Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
The Prevalence of Sarcopenia in Bipolar Disorder
Authors Bulbul F , Koca I, Tamam L , Demirkol ME , Cakmak S , Ersahinoglu E
Received 14 January 2020
Accepted for publication 27 March 2020
Published 8 April 2020 Volume 2020:16 Pages 915—921
DOI https://doi.org/10.2147/NDT.S245721
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Feridun Bulbul,1 Irfan Koca,2 Lut Tamam,1 Mehmet Emin Demirkol,1 Soner Cakmak,1 Emre Ersahinoglu1
1Department of Psychiatry, School of Medicine, Çukurova University, Adana, Turkey; 2Department of Physical Medicine and Rehabilitation, Fizyoclinic Wellness Center, Gaziantep, Turkey
Correspondence: Feridun Bulbul Tel +90 532 3828857
Email [email protected]
Background: Sarcopenia (SP) is a syndrome described as generalized and progressive loss of muscle mass and strength that may cause fall, fractures, disability and death. Oxidative stress might be a probable etiologic factor in SP as well. SP is a comorbid syndrome that is seen in chronic illnesses. If these two considerations are taken together, one may also think that SP could be also seen in bipolar disorder (BD), because it is a chronic disorder and oxidative stress was related to both illnesses. In our study, we proposed to investigate the prevalence of SP in BD patients.
Methods: We recruited 111 consecutive BD patients who registered in Mood Disorders Unit of Çukurova University. Blood tests were taken from patients to exclude the possible confounding factor related to SP. Socio-demographic variable forms were filled out. Every patient underwent physical mass, strength, and performance tests for the diagnosis of SP, which was determined by the criteria of European consensus.
Results: The mean age of the patients was 38.00 ± 11.44 years (18– 68). Among the participants 69 (62.2%) were female, and 42 (37.8%) were male. Pre-SP was 6.3% (n=7), SP was 9.0% (n=10), and severe SP was 1.8% (n=2) in BD patients. The prevalence of pre-SP, SP and severe SP in BD patients was 7.1%, 16.7% and 2.4% in men and 5.8%, 4.3% and 1.4% in women, respectively. Although it was not marginally significant, a difference was observed in SP patients as they had more median psychotic features and median number of episodes per year for BD.
Conclusion: This is the first study that investigated SP in BD patients. Sarcopenia was found more frequently in BD patients than in the general population.
Keywords: sarcopenia, prevalence, depression, bipolar disorder
Introduction
Sarcopenia (SP) is a comorbid syndrome described as the progressive loss of muscle mass and strength that may induce disability, fractures, fall and death. Although SP can be seen at an early age secondary to chronic diseases, sedentary life and malnutrition, it is primarily common at age 65 and over.1 It may develop due to conditions such as inactivity, malnutrition and cachexia in young individuals.2 European Working Group on Sarcopenia in Older People (EWGSOP) described sarcopenia as a syndrome qualified by a generalized and progressive loss of skeletal muscle mass and strength at risk of reverse outcomes like a poor quality of life, physical disability and death.3
The incidence of sarcopenia increases with age. From the beginning of the fourth decade, muscle mass decreases linearly. The rate of loss is higher in men than in women.4 The sarcopenia prevalence varies relying on the method utilized.5 The sarcopenia prevalence in the community in relation to age-related variations and regional was reported to be 1–29%, 14–33% in longtime care populations, and 10% in hospital-based acute care populations.6
Physical inactivity, inadequate protein uptake, changes in the neuroendocrine system and increased inflammation have been shown to be the causes of sarcopenia.7,8 In many studies, it has been reported that muscle loss occurs as a result of increased exposure to reactive oxygen products and increased oxidative stress.9–11 Sarcopenia adversely affects the quality of life.10 To our knowledge, nutrition and physical exercise are the most important elements in all protection levels.12
Bipolar disorder (BD) is one of the most incapacitate medical diseases, and is considered worldwide to be a public health trouble. As a psychiatric illness, BD is qualified by recurrent depression episodes and mania & hypomania. It is crucial to underline that BD patients pass on most of their lives symptomatic, and the larger part of that time is passed on subject to the symptoms of depression.13 In many studies, it has been reported that reactive oxygen products and increased oxidative stress in BD.13–16
In recent years male patients with senile depressive reported that they had increased SP prevalence.15 BD patients are often depressed.16 Oxidative stress might be a probable etiologic factor in SP as well. If these two taken together, one may also think that SP could be also seen in bipolar disorder (BD), because it is a chronic disorder and oxidative stress was related with both illness. In our study, we purposed to investigate the prevalence of SP in BD patients.
