Back to Journals » Journal of Pain Research » Volume 16
The Preemptive Analgesic Effect of Capsaicin Involves Attenuations of Epidermal Keratinocytes Proliferation and Expression of Pro-Inflammatory Mediators After Plantar Incision in Rats
Authors Guo R , Qiu H, Li H , Ma D, Guan Y, Wang Y
Received 3 November 2022
Accepted for publication 8 January 2023
Published 19 January 2023 Volume 2023:16 Pages 141—149
DOI https://doi.org/10.2147/JPR.S395065
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Ruijuan Guo,1 Huanrong Qiu,1 Huili Li,2 Danxu Ma,2 Yun Guan,3 Yun Wang2
1Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, People’s Republic of China; 2Department of Anesthesiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100020, People’s Republic of China; 3Department of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD, 21205, USA
Correspondence: Yun Wang, Department of Anesthesiology, Beijing Chaoyang Hospital, Capital Medical University, No. 8, Gongtinan Road, Chaoyang District, Beijing, 100020, People’s Republic of China, Tel +86-010-85231330, Fax +86-10-65077808, Email [email protected]
Purpose: Subcutaneous infiltration of capsaicin, which initially activates transient receptor potential vanilloid 1 (TRPV1) receptors, can subsequently desensitize TRPV1-expressing nociceptors and induce analgesia in different pain models. Yet, whether the modulation of keratinocytes may also contribute to the analgesic action of capsaicin treatment remains unclear. In a rat model of postoperative pain, we tested the hypothesis that subcutaneous injection of capsaicin inhibited the proliferation of epidermal keratinocytes and their expression of pronociceptive inflammatory mediators after plantar incision.
Methods: The plantar incision model was carried out in the current study. Behavioral tests were used to evaluate postoperative pain-related behaviors in rats. Immunohistochemistry was used to investigate epidermal keratinocytes proliferation and expression of pro-inflammatory mediators in keratinocytes in rats.
Results: Behaviorally, plantar incision induced robust postoperative pain hypersensitivity. However, subcutaneous pretreatment of capsaicin (1%) but not the vehicle, prevented the development of postoperative pain. There was an increased proliferation of keratinocytes and the expressions of interleukin-1β (IL-1β) and tumour necrosis factor-alpha (TNF-α) in keratinocytes at 3 d and 7 d after plantar incision. However, these changes were also significantly attenuated by capsaicin pretreatment.
Conclusion: Our findings suggest that capsaicin pretreatment may inhibit incision-induced keratinocytes proliferation and reduce their expression of pronociceptive inflammatory mediators under postoperative pain conditions, which represents a peripheral non-neuronal mechanism of capsaicin-induced analgesia.
Keywords: capsaicin, keratinocyte, postoperative pain, inflammatory mediators
Introduction
Postoperative pain remains a spreading challenge in surgical patients. This is emphasized by the fact that many patients complain of experiencing moderate-to-severe pain after surgery despite the existence of intensive and multi-modal pain management. It indicates the great need to dissect the mechanisms of postoperative pain, in order to develop new analgesics to improve postoperative pain management.
The central and peripheral mechanism underlying postoperative pain have been emphasized over the past decades. As for the central mechanism, studies have confirmed that the pain transmission pathway in the spinal cord could be blocked through different administration methods, such as oral administration, local administration, or intrathecal administration, to produce analgesic effect.1–4 For peripheral mechanisms, it has been demonstrated that neuroimmune plays a vital role in skin disease, burn injury, and wound healing.5–8 However, the role of the skin neuroimmune system in incisional pain is not fully understood. Keratinocytes are an important component of the epidermis and form a mechanical barrier to protect the organisms. Keratinocytes have been shown to participate in the skin neuroimmune process.9–11 For instance, wound healing requires the activation of keratinocytes, macrophages, endothelial cells, fibroblasts, and platelets.12 Our previous study demonstrated that keratinocytes are also involved in the maintenance of postoperative pain by proliferation and secreting pronociceptive inflammatory factors such as interleukin-1β (IL-1β) and tumour necrosis factor-alpha (TNF-α).13 Capsaicin is a chili pepper extract and a member of the vanilloid family. Subcutaneous infiltration of capsaicin, which initially strongly activates transient receptor potential vanilloid 1 (TRPV1) receptors, can later induce an analgesic effect in different pain models.14–16 The underlying mechanism might be associated with selective desensitization of TRPV1-expressing nociceptors and peripheral nerve degeneration, depending on the concentration and treatment protocols. Keratinocytes also express TRPV1 receptors.17 Yet, it remains unclear whether the modulation of keratinocytes may also contribute to the analgesic action of subcutaneous infiltration of capsaicin. In a rat model of postoperative pain, we tested the hypothesis that capsaicin pretreatment could inhibit epidermal keratinocytes proliferation and reduce their expression of pronociceptive inflammatory mediators after plantar incision.
