Back to Journals » ClinicoEconomics and Outcomes Research » Volume 10
The preventive effect on respiratory tract infections of Oscillococcinum®. A cost-effectiveness analysis
Authors Colombo GL , Di Matteo S, Martinotti C , Oselin M , Bruno GM , Beghi GM
Received 19 June 2017
Accepted for publication 17 November 2017
Published 23 January 2018 Volume 2018:10 Pages 75—82
DOI https://doi.org/10.2147/CEOR.S144300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Dean Smith
Giorgio L Colombo,1,2 Sergio Di Matteo,2 Chiara Martinotti,2 Martina Oselin,2 Giacomo M Bruno,2 Gianfranco M Beghi3
1Department of Drug Sciences, University of Pavia, Pavia, Italy; 2S.A.V.E. Studi Analisi Valutazioni Economiche S.r.l., Health Economics & Outcomes Research, Milan, Italy; 3Unit of Pulmonary Rehabilitation, Hospital of Casorate Primo, Pavia, Italy
Background: Anas barbariae hepatis et cordis extractum 200K (Oscillococcinum®) is used to treat and prevent seasonal colds and airway inflammatory affections, improve symptom control, and reduce the frequency of respiratory tract infection (RTI) episodes. The objective of this controlled observational study is to investigate, from the Italian National Health Service (NHS) point of view, the role of Anas barbariae hepatis et cordis extractum 200K in preventing RTIs and estimate the annual average cost per patient due to visits and medicines in a real-world setting, investigating whether this method of treatment can bring savings for the NHS.
Methods: Data from a single center from 2002 to 2011 were used. The analysis examined 455 patients who suffered from respiratory diseases. Of the total number of patients, 246 were treated with Anas barbariae hepatis et cordis extractum 200K while 209 were not treated (Control group). All the data concerning RTI episodes, pharmacological treatments, and pneumological visits were extracted from the database.
Results: It was found that, regardless of the diagnosis, the frequency of RTI episodes was always lower in patients treated with Anas barbariae hepatis et cordis extractum 200K; the difference between the numbers of events occurring was statistically significant in every class of patients (p<0.001). The costs that the NHS had to incur were significantly lower in the classes of patients treated (p<0.001).
Discussion: The results indicate that Anas barbariae hepatis et cordis extractum 200K has a preventive effect on the onset of RTI episodes. The analysis shows that treating patients with Anas barbariae hepatis et cordis extractum 200K lowers costs for the NHS; this is primarily due to the fact that the medication causes fewer episodes of RTI to develop. This study suggests that the treatment with Anas barbariae hepatis et cordis extractum 200K could be helpful in preventing RTIs and improving the health status of patients who suffer from respiratory diseases, and it could lead to savings to the Italian NHS.
Keywords: Oscillococcinum, prevention, respiratory tract infection, cost-effectiveness
Background
Homeopathy is a 200-year-old form of integrative medicine that was developed in Germany. The discipline is based on the theory “like cures like”; meaning substances that cause symptoms in a healthy person have the ability to cure an ill person suffering from the same symptoms (when administered in homeopathic potencies). Homeopathy is also based on the belief that molecules in highly diluted substances retain a signature of the original substance. Specifically, the techniques used to prepare homeopathic remedies include a process of repeated dilution and agitation, known as “potentization” or “dynamization”, of the substance in hydroalcoholic solutions or in other excipients and the “succession” of the product into different grades.1 Homeopathic medicines are derived from substances that come from herbs, minerals, or animals, such as red onion, arnica, crushed whole bees, belladonna. Homeopathic medicines are often formulated as sugar pellets that must be placed under the tongue to be absorbed by the body; they may also be made available in other forms, such as drops, ointments, gels, creams, and tablets. Treatments are “individualized” or tailored to each person–it is not uncommon for different people with the same condition to receive different treatments.2 This lack of prescribing standards is one of the usually debated points in homeopathy as well as the fact that several effectiveness analyses on the subject have led to the conclusion that homeopathy cannot be viewed as an evidence-based form of therapy since the clinical effects that these medicines produce are neither superior to the control nor significantly different from the placebo.3 Despite its controversial nature, the clinical use of these products has increased in recent years. According to the 2015 Italian National Institute of Statistics (ISTAT) research based on the information collected in a survey conducted in 2013, in Italy, homeopathic medicines are regularly used by about 2,452,000 people, ~4.1% of the whole population, placing Italians in third place in Europe after the citizens of France and Germany. The same statistical data showed that about 20,000 physicians prescribe, at least once a year, homeopathic medicines.4 Over 70% of homeopathy users are individuals affected by multiple chronic pathologies at the same time; they are, therefore, patients who take multi-pharmacological treatments, who are subject to