Patients and Methods
Study Design
Patients with bipolar disorder who attended the psychiatry outpatient clinic of Çukurova University Faculty of Medicine between 1 October 2019 to 30 December 2019 were included in this study. Patients diagnosed with bipolar disorder according to Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) diagnostic criteria were used in the study.17 Patients who were extremely fond of cachexia, mental retardation, chronic kidney and liver disease, history of infection, malignancy, and trauma in the recent 1 month and did not follow the instructions were excluded. The sample size was determined after power analysis. The study data were composed from 111 patients who were consecutively recruited for the investigation. The study protocol was approved by the Çukurova University Local Research Ethics Committee (Approval number: TR-92/11). Written informed consent was taken from all participants, and the study was guided in consistency with the Declaration of Helsinki. Sociodemographic data form created by us was filled out. The walking speed of the participants (walking time of 6 m) muscle strength (measured by CAMRY Digital Hand Dynamometer) and muscle mass (measured by Tanita body analyzer) were measured. The scores measured in bipolar disorder patients were measured according to the diagnostic criteria of sarcopenia, and the frequency of sarcopenia was found, and the relationship between sarcopenia and BD manic and depressive episodes was investigated. The blood tests were taken from patients to except the probable confounding factor connected with SP.
Anthropometric Parameters
Current waist circumference (WC), height, weight, body mass index (BMI), calf circumferences (CC), and bilateral mid-upper arm (MAC) of all participants were evaluated utilizing a precision digital scale up to 0.1 kg and a standardized gauge up to 0.1 cm. Participants take off heavy clothes, shoes and socks before measurements were taken. WC was assessed in the middle of the smallest abdomen or in obese subjects between the iliac crest and the lowest rib. BMI was described as body weight in kilograms divided by height squared in meters. MAC was evaluated at the middle point between the acromion and the olecranon process while internally rotating and elevating the arm. All evaluations were guided by trained staff.
Body Composition
For evaluation of body composition parameters, a bioelectrical impedance analyzer (Tanita SA165 A0950 U-3, Netherlands) was utilized. After fasting for a minimum of 2 h and with an empty bladder, bioelectrical impedance analysis (BIA) was done. Total skeletal muscle mass was measured with the predictive equation defined by Janssen et al.18 Skeletal muscle mass index (SMMI) was measured as whole SMM (kg)/height squared (m2).19 Cut-off rates described by Bahati et al for the Turkish population were utilized to define low SMMI as 7.4 and 9.2 kg/m2 in females and males.20
Physical Performance
For normal speed of gait evaluation, an 8-m-long hallway was utilized in which 4-m tape was noted from the end of the second meter to the end of the sixth meter. Therefore, the hallway was divided into three divisions: the first 2 m as acceleration zone, the second 4 m timing area and the third 2 m deceleration zone. Participants were requested to walk with their regular pace. Time to pass through the central area was evaluated with a stopwatch. If the assessed rate is less than 0.8 m/s, it is considered as low gait speed.
Muscle Strength
Grip strength of the dominant hand was used to define muscle strength. Jamar dynamometer with an authorized protocol was utilized for this.21,22 The evaluation was done in sitting position, wrist in neutral position and elbow in 90º flexion. In the sitting position, participants squeezed as much as probable three times (30-s rest intervals), and the highest was listed as the grip strength. The cut-off thresholds for handgrip strengths of 20 and 30 kg for women and men, respectively, by suggested for the Turkish population by Bahat et al were utilized for the sarcopenia diagnosis.20
Sarcopenia Definition
The EWGSOP criteria were utilized for the age-related sarcopenia diagnosis.23 According to this description, sarcopenia is defined as low skeletal muscle mass with low muscle strength and low physical performance (decreased walking speed).