Materials and Methods
Animals
A total of 84 adult male Sprague–Dawley rats (weighing 280–300 g) from Huafukang Company were used in this experiment. All rats were housed on a 12h light/12h dark cycle. There was enough food and water, and the rats were allowed to find food and water freely. Moreover, experiments were approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University (Beijing, China) and were performed in compliance with the recommendations of the Guide for Animal Experimentation of the International Association for the Study of Pain.
Antibodies
The primary antibodies used in the current study were as follows: Cytokeratin Pan Type I/II Monoclonal Antibody (AE1/AE3) (MA1-82041, 1:100, Thermo Fisher Scientific, Waltham, MA, UK); Proliferating cell nuclear antigen (PCNA) (HX16901, 1:100, huaxingbio, Beijing, China); IL1β (HX15318, 1:200, huaxingbio, Beijing, China); TNFα (HX19392, 1:200, huaxingbio, Beijing, China). The sources of secondary antibodies were as follows: Cy3 conjugated Goat Anti-rabbit IgG (GB21303, servicebio, 1:300, Wuhan, China), Alexa FluorVR 488-conjugated AffiniPure Goat Anti-mouse IgG (GB25301, servicebio, 1:400, Wuhan, China).
Surgical Procedures and Drugs Administration
Animals were anesthetized with 2–3% isoflurane for plantar incision in right hind paw. Capsaicin (HY-10448, MedChemexpress, NJ, USA) is diluted to a concentration of 1% by a mixture of 80% saline, 10% ethanol, and 10% Tween-80 (T8360, Solarbio, Beijing, China). The mixture of 80% saline, 10% ethanol, and 10% Tween-80 was used as a vehicle. 200 μL capsaicin or vehicle was subcutaneously infiltrated by a 1 mL syringe with a 25 g needle in the right-hind paw 30 min before plantar incision, starting 0.5 cm from the proximal edge of the heel and extending toward the toes. The skin and fascia were cut for a 2 cm longitudinal incision. The flexor muscle under the fascia was incised longitudinally and stretched with forceps last for 30 s. 5–0 silk sutures were used to close the skin and fascia. Antibiotic ointment covered the wounded skin to prevent infection.
Behavior Tests
Spontaneous foot lifting (SFL), paw withdrawal threshold (PWT), and paw withdrawal latencies (PWL) were carried out to evaluate pain behaviors. All behavioral tests were completed by a trained assistant who was blinded to the experimental group.
SFL
SFL was tested as previously described.18 Rats were allowed to habituate for 30 min in a quiet environment. A hand tally counter was used to record the rapid lifting of the incisional paw. A stopwatch was applied to record the duration of prolonged (>1 second) paw elevation of the incisional paw. Rats were observed closely for a 5-min period every 10 min. The value of SFL was recorded four 4 times. The mean frequency of SFL (number of counts/20 min) and mean duration of paw lifts (sec/20 min) were calculated.
PWT
The rats were placed on a grid, restricted by the individual chamber, and adapted for 30 min. Von Frey filaments (Danmic Global, San Jose, CA, USA) were used to test PWT around the incision. As previously described,19 rapid lifting, licking, or shaking of the right paw recorded positive responses. An up-down method was used to determine the PWT.
PWL
PWL was used to measure hyperalgesia in heat by Hargreaves Test (Model 390G, IITC, USA). Rats were placed under individual chambers on a glass plate with a constant temperature of 30 degrees and acclimated for 30 min. A heat source underneath the glass plate was used for the wounded paw. Each rat was tested 4 times, and the mean value was taken. The interval between each measurement was 5 min. The light intensity was set at 34%. Cut-off time point was set at 30 s to avoid tissue damage.