frequent clinical relapses or do not respond to specific conventional pharmacological treatments. According to data from the international scientific literature, the clinical conditions that are commonly treated with homeopathic medicines are: respiratory or dermatological allergies, gastrointestinal disorders, obstetric and gynecological diseases, otolaryngology diseases, dermatological diseases, inflammatory syndromes, respiratory diseases, circulatory disorders, headaches. About one-quarter of the patients are children <14 years old, who use homeopathic medicines especially to treat acute relapsing diseases of the upper respiratory tract.5 In regard to this, an observational longitudinal study carried out in Italy from 1998 to 2008 analyzed the socio-demographic characteristics and the outcomes of a pediatric population affected by different diseases like the previously mentioned, the most frequent of which were respiratory diseases. The results seem to confirm that homeopathic medicine produces a positive therapeutic response, particularly in children presenting respiratory diseases, since the study demonstrated that 68% of children with respiratory disease had a significant improvement or achieved a resolution of their problems.6 Based on the homeopathic principle of “isopathy”, a medicine derived from the causative agent of the disease, or from a product of the disease process, is used to treat the condition;7 a specific extract of wild duck’s heart and liver, which may be reservoirs and vectors of influenza viruses and infective agents responsible for respiratory tract infections (RTIs) occurring in the sinuses, throat, and airways, is used to prevent and treat influenza and the viruses that cause influenza-like syndromes, such as cough, fever, chills, and muscle pain.8,9 This product is patented with the name of Anas barbariae hepatis et cordis extractum 200K and made by the “Korsakovian” method. The extract of Anas barbariae hepatis and cordis extractum is shaken in a flask and then poured off. A mixture of water/alcohol is added to dilute the liquid, which remains on the walls of the flask (~1%). This new dilution is succussed and poured off. The process is carried out 200 times, to give a “200K” dilution or “potency”.10 Its high potency, though, is one of the most disputed aspects of homeopathy: from the physico-chemical point of view, these solutions, with high probability, do not contain even a single molecule of the original substance, so they do not have a clinical effect. However, recent studies undertaken to clarify the efficacy of homeopathic medicines have shown that highly diluted homeopathic products can produce physiological effects.11 Anas barbariae hepatis et cordis extractum 200K is regularly used during the winter months to treat and prevent seasonal colds and airway inflammatory conditions, improving symptom control and reducing the frequency of RTI episodes. It is commercially available as an over-the-counter product in many countries. In Italy, homeopathic medicines, as well as Anas barbariae hepatis et cordis extractum 200K, are all purchased directly by the citizen, so this expense is not borne by the National Health Service (NHS). These medicines would, therefore, reduce health care expenditure and extend patients’ lives; at least this is what has been demonstrated by Kooreman and Baars. Their study shows that patients treated with homeopathic medicines tend to have lower mortality rates: their mortality rate is lower by up to 30% than those treated with conventional medicine. The treatment’ costs incurred on patients who take homeopathic products are 7% lower compared with those who take conventional drugs. The lower total costs result from lower hospital and pharmaceutical costs (prescription medicines), therefore this can be translated into a lower demand for health care.12 The aim of this paper is to confirm this trend. The article presents the results of a retrospective controlled observational study designed to examine changes in health, where health changes mean a reduction in the average number of RTI episodes per year and per patient during the study duration, as well as to estimate the annual average cost per patient due to visits and medicines, of a cohort of patients undergoing homeopathic treatment vs a control group of untreated patients, in a real-world setting.
Methods
Patients
To conduct the analysis, the same database employed by Beghi et al in their work was used.13 In this database, from January 1, 2002 to December 31, 2011, data on 459 patients who suffered from respiratory diseases (221 males, 48.1%; 238 females, 51.9%; age range: 1–84 years; mean ± SD: 30.9±25.2 years) were recorded. These patients were referred to a respiratory diseases specialist’s office in Agnadello (Cremona, Italy). To be included in the analysis, it was necessary for patients to have experienced at least 3 episodes of RTIs in the year preceding the start of treatment or observation, and at least 1 year of follow-up after the start of treatment/observation. For RTIs we mean ear, airways, throat or lungs infections, sore throat, tonsillitis, laryngitis, sinusitis, cough, cold, etc. The exclusion criteria from the study were as follows: having been subjected to administration of influenza vaccines or of any other kind of vaccine, or suffering from psychiatric disorders as well as being unable to follow the prescriptions.