Statistical Analysis
All analyses were performed by using SPSS (version 25, USA). P values less than 0.05 were considered as significant. Descriptive analyses were performed using mean and standard deviation (mean ± SD) for normally distributed variables, and median and (min-max) values for normally non-distributed variables. Normally distributed numerical variables were compared with Independent samples t test and non-normally distributed numerical variables were compared with Mann–Whitney U-test. For correlation analysis, Spearman’s test was utilized for normally distributed numerical variables and Spearman correlation tests for non-normally distributed variables.
Results
The mean age of the patients was 38.00 ±11.44 years (18–68). Among the participants 69 (62.2%) were female, and 42 (37.8%) were male. Pre-SP was 6.3% (n=7), SP was 9.0% (n=10), and severe SP was 1.8% (n=2) in BD patients. The prevalence of Pre-SP, SP and severe SP in BD patients was 7.1%, 16.7% and 2.4% in men and 5.8%, 4.3% and 1.4% in women, respectively. A comparison of demographic and anthropometric parameters of groups in BD patients is shown in Table 1.
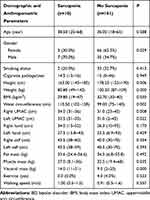 |
Table 1 Comparison of Demographic and Anthropometric Parameters of Groups in BD |
The percentage of males in the sarcopenia group and the percentage of females in the non-sarcopenia group were statistically higher (p=0.029). The height, weight, BMI, muscle and visceral values were significantly lower in the sarcopenia group compared to the non-sarcopenia group (p<0.05). However, it was found that waist circumference, right Upper-middle arm circumference (UMAC) and left UMAC values were higher in the sarcopenia group compared to the non-sarcopenia group (p<0.05). In addition, there was no statistically significant difference between the groups in terms of age, smoking status, right hand, left hand, right calf, left calf, fat, exercise, and walking speed (p>0.05) (Table 1).
A comparison of laboratory parameters of groups in BD patients is shown in Table 2. The hemoglobin (Hb), creatinine, uric acid, ALT, and AST values were significantly higher in the sarcopenia group compared to the non-sarcopenia group (p<0.05) (Table 2).
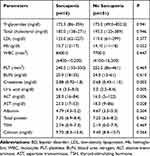 |
Table 2 Comparison of Laboratory Parameters of Groups in BD Patients |
A comparison of parameters related to bipolar disorder manic and depressive episodes of groups in BD patients is shown in Table 3. SP diagnosed patients had a statistically significant total number of episodes than no-SP patients (p=0.032). There was a positive correlation between sarcopenia and total episode (r=0.206, p=0.031). Although it was not marginally significant, another difference has been observed in SP patients who had more median psychotic feature and median number of episodes per year. In addition, no statistically significant difference was found between the groups in terms of disease duration, drug amount, hospitalization status, number of hospitalizations, first episode, number of episodes per year, psychotic feature status, and family psychiatric illness (p>0.05) (Table 3).
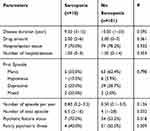 |
Table 3 Clinical and Psychiatric Parameters in Patients with Bipolar Disorder According to the Presence/Absence of Sarcopenia |
Correlation coefficients between sarcopenia and parameters of BD patients are shown in Table 4. A positive correlation was found between sarcopenia and gender, Hb, creatinine, uric acid, ALT, AST, height, weight, BMI, right UMAC, left UMAC, visceral, waist circumference and number of total episode parameters (Table 4).
 |
Table 4 Correlation Coefficients Between Sarcopenia and Parameters in BD Patients |
Discussion
To our knowledge, this is the first study that investigated SP in BD patients. In our study, Pre-SP, SP and severe SP prevalence in BD patients were 6.3%, 9.0% and 1.8%, respectively.