Immunofluorescence
The rats were euthanized by carbon dioxide. The right-hind paw skin including the incision was collected. The tissues were paraffin sectioned, and the antibodies were incubated. Tissues that were placed in a wet box were incubated on slides with primary antibody overnight at 4°C. Slides with tissues were washed three times with PBS (pH 7.4) for 5 min every time and then incubated at room temperature for 50 min with a secondary antibody. PBS buffer was used to wash the tissues three times. DAPI solution was incubated at room temperature for 10 min. After that, slides with tissues were incubated for 5 min, with a spontaneous fluorescence quenching reagent.
Images magnified 200 times were collected by Ortho-Fluorescent Microscopy (Nikon) and were processed with Nikon DS-U3 imaging system. The average thickness of epidermis within 1000 μm from the area of the incision position was measured. The number of PCNA positive keratinocytes was counted by manual. The TNFα/IL-1β expression in keratinocytes between groups was represented by fluorescence intensity. A technician who was blind to the groups completed the cell count, fluorescence intensity, and epidermal thickness.
Statistical Analysis
All data were analyzed using GraphPad Prism 6. Qualitative data were presented as mean ± standard error and analyzed by two-way analysis of variance (ANOVA). For comparisons of intergroup and intragroup, Bonferroni multiple-comparison tests were used. A P value of <0.05 was the significance threshold for all analyses.
Results
Intra-Plantar Capsaicin Pretreatment Attenuated Post-Surgical Pain
We measured PWT, PWL, and SFL in rats at 1 d before incision and 3 h, 1 d, 3 d, 5 d, 7 d, and 14 d after incision (Figure 1A – D). In the vehicle-treated group, the decrease of PWT to mechanical stimuli and PWL to heat stimuli of the ipsilateral hind paw reached a peak level at 3 h after plantar incision (P < 0.0001, n = 6, Figure 1A and B). The PWT gradually increased from 1 d (P < 0.0001, n = 6) to 3 d (P < 0.01, n = 6) after injury, and returned to pre-injury baseline by 5 d post-injury. Similarly, the PWL gradually increased from 1 d (P < 0.0001, n = 6) to 5 d (P < 0.001, n = 6) post-injury and recovered to the baseline by 7 d post-injury. Importantly, PWT and PWL did not significantly decrease from the baseline in the capsaicin-treated group after plantar incision (P > 0.05, n = 6, Figure 1A and B).
The increase of SFL frequency and duration indicate that spontaneous pain reached the peak level at 3 h post-injury (P < 0.0001, n = 6) and then returned to pre-injury baseline at 5 d after an incision in the vehicle-treated group (P > 0.05, n = 6, Figure 1C and D). In contrast, SFL frequency and duration were not significantly increased from the pre-injury baseline in the capsaicin-treated group (P > 0.05, n = 6).
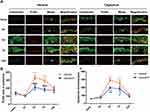
Local Capsaicin Pretreatment Reduced the Proliferation of the Epidermal Keratinocytes After Plantar Incision
The number of PCNA cells with positive cytokeratin labeling was counted to examine the keratinocyte proliferation after the plantar incision. The skin samples were harvested from naïve rats and different groups of rats at 3 h, 3 d, 7 d, and 14 d after the incision. Compared to that in the naïve group, the numbers of PCNA cells were significantly increased at 3 d and 7 d post-injury (P < 0.0001) in the vehicle-treated group but not in the capsaicin-treated group (n = 4–6/group, Figure 2A and B).
After incision, epidermal thickness increased and reached the peak level at 3 d (P < 0.0001) after the incision and returned to the baseline value at 14 d after the incision. Importantly, the increase in epidermal thickness in the capsaicin-treated group after the plantar incision was significantly less than that in the vehicle-treated group at 3 d (P < 0.01, n = 4–6) and 7 d (P < 0.05, n = 4–6) post-injury (Figure 2C).
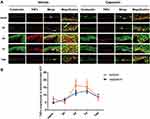
Capsaicin Pretreatment Suppressed the Increased TNFα Expression in Epidermal Keratinocytes After Plantar Incision
In vehicle-treated rats, the TNFα expression in keratinocytes in the epidermal incision margin skin was also significantly increased at 3 d (P < 0.05) and 7 d (P < 0.05, n = 4–6/time point) after incision and returned to the pre-injury level at 14 d (Figure 3A and B). However, it did not significantly change in the capsaicin-treated group after the incision (P > 0.05, n = 4–6/time point, Figure 3B).