Ethics
This study was notified to the regional Independent Ethical Committee of Lombardy region. According to the Italian regulation (“Guidelines for the classification of observational study of drug”; May 20, 2008) no approval of the Committee is needed for observational studies. All the subjects gave their written informed consent for the use of their data in this study. The study protocol conforms to the ethical guidelines of the “World Medical Association (WMA) Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964.
First visit
At the time of enrollment, during the first visit, the state of health and the type of symptoms presented by each patient were evaluated and recorded. The presence of RTI was diagnosed by a physical examination, which could be due to ear pain, sinusitis, tonsillitis, larynx inflammation, acute otitis media, red pharyngeal mucosa, or acute inflammation of the throat. The demographic details and clinical diagnosis of every patient were registered. In particular, based on the clinical diagnosis of each patient, all patients were subdivided into 5 macro-categories: recurrent respiratory infection (RRI), RTI, allergic respiratory diseases, asthma, and COPD. Based on this, it should be noted that from the total number of patients (N=459), 4 were excluded from the analysis (N=455, total number of patients analyzed) since, based on their clinical diagnosis, they would have had to be a part of a 6th category that, due to the small number of patients in that category, would not have had a significant impact on the final results. In addition, according to age, within each macro-category, patients were split into 2 additional micro-categories: adults (age >12 years) and pediatric patients (age ≤12 years). When analyzing the results of each macro-category, data were studied taking into consideration patients of all ages at first (adult + pediatric), then focusing on the pediatric population only.
Treatment
Out of the 455 analyzed patients, 246 subjects (54.1%) were treated with a homeopathic treatment (Anas barbariae hepatis et cordis extractum 200K) in addition to symptomatic drugs, while 209 patients (45.9%) were not treated with Anas barbariae hepatis et cordis extractum 200K but only with symptomatic drugs (Control group). At the time of enrollment, the physician instructed all patients to take 1 dose of Anas barbariae hepatis et cordis extractum 200K (contains 1 g, about 200 pillules impregnated with 0.01mL of the specific extract of duck’s heart and liver) a week for 8 months (from September to April), and to repeat the treatment in the subsequent years, avoiding the administration of any type of vaccine during the entire duration of observation. The patients purchased and took the medicine on their own account. Subsequently, during consultation visits and/or by contacting over telephone, the physicians evaluated the patients’ adherence to the treatment, by checking if the patients took the medicine or not. A total of 209 subjects were found to be non-compliant to the homeopathic treatment; due to the fact that they did not take the medicine as recommended, these patients formed the Control group. Table 1 shows the characteristics of the patients being examined. RRI was diagnosed only among children, while only adults were affected by COPD. The presence of RTI, allergic respiratory diseases, and asthma was significantly related to age; in particular, in all the 3 categories, the disease was highly frequent among older patients.
Follow-up
At each subsequent yearly consultation, the patient’s state of health was evaluated and a data form was completed containing the patient’s clinical details and diagnosis. The duration of the observation period varied between 1 and 10 years, with a mean ± SD value of 5.3±2.6 years.