It is thought that the money spent by the healthcare system in the USA in 2000 for sarcopenia and sarcopenia-related diseases is approximately 18 billion dollars and constitutes 1.5% of total health expenditures.24,25 Therefore, in our country, whose life expectancy is rapidly increasing, sarcopenia is an important health problem. The incidence of sarcopenia increases with age. Studies have reported that the prevalence of sarcopenia is between 5% and 13% between 60 and 70 years of age, between 11% and 50% in 80 years of age and over or between 8 and 40% in 60 years of age and over.26,27 The incidence of sarcopenia is low in the young population (20–39 years). In a study by Krzymińska-Siemaszko et al, the incidence of sarcopenia in the young population was 16.28% for males and 2.62% for females.28 In our study, although the mean age was 38.00 ± 11.44, Pre-SP was 6.3%, SP was 9.0%, and severe SP was 1.8% in BD patients. Ozturk et al reported that the prevalence of sarcopenia was 21.8% in men and 8.7% in women.29 In our study, the prevalence of Pre-SP, SP and severe SP in BD patients was 7.1%, 16.7% and 2.4% in men and 5.8%, 4.3% and 1.4% in women, respectively. However, the percentage of males in the sarcopenia group and the percentage of females in the non-sarcopenia group were statistically higher.
Although only a few previous studies portrayed the relation between quality of life and muscle strength, Ozturk et al showed that both gait speed and muscular strength were positively correlated in patients with sarcopenia.29 However, in the current study, there was a negative correlation between SP and muscle strength, but no correlation with walking speed in BD patients.
Although it is well known that most of medical disorders were affected by body composition, it is also reported that body composition is related to the formation of general mental disorders, such as anxiety & depression.30 However, the probable act of muscle, that is the elements of body composition crucial for well-being and vitality, remains substantially undiscovered. The aim of the current study is to investigate the association between sarcopenia and bipolar disorder. The currency for this basis arises from the proof of general pathophysiological pathways for anxiety, depression and sarcopenia that include inflammation and oxidative stress, neurotrophins, and modulated by lifestyle behaviors. In a sectional Korean study, individuals with self-reported depression or those using antidepressants, also including women and men aged 60 years and older had lower appendicular skeletal muscle mass than those free of antidepressant use or depression.31 In another research, this time including hospitalized patients, those diagnosed with sarcopenia were more probably to suffer from depression, prolongation of hospital stay, were at a larger risk of non-elective readmission and had a larger mortality rate.32 In our study, hospitalization status and the number of hospitalizations were found to be lower in sarcopenia patients than those without sarcopenia in BD.
Among depressed patients with metabolic syndrome suffering from so-called metabolic depression, particularly waist circumference drives the association.33 Kokkeler et al reported that the larger WC had a negative effect on the result of late‐life depression in patients suffering from only sarcopenia.34 Low protein intake and reduce physical activity could eventuate in loss of muscle mass and strength, which results in fat infiltration.35 Patients with bipolar depression experience episodes of depression for the majority of their lives.36–38 Therefore, it has been shown in the studies that body mass index [BMI], total fat mass, visceral fat, upper-middle arm circumference, waist circumference and number of total episode increases were observed as a result of low physical activity and irregular nutrition, while muscle mass decreased.38–40 Most of these findings are also seen in elderly patients with sarcopenia.2,23,35 Similar to the literature, in our study, the height, weight, BMI, muscle and visceral values were significantly lower in the sarcopenia group compared to the non-sarcopenia group in BD. However, it was found that waist circumference, right Upper-middle arm circumference (UMAC) and left UMAC values were higher in the sarcopenia group compared to the non-sarcopenia group. SP diagnosed patients had a statistically significant total amount of episodes than no-SP patients. There was a positive correlation between sarcopenia and total episode. Although it was not marginally significant another difference has been observed in patients with SP had more median psychotic feature and median number of episodes per year in BD.
Limitations
There are some limitations to our study: First, sampling was not randomized. Second, neurotrophins and oxidative stress parameters could not be evaluated. Third, for the patient group of participants, it would be better to increase the number of participants for further studies.