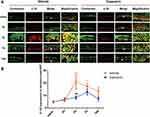
Capsaicin Pretreatment Suppressed the Increased IL-1β Expression in Epidermal Keratinocytes After Incision
The IL-1β expression in keratinocytes in the epidermal incision margin skin was significantly increased at 3 d (P < 0.001) and 7 d (P < 0.01) after incision but returned to the pre-injury baseline at 14 d (P > 0.05, n = 4–6/time point, Figure 4A, B). This change did not occur in capsaicin-treated group after incision (P > 0.05, n = 4–6/time point, Figure 4B). The level of co-expression of cytokeratin and IL-1β at 3 d after incision in the capsaicin-treated group was significantly lower than that in the vehicle-treated group (P < 0.01, Figure 4B).
Discussion
Our current study demonstrated that subcutaneous capsaicin pretreatment inhibited the incision-induced epidermal keratinocytes proliferation and reduced the expression of pro-nociceptive inflammatory mediators in keratinocytes, which may partially contribute to its preemptive analgesic effect on postoperative pain.
In the current study, we measured the SFL, PWT, and PWL to evaluate postoperative pain-related behaviors in rats. In the vehicle-treated group, plantar incision-induced pain behaviors were similar to that observed in our previous study.13 Strikingly, 1% capsaicin pretreatment prevented the expression of postoperative pain after plantar incision, indicating that capsaicin induced a preemptive analgesic effect. These findings are in line with previous results.16,19,20 Different concentrations of capsaicin were tested for its pain-inhibitory actions, and we used 1% capsaicin in the current study based on previous findings. For example, single intra-plantar administration of 1% capsaicin reduced the innervation of the dermis and epidermis and inhibited the development of postoperative pain behavior after plantar incision.16 Another study further demonstrated that a single application of 1.5% capsaicin (100 μL) on the plantar surface also resulted in significant analgesia against plantar incision injury in rats.21 Moreover, repeated injections of 0.1% capsaicin (20 μL) into the epidermis effectively reduced the number of epidermal nerve fibers and induced hypoalgesia in humans.20
Wound healing involves hemostasis, inflammation, proliferation, and remodeling. Keratinocytes are important epidermal cells involved in proliferation, and PCNA was used as an index of cell proliferation. Our previous study showed that postoperative pain was associated with a significant increase in PCNA in the epidermis around the plantar incision site.13 Capsaicin was reported to display anti-proliferative activity, such as inhibiting the proliferation of human small-cell lung cancer and breast cancer.22,23 Capsaicin was shown to reduce the activation of epidermal cell proliferation around the incision site in the hairy skin of rats,24 but it did not impact wound healing.19 In the current study, the thickness of keratinocytes and the expression of PCNA in glabrous skin at 3 d after incision were significantly less in the capsaicin-treated group, as compared to that in the vehicle-treated group. Collectively, these results suggest that capsaicin pretreatment before incision could effectively inhibit the proliferation of keratinocytes around the injury area.
TNFα and IL-1β are common inflammatory factors with strong pro-inflammatory activities. Our previous studies showed that keratinocytes around the plantar incision secrete several pro-inflammatory factors including TNFα and IL-1β.13 Capsaicin showed anti-inflammatory effects in a variety of models.25–27 Here, the secretion of pro-inflammatory factors by keratinocytes around the incision area was significantly reduced in capsaicin-treated rats, as compared with that in vehicle control, especially at 3 d after plantar incision. Exposures to high doses of capsaicin would cause neuronal desensitization due to depleting neuropeptides and inactivation of TRPV1 receptor,28,29 which may last from days to weeks.30,31 The inactivation of TRPV1 receptors by TRPV1 blockers also inhibited skin thickening and the expression of pro-inflammatory cytokines caused by ultraviolet radiation in hairless mice.32 Desensitization caused by a high concentration of capsaicin was initially due to an inhibition of voltage-gated calcium channels, but prolonged desensitization may result from the degeneration of epidermal nerve fibers.33,34 Plantar injection of a high concentration of capsaicin was shown to cause degeneration and loss of epidermal nerve fibers.19 Based on the aforementioned evidence, we postulate that the injection of 1% capsaicin may desensitize TRPV1 receptors in skin keratinocytes, which in turn inhibits their expression and release of pro-inflammatory factors around the incision area and hence partially contribute to the reduced postoperative pain.