Outcome measures
The primary outcome measure for assessing the effectiveness of the preventive treatment with homeopathic medicine was the average number of RTI episodes per year and per patient in the years of observation in both groups (Anas barbariae hepatis et cordis extractum 200K and Control) in each of the 5 categories. The secondary outcome of the study was the evaluation of the annual average cost per patient due to the number of RTI episodes that subjects suffered. This cost was generally calculated for the whole cohort of patients in the study and then specifically for each of the 5 categories, taking into consideration both the total number of patients and the subgroup of pediatric patients. In order to determine the annual average cost per patient due to the number of RTI episodes that subjects suffered, the parameters that were used were: the cost of the drug obtained from the Italian Prontuario Farmaceutico, the cost of the visits to which the patient was subjected, and the number of RTI episodes occurring during the several years of observation; finally, the calculation of the average annual cost was weighed for the years of observation for each patient. The analysis conducted is conservative and it has been carried out from the Italian NHS’ point of view; for both the groups of patients (Anas barbariae hepatis et cordis extractum 200K and Control), the same symptomatic drugs reimbursed by the NHS were economically valued in order to bring out the differential linked to fewer episodes of RTI. Since homeopathic medicines such as Anas barbariae hepatis et cordis extractum 200K, as well as symptomatic Group C drugs are an out-of-pocket expense in Italy, they are borne by the patient, and have neither been included nor considered in the evaluation. Group A drugs, reimbursed by the NHS, which were used to determine the total expenditure, according to the prescriptions registered in the database, are as follows: amoxicillin + clavulanic acid, prednisone, ibuprofen, levofloxacin, azithromycin dihydrate, montelukast sodium, and ceftriaxone. The cost of each pneumological visit was € 17.90.14 Inferential statistical tests were performed in order to capture possible statistically significant differences between the 2 groups of patients (Anas barbariae hepatis et cordis extractum 200K vs Control). Statistical comparisons were performed using Cochran–Mantel–Haenszel Tests, stratified by pathology and Wilcoxon/Kruskal-Wallis (W/K-W) tests (Rank Sums) for categorical and continuous variables, respectively. The first analysis permitted us to investigate, by comparison of proportions, the confounding effect of the pathology. Treatment group differences, for annual cost average data, were instead assessed, due to the high variability and lack of normality of the information, by the W/K-W procedure. Two-sided tests were performed at the 5% level. The software used for statistical analysis was JMP10 (SAS Institute, Inc, Cary, NC, USA).
Results
RTI episodes
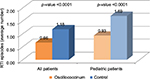
By analyzing all the 5 diagnostic categories together, it was found that, taking into consideration both the entire population of patients, and then only the pediatric population, despite the greater number of patients having taken Anas barbariae hepatis et cordis extractum 200K (All patients: Anas barbariae hepatis et cordis extractum 200K N=246, Control N=209. Pediatric patients: Anas barbariae hepatis et cordis extractum 200K N=107, Control N=62), the number of RTI episodes that occurred during the years of observation was definitely lower in the treated patients; the difference between these numbers was statistically significant in both the classes of patients (p<0.001) (Figure 1). To determine statistical significance, comparisons were made with the non-parametric test of W/K-W due to the established heterogeneity between group variances. The t-test has also taken this into account; in fact, the probabilistic value was obtained by applying the Welch’s t-test or unequal variances t-test. These results prove the evident effectiveness of Anas barbariae hepatis et cordis extractum 200K in reducing the incidence of RTI episodes. In particular, it has been shown that Anas barbariae hepatis et cordis extractum 200K is especially effective among the adult population. Pediatric patients (N=107), included in the total number of patients (N=246), reported on average 0.93 episodes, which means that adult patients (N=139), considered alone, had a significantly lower number of episodes, having been 0.66 episodes in the total population. Considering all patients, the CI value obtained was between 1.06 and 1.30 for the Control group; between 0.58 and 0.75 for the Anas barbariae hepatis et cordis extractum 200K group. The SD value was 1.18 for the Control group and 0.67 for the Anas barbariae hepatis et cordis extractum 200K group. Considering pediatric patients alone, the CI value obtained was between 1.42 and 1.96 for the Control group; between 0.78 and 1.09 for the Anas barbariae hepatis et cordis extractum 200K group. The SD value was 1.06 for the Control group and 0.80 for the Anas barbariae hepatis et cordis extractum 200K group.
Table 2 shows the average number of RTI episodes that manifested in the several years of observation in the 5 categories of patients. It can be seen that, regardless of the diagnosis, the number of RTI episodes was always lower in the Anas barbariae hepatis et cordis extractum 200K (Oscillococcinum®; Boiron, Messimy, France) patients; the difference between these numbers, Anas barbariae hepatis et cordis extractum 200K vs Control, was statistically significant in every class of patients.
Costs
As shown in Figure 2, when evaluating all the 5 diagnostic categories together, it has been found that taking into consideration both the entire population of patients, and then only the pediatric population, the costs that the NHS had to incur were significantly lower in the classes of patients treated. These lower costs were certainly due to the reduction in the occurrence of RTI events due to the intake of Anas barbariae hepatis et cordis extractum 200K but also attributable to the reduced need for symptomatic medication as the patient’s health conditions improved. The differences between these costs was statistically significant in both the classes of patients (p<0.0001). The SD value was 62.28 for the Control group and 24.95 for the Anas barbariae hepatis et cordis extractum 200K group when considering all patients; while it was 30.07 for the Control group and 15.38 for the Anas barbariae hepatis et cordis extractum 200K group when considering pediatric patients.
