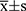Back to Journals » International Journal of General Medicine » Volume 16
The Predictive Value of Heparin-Binding Protein and D-Dimer in Patients with Sepsis
Authors Tang J, Yuan H, Wu YL, Fu S , Pan XY
Received 20 February 2023
Accepted for publication 10 May 2023
Published 6 June 2023 Volume 2023:16 Pages 2295—2303
DOI https://doi.org/10.2147/IJGM.S409328
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Jian Tang,1 Hong Yuan,2 Yun Long Wu,1 Shui Fu,3 Xiao Yong Pan4
1Department of Intensive Care, First People’s Hospital of Linping District, Hangzhou, Zhejiang Province, People’s Republic of China; 2Department of Cardiology, First People’s Hospital of Linping District, Hangzhou, Zhejiang Province, People’s Republic of China; 3Clinical Laboratory Department, First People’s Hospital of Linping District, Hangzhou, Zhejiang Province, People’s Republic of China; 4Clinical Laboratory Department, The People’s Hospital of Cangnan Zhejiang, Wenzhou, Zhejiang Province, People’s Republic of China
Correspondence: Xiao Yong Pan, Clinical Laboratory Department, The People’s Hospital of Cangnan Zhejiang, Wenzhou, Zhejiang Province, People’s Republic of China, Email [email protected]
Objective: To explore the serial measurement of heparin-binding protein and D-dimer in the prediction of 28-day mortality and efficacy evaluation of critically-ill patients with sepsis.
Methods: We recruited a total of 51 patients with sepsis in the ICU of our hospital. They were divided into a survival group or a death group according to their prognosis 28 days after treatment. The HBP and D-dimer levels in these patients were determined on the 1st (24h), 3rd, and 5th days. Besides, the sequential organ failure assessment (SOFA) score of these patients was recorded at admission. The patients in both groups were subjected to comparison regarding HBP and D-dimer levels and SOFA scores within 24h of admission. Additionally, a correlation between the levels of HBP and D-dimer and the SOFA score was statistically measured, while the predictive effectiveness of these factors for the prognosis of patients with sepsis was also determined. Moreover, the dynamic changes in HBP and D-dimer during the treatment of both groups were analyzed.
Results: The HBP and D-dimer levels and the SOFA scores in the survival group were considerably lower than those in the death group, and the differences were statistically significant (P< 0.05). Additionally, the levels of HBP and D-dimer in sepsis patients were positively correlated with the SOFA score (P< 0.05). The area under the curve (AUC) of HBP, D-dimer, and their combination in predicting the prognosis of patients with sepsis was 0.824, 0.771, and 0.830, respectively. Besides, the sensitivity and specificity of their combination in predicting the prognosis of patients with sepsis were 68.42% and 92.31%, respectively. The HBP and D-dimer levels presented a downward trend in the survival group during treatment, while they exhibited an upward trend in the death group.
Conclusion: HBP and D-dimer realize high predictive effectiveness for the prognosis of patients with sepsis, while the combined use of these two factors achieves superior effectiveness. Thus, they can be applied to the prediction of 28-day mortality and efficacy evaluation of sepsis patients.