Conclusion
This is the first study that researched the prevalence of SP in BD patients. High frequency of sarcopenia in BD is interesting. Sarcopenia was found more frequently in BD patients than in the general population. In our next studies, we aim to clarify what measures will be evaluated in the treatment of sarcopenia. However, reduced sarcopenia in overweight is generally expected. A number of exacerbation episodes related to sarcopenia may indicate more sedentary behavior and this is important. Finally, as BD was described a degenerative and chronic disorder and he degeneration was demonstrated in gray and white matter in the brain,41 the outcomes of the study may add a new point of view of degeneration seen in BD is muscles.
Disclosure
Our study was previously presented at a conference (https://www.tandfonline.com/doi/abs/10.1080/10177833.2014.11790943) as “preliminary results”. Since there was not enough data to be published in the article, the current study was redesigned by obtaining the permission of the clinical ethics committee from another center. Evaluations were made on new and more patients. Therefore, it has become a new study that is not related to the previous study. The authors declared no conflicts of interest in this study.
References
1. Dişcigil G, Sökmen ÜN. Yaşlılıkta sarkopeni. J Turk Family Physician. 2017;8:49–54.
2. Yaman H, Vural R. Management of sarcopenia in elderlies. Turk J Family Med Primary Care. 2016;10:243–249. doi:10.21763/tjfmpc.271330
3. Cruz-Jentoft A, Baeyens JP, Bauer JM et al. European working group on sarcopenia in older people: sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age ageing 2010;39:412–423.
4. Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623. doi:10.1097/BOR.0b013e328358d59b
5. Bautmans I, Van Puyvelde K, Mets T. Sarcopenia and functional decline: pathophysiology, prevention and therapy. Acta Clin Belg. 2009;64:303–316. doi:10.1179/acb.2009.048
6. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age ageing 2014;43:748–759.
7. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi:10.1093/ageing/afq034
8. An KO, Kim J. Association of sarcopenia and obesity with multimorbidity in Korean adults: a nationwide cross-sectional study. J Am Med Dir Assoc. 2016;17(960):e1–e7. doi:10.1016/j.jamda.2016.07.005
9. Kuyumcu ME, Halil M, Kara Ö, et al. Ultrasonographic evaluation of the calf muscle mass and architecture in elderly patients with and without sarcopenia. Arch Gerontology Geriatrics Gerontology Int. 2016;65:218–224. doi:10.1016/j.archger.2016.04.004
10. Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing. 2013;42:378–384.
11. Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35:409–415. doi:10.1093/ageing/afl024
12. Savaş S. Prevention of sarcopenia. Ege J Med. 2015;54:46–50
13. Valvassori SS, Bavaresco DV, Feier G, et al. Increased oxidative stress in the mitochondria isolated from lymphocytes of bipolar disorder patients during depressive episodes. Psychiatry Res. 2018;264:192–201. doi:10.1016/j.psychres.2018.03.089
14. Ng QX, Ramamoorthy K, Loke W, et al. Clinical role of aspirin in mood disorders: a systematic review. Brain Sci. 2019;9:296. doi:10.3390/brainsci9110296
15. Pasco JA. Age-Related Changes in Muscle and Bone. In: Duque G, editor. Osteosarcopenia: Bone, Muscle and Fat Interactions. Cham: Springer; 2019:45–71.
16. Rowland T, Perry BI, Upthegrove R, et al. Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: systematic review and meta-analyses. Br J Psychiatry. 2018;213:514–525. doi:10.1192/bjp.2018.144
17. Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub; 2013.
18. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–471. doi:10.1152/jappl.2000.89.2.465
19. Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontology Ser A. 2002;57:M772–M7. doi:10.1093/gerona/57.12.M772
20. Bahat G, Tufan A, Tufan F, et al. Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin nutr. 2016;35:1557–1563. doi:10.1016/j.clnu.2016.02.002
21. Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand Grip Strength: age and gender stratified normative data in a population-based study. BMC Res Notes. 2011;4:127. doi:10.1186/1756-0500-4-127
22. Mathiowetz V. Comparison of Rolyan and Jamar dynamometers for measuring grip strength. Occup Ther Int. 2002;9:201–209. doi:10.1002/oti.165
23. Akishita M, Kozaki K, Iijima K, et al. Chapter 1 Definitions and diagnosis of sarcopenia. Geriatrics Gerontology Int. 2018;18:7–12. doi:10.1111/ggi.13311
24. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi:10.1111/j.1532-5415.2004.52014.x
25. Walrand S, Guillet C, Salles J, Cano N, Boirie Y. Physiopathological mechanism of sarcopenia. Clin Geriatr Med. 2011;27:365–385. doi:10.1016/j.cger.2011.03.005
26. Morley J. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452. doi:10.1007/BF02982705
27. Van Kan GA. Epidemiology and consequences of sarcopenia. JNHA. 2009;13:708–712. doi:10.1007/s12603-009-0201-z
28. Krzymińska-Siemaszko R, Fryzowicz A, Czepulis N, Kaluźniak-Szymanowska A, Dworak L, Wieczorowska-Tobis K. The impact of the age range of young healthy reference population on the cut-off points for low muscle mass necessary for the diagnosis of sarcopenia. Eur Rev Med Pharmacol Sci. 2019;23:4321–4332. doi:10.26355/eurrev_201905_17938
29. Öztürk ZA, Türkbeyler İH, Abiyev A, et al. Health‐related quality of life and fall risk associated with age‐related body composition changes; sarcopenia, obesity and sarcopenic obesity. Intern Med J. 2018;48:973–981. doi:10.1111/imj.13935
30. Pasco JA, Williams LJ, Jacka FN, et al. Sarcopenia and the common mental disorders: a potential regulatory role of skeletal muscle on brain function? Curr Osteoporos Rep. 2015;13:351–357. doi:10.1007/s11914-015-0279-7
31. Kim NH, Kim HS, Eun CR, et al. Depression is associated with sarcopenia, not central obesity, in elderly Korean men. J Am Geriatr Soc. 2011;59:2062–2068. doi:10.1111/j.1532-5415.2011.03664.x
32. Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin nutr. 2013;32:772–776. doi:10.1016/j.clnu.2013.01.010
33. Marijnissen R, Vogelzangs N, Mulder M, Van Den Brink R, Comijs HC, Voshaar RO. Metabolic dysregulation and late-life depression: a prospective study. Psychol Med. 2017;47:1041–1052. doi:10.1017/S0033291716003196
34. Kokkeler KJ, van den Berg KS, Comijs HC, Oude Voshaar RC, Marijnissen RM. Sarcopenic obesity predicts nonremission of late‐life depression. Int J Geriatr Psychiatry. 2019;34:1226–1234. doi:10.1002/gps.5121
35. Buch A, Carmeli E, Boker LK, et al. Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—An overview. Exp Gerontol. 2016;76:25–32. doi:10.1016/j.exger.2016.01.008
36. Hirschfeld RM. Differential diagnosis of bipolar disorder and major depressive disorder. J Affect Disord. 2014;169:S12–S16. doi:10.1016/S0165-0327(14)70004-7
37. Douglas KM, Gallagher P, Robinson LJ, et al. Prevalence of cognitive impairment in major depression and bipolar disorder. Bipolar Disord. 2018;20:260–274. doi:10.1111/bdi.12602
38. Tondo L, Vazquez GH, Baldessarini RJ. Depression and Mania in Bipolar Disorder. Curr Neuropharmacol. 2017;15:353–358. doi:10.2174/1570159X14666160606210811
39. Vancampfort D, Sienaert P, Wyckaert S, et al. Health-related physical fitness in patients with bipolar disorder vs. healthy controls: an exploratory study. J Affect Disord. 2015;177:22–27. doi:10.1016/j.jad.2014.12.058
40. Vancampfort D, Stubbs B, Sienaert P, et al. A comparison of physical fitness in patients with bipolar disorder, schizophrenia and healthy controls. Disabil Rehabil. 2016;38:2047–2051. doi:10.3109/09638288.2015.1114037
41. Frey BN, Zunta-Soares GB, Caetano SC, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? Eur Neuropsychopharmacol. 2008;18:717–722. doi:10.1016/j.euroneuro.2008.04.015
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