  | Figure 2 Annual average cost per patient. Note: Oscillococcinum® manufactured by Boiron, Messimy, France. |
When the costs were assessed by splitting the patients into the 5 diagnostic categories, significantly lower costs in the patient population being treated were confirmed (Table 3). Comparing the number of episodes manifested and the calculated costs, it is possible to see that there is a correlation between the two; for example, RTI and allergic respiratory diseases averaged fewer episodes and at the same time, lower costs. The considerably higher costs in the COPD category are mainly attributable to the greater need for symptomatic medications, that are also more expensive than the symptomatic medications of the other groups of patients, rather than to the number of RTI episodes, that for example are higher in RRI patients, who however have lower costs.
Discussion
The main objectives of this retrospective observational study were to analyze the effects of the extract of duck’s liver and heart in the prevention of RTIs (Anas barbariae hepatis et cordis extractum 200K) and to estimate the annual average cost per patient, due to visits and medicines, of a cohort of patients undergoing homeopathic treatment vs a control group of untreated patients, in a real-world setting. The results indicate that Anas barbariae hepatis et cordis extractum 200K has a preventive effect on the onset of RTI episodes. Although the role of this medicine has often been debated,9 the protective effect observed in this analysis is consistent with other studies that have documented its effect on the treatment of flu and flu-like symptoms.15,16 These results are of great importance, not only because any gain in health offered by homeopathic medicines alone or in combination with symptomatic drugs could be considered of value to the health care system at the time of managing the seasonal epidemics that cause RTIs every year, but also because it could help in reducing the yearly expenditures of the NHS in treating these episodes. During the period of observation, patients treated with Anas barbariae hepatis et cordis extractum 200K had a lower number of RTI events than that of untreated patients. The difference between the sum of the number of episodes reported in all categories of patients, Anas barbariae hepatis et cordis extractum 200K vs Control, was statistically significant in every class of patients (p<0.001). Although pediatric patients represent a minority of the population within the study, they have, on average, the greatest number of RTI episodes. Presumably, this is not attributable to the poor efficacy of homeopathic medicines, but this observation is consistent with the pathophysiology of RTIs which are much more prevalent in the pediatric group than in adults. The exact mechanisms responsible for these reported effects are not clear yet; hence, more evidence is needed. From an economic point of view, the analysis suggests that treating patients with Anas barbariae hepatis et cordis extractum 200K has lower costs primarily due to the fact that the medication causes fewer episodes of RTI to develop, so the patient, having a better health condition, needs lesser amounts of symptomatic drugs, and consequently the costs necessary to treat the disease are lower. Being the analysis point of view that of the NHS, only the costs that the health care system has to face were taken into account, while the costs at the expense of the patients were not considered. Thus, for both the groups of patients (Anas barbariae hepatis et cordis extractum 200K vs Control), the same symptomatic medications reimbursed by the NHS, the so-called Group A drugs, were economically valued. The results of the analysis pointed out that through the use of homeopathic care, it is possible for the NHS to save costs. Deliberately, neither Anas barbariae hepatis et cordis extractum 200K nor symptomatic Group C drugs were valued; all of them are a part of the out-of-pocket expense borne by the patient. This study has several limitations, one of the concerns being its observational design that, as documented by Concato et al,17 is susceptible to inherent biases associated with the collection of non-random data. Indeed, non-randomized assignment of subjects into the treatment and control group may have involved potential selection bias. Moreover, the use of an untreated control group, rather than one treated with placebo, might be questionable. This study, however, shows a snapshot of the use and the therapeutic effectiveness of this homeopathic medicine in a real-world setting, and the results obtained need to be confirmed by large randomized controlled studies. Furthermore, considering the preventive effect of Anas barbariae hepatis et cordis extractum 200K on the onset of RTI episodes shown in the study and subsequent reduction in the yearly expenditures of the NHS to treat these episodes, it appears a good assessment of the relationship between benefits and costs that could be examined in further economic evaluations. In particular, it might be interesting to develop cost–benefit analyses in order to compare the therapeutic effectiveness and potential savings provided by this homeopathic medicine to other alternatives, and in other contexts.