Keywords: sepsis, heparin-binding protein, HBP, D-dimer, sequential organ failure assessment score, SOFA, prognosis
Introduction
Sepsis is an inflammatory response syndrome that is caused by numerous infectious factors and mediated by various inflammatory reaction substances. The disease is a life-threatening organ dysfunction caused by maladjustment of the host’s immune response to infection.1,2 It affects millions of people all over the world every year, accounting for between one-sixth and one-third of all deaths. Cases of sepsis have witnessed increasing incidence in recent years,3 and it has become a global burden in critical medicine. Patients with sepsis usually present rapid progression and it is difficult to achieve favorable outcomes. Therefore, early diagnosis, efficacy monitoring, and prognosis assessment are of great clinical significance for the prevention of sepsis.4 Biomarkers have been used to assist with the diagnosis of sepsis as well as determining the severity of the disease.5 In the past decade, researchers have verified that conventional cell blood count (CBC) parameters are effective biomarkers for the screening of septicemia.6 Besides, emerging biological markers such as pentraxin-3 (PTX3),7 clusterin,8 IL-18,9 and circRNA-miRNA-lncRNA networks10 can also be used to obtain prognostic information on patients with sepsis, thereby providing guidance and monitoring for relevant treatment. Heparin-binding protein (HBP) is a novel biomarker related to infectious diseases. As an acute phase protein (APP), HBP can be used to effectively assess the severity of sepsis. Besides, it is more crucial in the early diagnosis and efficacy monitoring of patients with septic shock.11,12 As revealed by a previous study,13 patients with sepsis suffer from varying degrees of coagulation dysfunction. D-dimer is a specific degradation product derived from the hydrolysis of cross-linked fibrin by fibrinolytic enzymes. Moreover, it is a molecular marker of hypercoagulability and secondary hyperfibrinolysis in the body. Thus, D-dimer expression effectively reflects the severity of sepsis, so it can be used as an index for the efficacy monitoring and prognosis assessment of sepsis. Sepsis is an infection associated with organ failure and it is a pathogenic factor that causes sequential organ failure. Clinically, the sequential organ failure assessment (SOFA) score (previously known as the sepsis-related organ failure assessment score) is generally applied to the prognosis assessment of patients with multiple organ failure. This assessment system dynamically reflects changes in organ function and has been widely used in the classification and prognosis assessment of critically ill patients.14–16 In this study, the HBP and D-dimer levels in patients with sepsis were detected and the SOFA score was calculated to establish a correlation between HBP and D-dimer levels and the SOFA score. Also, we aimed to identify the predictive value of these factors for assessing the severity of sepsis and relevant efficacy.
Materials and Methods
Samples
This study was a prospective study. We included a total of 51 patients with sepsis who were admitted to the ICU of our hospital from January 2019 to September 2022. The cohort comprised 27 males and 24 females, with an age of 55.24±16.24 years, ranging from 24 to 88 years old. These patients were diagnosed and treated for sepsis according to existing conventions.17 The inclusion criteria included:① Patients aged over 18 years; ② Patients admitted to the ICU for the first time; ③ Patients with complete clinical data. The exclusion criteria were: ① Patients who died of illness within 24h; ② Patients requiring large-volume blood transfusions; ③ Patients with blood system diseases; ④ Patients with advanced-stage tumors; ⑤ Patients with severe organ dysfunction or immunodeficiencies. The patients were divided into a survival group (n = 38) and a death group (n = 13), according to their prognosis 28 days after treatment. The enrollment and research processes are presented in Figure 1.
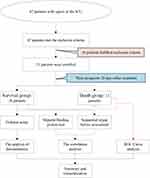 |
Figure 1 Flowchart of the recruitment and review process for patients with sepsis in intensive care units. |
Instruments and Consumables
A Jet-iStar3000 automatic immune analyzer and heparin-binding protein detection reagents (batch number: HBP2206005F) were purchased from Joinstar Biomedical Technology Co., Ltd. (China). Besides, a Sysmex CS5100 automatic coagulation analyzer and D-dimer detection reagents (reagent batch number: 568,816; buffer batch number: 569,216; supplement batch number: 56,938,816; diluent batch number: 5,689,816; calibrator batch number: 569,416) were procured from Sysmex Medical Electronics (Shanghai) Co., Ltd.
Methods
On the 1st, 3rd, and 5th day after admission, peripheral blood samples were collected and placed in a vacuum blood collection tube with sodium citrate in a 1:9 ratio, two tubes per patient. After the samples were statically cultured for 30min, they were centrifuged at 2000g for 10min. Then, the levels of HBP and D-dimer were detected using theJet-iStar3000 automatic immune analyzer and Sysmex CS5100 automatic coagulation analyzer, respectively. Subsequently, the SOFA scores for all patients were calculated within 24h. The overall SOFA score is based on six individual scores for the respiratory, hepatic, coagulation, cardiovascular, renal, and neurological systems. The score of each organ function was 0–4 points, where lower scores indicated lower disease severity.
Statistical Analysis
Data processing was performed using SPSS 26.0. Besides, the Shapiro–Wilk test was applied to confirm whether the continuous variables followed a normal distribution. The data with normal distribution were expressed as 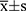 . Additionally, the independent samples t-test was performed to draw a comparison between the two groups and the chi-square test was carried out to compare the rates. Moreover, the enumeration data were analyzed using the Mann–Whitney Wilcoxon rank sum test. The correlation between HBP and D-dimer levels and the SOFA scores was tested with the Pearson correlation coefficient as a basis. Also, receiver operating characteristic (ROC) curves were plotted to analyze the predictive value of these factors for the prognosis of patients with sepsis. P< 0.05 indicated that the difference was statistically significant.
