biol. 2010;11(3):196–207. doi:10.1038/nrm2851
79. Smith S, Fox J, Mejia M, et al. Histone deacetylase inhibitors selectively target homology dependent DNA repair defective cells and elevate non-homologous endjoining activity. PLoS One. 2014;9(1):e87203. doi:10.1371/journal.pone.0102335
80. Jiang Z. Phase III trial of childamide, a subtype-selecetive histone deacetylase HDAC inhibitor, in combination with exemestane in patients with hormone receptor-positive advanced breast cancer.
81. Luo Y, Wang H, Zhao X, et al. Valproic acid causes radiosensitivity of breast cancer cells via disrupting the DNA repair pathway. Toxicol Res (Camb). 2016;5(3):859–870. doi:10.1039/c5tx00476d
82. Zhang Y, Martin SG. Redox proteins and radiotherapy. Clin Oncol (R Coll Radiol). 2014;26(5):289–300. doi:10.1016/j.clon.2014.02.003
83. Song CW, Lee H, Dings RP, et al. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;2:362. doi:10.1038/srep00386
84. Xiao Y, Zhang S, Hou G, Zhang X, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of diabetic women with luminal subtype breast cancer. Tumour Biol. 2014;35(3):2035–2045. doi:10.1007/s13277-013-1270-5
85. Bayraktar S, Hernadez-Aya LF, Lei X, et al. Effect of metformin on survival outcomes in diabetic patients with triple receptor-negative breast cancer. Cancer. 2012;118(5):1202–1211. doi:10.1002/cncr.26439
86. Zhang Y, Storr SJ, Johnson K, et al. Involvement of metformin and AMPK in the radioresponse and prognosis of luminal versus basal-like breast cancer treated with radiotherapy. Oncotarget. 2014;5(24):12936–12949. doi:10.18632/oncotarget.2683
87. Massari NA, Nicoud MB, Medina VA. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol. 2018. doi:10.1111/bph.14535
88. Rivera ES, Cricco GP, Engel NI, Fitzsimons CP, Martin GA, Bergoc RM. Histamine as an autocrine growth factor: an unusual role for a widespread mediator. Semin Cancer Biol. 2000;10(1):15–23.
89. Martinel Lamas DJ, Cortina JE, Ventura C, et al. Enhancement of ionizing radiation response by histamine in vitro and in vivo in human breast cancer. Cancer Biol Ther. 2015;16(1):137–148. doi:10.4161/15384047.2014.987091
90. Yao JX, Yao ZF, Li ZF, Liu YB. Radio-sensitization by Piper longumine of human breast adenoma MDA-MB-231 cells in vitro. APJCP. 2014;15(7):3211–3217.
91. Gu Q, He Y, Ji J, et al. Hypoxia-inducible factor 1α (HIF-1α) and reactive oxygen species (ROS) mediates radiation-induced inv asiveness through the SDF-1α/CXCR4 pathway in non-small cell lung carcinoma cells. Oncotarget. 2015;6(13):10893–10907. doi:10.18632/oncotarget.3535
92. Domanska UM, Boer JC, Timmer-Bosscha H, et al. CXCR4 inhibition enhances radiosensitivity, while inducing cancer cell mobilization in a prostate cancer mouse model. Clin Exp Metastasis. 2014;31(7):829–839. doi:10.1007/s10585-014-9673-2
93. Ali MM, Kumar S, Shankar A, et al. Effects of tyrosine kinase inhibitors and CXCR4 antagonist on tumor growth and angiogenesis in rat glioma model: MRI and protein analysis study. Transl Oncol. 2013;6(6):660–669.
94. Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carc inoma. Int J Cancer. 2008;122(1):91–99. doi:10.1002/ijc.23083
95. Zhou KX, Xie LH, Peng X, et al. CXCR4 antagonist AMD3100 enhances the response of MDA-MB-231 triple-negative breast cancer cells to ionizing radiation. Cancer Lett. 2018;418:196–203. doi:10.1016/j.canlet.2018.01.009
96. Buchholz TA, Huang EH, Berry D, et al. Her2/neu-positive disease does not increase risk of locoregional recurrence for patients treated with neoadjuvant doxorubicin-based chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(5):1337–1342. doi:10.1016/j.ijrobp.2004.02.018
97. Ribelles N, Perez-Villa L, Jerez JM, et al. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. BCR. 2013;15(5):R98. doi:10.1186/bcr3559
98. Park S, Koo JS, Kim MS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21(1):50–57. doi:10.1016/j.breast.2011.07.008
99. Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358(13):1409–1411. doi:10.1056/NEJMc0801440
100. Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer. 2012;118(8):1982–1988. doi:10.1002/cncr.26484
101. Cao N, Li S, Wang Z, et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res. 2009;171(1):9–21. doi:10.1667/RR1472.1
102. Duru N, Fan M, Candas D, et al. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18(24):6634–6647. doi:10.1158/1078-0432.CCR-12-1436
103. Giordano A, Gao H, Anfossi S, et al. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther. 2012;11(11):2526–2534. doi:10.1158/1535-7163.MCT-12-0460
104. Arias-Romero LE, Villamar-Cruz O, Huang M, Hoeflich KP, Chernoff J. Pak1 kinase links ErbB2 to β-catenin in transformation of breast epithelial cells. Cancer Res. 2013;73(12):3671–3682. doi:10.1158/0008-5472.CAN-12-4453
105. Schade B, Lesurf R, Sanguin-Gendreau V, et al. β-Catenin signaling is a critical event in ErbB2-mediated mammary tumor progression. Cancer Res. 2013;73(14):4474–4487. doi:10.1158/0008-5472.CAN-12-3925
106. Oliveras-Ferraros C, Corominas-Faja B, Cufi S, et al. Epithelial-to-mesenchymal transition (EMT) confers primary resistance to trastuzumab (Herceptin). Cell Cycle. 2012;11(21):4020–4032. doi:10.4161/cc.22225
107. Taliaferro-Smith L, Oberlick E, Liu T, et al. FAK activation is required for IGF1R-mediated regulation of EMT, migration, and invasion in mesenchymal triple negative breast cancer cells. Oncotarget. 2015;6(7):4757–4772. doi:10.18632/oncotarget.3023
108. Wilson C, Nicholes K, Bustos D, et al. Overcoming EMT-associated resistance to anti-cancer drugs via Src/FAK pathway inhibition. Oncotarget. 2014;5(17):7328–7341. doi:10.18632/oncotarget.2397
109. Hao H, Naomoto Y, Bao X, et al. Focal adhesion kinase as potential target for cancer therapy (Review). Oncol Rep. 2009;22(5):973–979.
110. Hou J, Zhou Z, Chen X, et al. HER2 reduces breast cancer radiosensitivity by activating focal adhesion kinase in vitro and in vivo. Oncotarget. 2016;7(29):45186–45198. doi:10.18632/oncotarget.9870
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.