Often, due to lack of robust evidence, the effectiveness of homeopathic medicines is questioned.3 RTIs usually have a strong impact on the psychophysical wellness of the patient, so it is understandable why the number of people using homeopathic medicines is increasing.18 This analysis suggests that the treatment with Anas barbariae hepatis et cordis extractum 200K could be used as a helpful tool in preventing RTIs and improving the health status of patients who suffer from respiratory diseases. The study also highlights that through the use of Anas barbariae hepatis et cordis extractum 200K, it could be possible to obtain savings for the Italian NHS.
Acknowledgments
The authors wish to sincerely thank Dr Luigi Alberto Marrari and Dr Lorenza Ferrari for their advice and cooperation. This work was done through the unconditional economic contribution of Laboratoires Boiron S.r.l.
Disclosure
Dr Beghi declares that he received a fee when he presented reports during scientific events of Boiron. The authors report no other conflicts of interest in this work.
References
Effectiveness of Homeopathy for Clinical Conditions: Evaluation of the Evidence – Overview Report. Available from: https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cam02i_homeopathyoverviewreport140408.pdf. Accessed May 23, 2017. | ||
NIH; National Center for Complementary and Integrative Health. Homeopathy. Available at: https://nccih.nih.gov/health/homeopathy. Accessed May 25, 2017. | ||
Ernst E. A systematic review of systematic reviews of homeopathy. Br J Clin Pharmacol. 2002;54(6):577–582. | ||
Annuario Statistico Italiano. ISTAT 2015 [Italian statistical yearbook. National Institute of Statistics 2015]. Available from: http://www.istat.it/it/archivio/156420. Accessed May 25, 2017. Italian. | ||
Dati SIMOH, Scuola Italiana di Medicina Omeopatica Hahnemanniana [SIMOH, Hahnemannian Homeopathic Medicine School, data]. Available from: http://www.omeopatiasimoh.org/. Accessed May 26, 2017. Italian. | ||
Rossi E, Bartoli P, Panozzo M, Bianchi A, Frè MD. Outcome of homeopathic treatment in paediatric patients: an observational study from 1998 to 2008. Eur J Integrative Med. 2010;2(3):115–122. | ||
Swayne J. International dictionary of homeopathy. Edinburgh: Churchill Livingstone; 2000. | ||
Watanabe Y, Ibrahim MS, Ellakany HF, Abd El-Hamid HS, Ikuta K. Genetic diversification of H5N1 highly pathogenic avian influenza A virus during replication in wild ducks. J Gen Virol. 2011;92(Pt 9):2105–2110. | ||
Mathie RT, Frye J, Fisher P. Homeopathic Oscillococcinum® for preventing and treating influenza and influenza-like illness. Cochrane Database Syst Rev. 2015;1:CD001957. | ||
Homeopathic Pharmacopoeia Convention of the United States. Monograph: Anas Barbariae Hepatis Et Cordis Extractum. PA, USA: Southeastern; 2012. | ||
Clausen J, van Wijk R, Albrecht H. Review of the use of high potencies in basic research on homeopathy. Homeopathy. 2011;100(4):288–292. | ||
Kooreman P, Baars EW. Patients whose GP knows complementary medicine tend to have lower costs and live longer. Eur J Health Econ. 2012;13(6):769–776. | ||
Beghi GM, Morselli-Labate AM. Does homeopathic medicine have a preventive effect on respiratory tract infections? A real life observational study. Multidiscip Respir Med. 2016;11:12. | ||
Gallarate Hospital (VA). Tariffario prestazioni ambulatoriali, Regione Lombardia. In vigore dal 01 marzo 2016. Delibere di riferimento: DGR X/2898 del 23.12.2014 e successive modifiche da regole di sistema annuali, tra cui: DGR 3993 del 04.08.2015, DGR 4702 del 29.12.2015 [Outpatient services price list, Lombardy Region. In force since March 1st 2016. Reference resolutions: DGR X/2898 of 23.12.2014 and subsequent amendments by annual system rules, including: DGR 3993 of 04.08.2015, DGR 4702 of 29.12.2015]. | ||
Vickers AJ, Smith C. Homoeopathic Oscillococcinum for preventing and treating influenza and influenza-like syndromes. Cochrane Database Syst Rev. 2000;2:CD001957. | ||
Marrari LA, Terzan L, Chaufferin G. Oscillococcinum for influenza treatment. Ann Ist Super Sanita. 2012;48(1):105–109. | ||
Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. | ||
Van Wassenhoven M, Ives G. An observational study of patients receiving homeopathic treatment. Homeopathy. 2004;93(1):3–11. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.