In this study, male rats, but not female rats, were used to investigate the effects of capsaicin pretreatment on the proliferation of keratinocytes and the production of pro-inflammatory factors, which might lead to related gender differences in results. In future research, it is necessary to research the peripheral mechanism of capsaicin pretreatment on postoperative pain in the plantar incision model in female rats. Besides, we focus on the secretion of pro-inflammatory factors by keratinocytes around the incision area by pre-administration of capsaicin in the current research. It remains unclear to what extent TRPV1 activation affects keratinocytes and changes in local neurogenic inflammation. Further researches need to be performed on these two aspects in future studies.
Conclusion
In summary, plantar incision-induced postoperative pain may be partially attributable to the production and release of pro-inflammatory factors from epidermal keratinocytes, providing new insights into the role of keratinocytes in postoperative pain. Importantly, pretreatment with capsaicin inhibited the proliferation and production of pro-inflammatory factors by keratinocytes, which represents one of the peripheral non-neuronal mechanisms of capsaicin-induced analgesia.
Acknowledgments
This work was supported by grants from the Beijing Natural Science Foundation (7202053), the National Natural Science Foundation of China (82171217, 81771181, 81571065), and the Scientific Research Common Program of Beijing Municipal Commission of Education (KM201910025018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siracusa R, Fusco R, Cordaro M, et al. The protective effects of pre- and post-administration of micronized palmitoylethanolamide formulation on postoperative pain in rats. Int J Mol Sci. 2020;21(20):7700. doi:10.3390/ijms21207700
2. Zhu Q, Mao LN, Liu CP, et al. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci Rep. 2016;6:19266. doi:10.1038/srep19266
3. Siracusa R, Monaco F, D’Amico R, et al. Epigallocatechin-3-gallate modulates postoperative pain by regulating biochemical and molecular pathways. Int J Mol Sci. 2021;22(13):6879. doi:10.3390/ijms22136879
4. Mecklenburg J, Patil MJ, Koek W, Akopian AN. Effects of local and spinal administrations of mu-opioids on postoperative pain in aged versus adult mice. Pain Rep. 2017;2(1):e584. doi:10.1097/PR9.0000000000000584
5. Zhang S, Edwards TN, Chaudhri VK, et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell. 2021;184(8):2151–2166. doi:10.1016/j.cell.2021.03.002
6. Walsh CM, Hill RZ, Schwendinger-Schreck J, et al. Neutrophils promote CXCR3-dependent itch in the development of atopic dermatitis. Elife. 2019;8. doi:10.7554/eLife.48448
7. Radek KA, Lopez-Garcia B, Hupe M, et al. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol. 2008;128(6):1525–1534. doi:10.1038/sj.jid.5701225
8. Cuddihy J, Wu G, Ho L, et al. Lactate dehydrogenase activity staining demonstrates time-dependent immune cell infiltration in human ex-vivo burn-injured skin. Sci Rep. 2021;11(1):21249. doi:10.1038/s41598-021-00644-5
9. Lee WJ, Shim WS. Cutaneous neuroimmune interactions of TSLP and TRPV4 play pivotal roles in dry skin-induced pruritus. Front Immunol. 2021;12:772941. doi:10.3389/fimmu.2021.772941
10. Buhl T, Ikoma A, Kempkes C, et al. Protease-activated receptor-2 regulates neuro-epidermal communication in atopic dermatitis. Front Immunol. 2020;11:1740. doi:10.3389/fimmu.2020.01740
11. Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128(5):1067–1070. doi:10.1038/jid.2008.88
12. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–1222. doi:10.1172/JCI32169
13. Guo R, Hao J, Ma D, et al. Persistent proliferation of keratinocytes and prolonged expression of pronociceptive inflammatory mediators might be associated with the postoperative pain in KK mice. Mol Pain. 2020;16:2068236236. doi:10.1177/1744806920927284
14. Dupoiron D, Jubier-Hamon S, Seegers V, et al. Peripheral neuropathic pain following breast cancer: effectiveness and tolerability of high-concentration capsaicin patch. J Pain Res. 2022;15:241–255. doi:10.2147/JPR.S341378
15. Vieira IF, de Castro AM, Loureiro M, et al. Capsaicin 8% for peripheral neuropathic pain treatment: a retrospective cohort study. Pain Physician. 2022;25(4):E641–E647.
16. Tran PV, Johns ME, McAdams B, et al. Global transcriptome analysis of rat dorsal root ganglia to identify molecular pathways involved in incisional pain. Mol Pain. 2020;16:2068207040. doi:10.1177/1744806920956480
17. Denda M, Fuziwara S, Inoue K, et al. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001;285(5):1250–1252. doi:10.1006/bbrc.2001.5299
18. Kabadi R, Kouya F, Cohen HW, Banik RK. Spontaneous pain-like behaviors are more sensitive to morphine and buprenorphine than mechanically evoked behaviors in a rat model of acute postoperative pain. Anesth Analg. 2015;120(2):472–478. doi:10.1213/ANE.0000000000000571
19. Uhelski ML, McAdams B, Johns ME, et al. Lack of relationship between epidermal denervation by capsaicin and incisional pain behaviours: a laser scanning confocal microscopy study in rats. Eur J Pain. 2020;24(6):1197–1208. doi:10.1002/ejp.1564
20. Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18(21):8947–8959. doi:10.1523/JNEUROSCI.18-21-08947.1998
21. Pospisilova E, Palecek J. Post-operative pain behavior in rats is reduced after single high-concentration capsaicin application. Pain. 2006;125(3):233–243. doi:10.1016/j.pain.2006.05.021
22. Chen M, Xiao C, Jiang W, et al. Capsaicin inhibits proliferation and induces apoptosis in breast cancer by down-regulating FBI-1-mediated NF-kappaB pathway. Drug Des Devel Ther. 2021;15:125–140. doi:10.2147/DDDT.S269901
23. Brown KC, Witte TR, Hardman WE, et al. Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS One. 2010;5(4):e10243. doi:10.1371/journal.pone.0010243
24. Martinez-Martinez E, Galvan-Hernandez CI, Toscano-Marquez B, Gutierrez-Ospina G, Rojas M. Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin. PLoS One. 2012;7(5):e36421. doi:10.1371/journal.pone.0036421
25. Chan TC, Lee MS, Huang WC, et al. Capsaicin attenuates imiquimod-induced epidermal hyperplasia and cutaneous inflammation in a murine model of psoriasis. Biomed Pharmacother. 2021;141:111950. doi:10.1016/j.biopha.2021.111950
26. Liu P, Hao J, Zhao J, et al. Integrated network pharmacology and experimental validation approach to investigate the therapeutic effects of capsaicin on lipopolysaccharide-induced acute lung injury. Mediators Inflamm. 2022;2022:9272896. doi:10.1155/2022/9272896
27. Jolayemi AT, Ojewole JA. Comparative anti-inflammatory properties of Capsaicin and ethyl-aAcetate extract of Capsicum frutescens Linn [Solanaceae] in rats. Afr Health Sci. 2013;13(2):357–361. doi:10.4314/ahs.v13i2.23
28. Lee SS, Sohn YW, Yoo ES, Kim KH. Neurotoxicity and long lasting analgesia induced by capsaicinoids. J Toxicol Sci. 1991;16(Suppl 1):3–20. doi:10.2131/jts.16.SupplementI_3
29. Comunanza V, Carbone E, Marcantoni A, Sher E, Ursu D. Calcium-dependent inhibition of T-type calcium channels by TRPV1 activation in rat sensory neurons. Pflugers Arch. 2011;462(5):709–722. doi:10.1007/s00424-011-1023-5
30. Nolano M, Simone DA, Wendelschafer-Crabb G, et al. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81(1–2):135–145. doi:10.1016/S0304-3959(99)00007-X
31. Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991;47(3):285–294. doi:10.1016/0304-3959(91)90217-L
32. Lee YM, Kang SM, Lee SR, et al. Inhibitory effects of TRPV1 blocker on UV-induced responses in the hairless mice. Arch Dermatol Res. 2011;303(10):727–736. doi:10.1007/s00403-011-1153-9
33. Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123(1):53–62. doi:10.1085/jgp.200308906
34. Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75(2):157–168. doi:10.1093/bja/75.2.157
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.