. Additionally, the independent samples t-test was performed to draw a comparison between the two groups and the chi-square test was carried out to compare the rates. Moreover, the enumeration data were analyzed using the Mann–Whitney Wilcoxon rank sum test. The correlation between HBP and D-dimer levels and the SOFA scores was tested with the Pearson correlation coefficient as a basis. Also, receiver operating characteristic (ROC) curves were plotted to analyze the predictive value of these factors for the prognosis of patients with sepsis. P< 0.05 indicated that the difference was statistically significant.
Results
Comparison of General Data Between Both Groups
There was no significant difference in gender, age, and primary disease between the two groups (P> 0.05). However, the ICU stay of patients in the survival group was significantly shorter than that of patients in the death group (P< 0.05), as presented in Table 1.
 |
Table 1 Comparison of General Data Between Both Groups |
Comparison of HBP and D-Dimer Levels and SOFA Score Between Both Groups
The levels of HBP and D-dimer and the SOFA score in the survival group were substantially lower than those in the death group (P<0.05), as Table 2 shows.
 |
Table 2 Levels of HBP and D-Dimer and SOFA Scores for Both Groups at Admission ( |
Correlation Analysis
Table 3 indicates that the levels of HBP and D-dimer were positively correlated with the SOFA score in patients with sepsis (P<0.05).
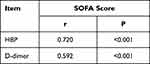 |
Table 3 Correlation Between the Levels of HBP and D-Dimer and the SOFA Score in Both Groups at Admission |
Predictive Value of HBP and D-Dimer Levels for the Prognosis of Patients with Sepsis
Results from the ROC curve analysis showed that the AUC of HBP, D-dimer, and their combination in predicting the prognosis of patients with sepsis was 0.824 (95% CI: 0.690–0.958), 0.771 (95% CI: 0.634–0.909), and 0.830 (95% CI: 0.700–0.960), respectively. Additionally, the sensitivity of HBP and D-dimer in predicting the prognosis of patients with sepsis was 69.23% and 68.42%, respectively. Also, the specificity of HBP and D-dimer in predicting the prognosis of sepsis patients was 69.23% and 76.92%. The sensitivity and specificity of their combination in predicting the prognosis of patients with sepsis were 68.42% and 92.31%, respectively, as Table 4 and Figure 2 illustrate.
 |
Table 4 Predictive Value of HBP and D-Dimer for the Prognosis of Patients with Sepsis |
 |
Figure 2 ROC curves of HBP, D-dimer, and their combination. |
Dynamic Changes in the Levels of HBP and D-Dimer in Both Groups
The levels of HBP and D-dimer exhibited a downward trend in the survival group during treatment, while they presented an upward trend in the death group (Figure 3).
 |
Figure 3 Dynamic changes in HBP and D-dimer levels in both groups within 5 days of admission. Note: (A) HBP; (B) D-dimer. |
Discussion
Sepsis is a common cause of death in critically ill patients and it involves individuals with a wide range of conditions. Patients with chronic diseases or immunodeficiencies, those who have undergone a splenectomy, and those aged under 1 year old or over 60 years old have a higher risk of contracting sepsis. Sepsis is commonly caused by pneumonia, urinary tract infections, abdominal infections, blood flow infections, skin or soft tissue infections, meningitis, viral infections, and other factors. Almost 50 million patients are newly diagnosed with sepsis worldwide every year, approximately 20% of all fatalities are related to sepsis and up to 50% of survivors suffer from physical and emotional pain in the long term.18 Since there are many complex influencing factors for sepsis, disease monitoring and prognosis assessment remain difficult problems in the ICU.5 Currently, sepsis risk, prognosis assessment, and disease monitoring are the primary focus of clinical research in this field. Accurate disease condition assessment and prognosis facilitate the provision of better treatment protocols and improve the diagnosis and management of this disease.
The occurrence of sepsis is influenced by several complex factors and its early clinical diagnosis and prognosis assessment pose serious challenges in the ICU. Although researchers have made several advances in the mechanism and clinical treatment of sepsis, the morbidity and mortality rates of this disease have not been successfully reduced. Early diagnosis and effective symptomatic treatment significantly improve clinical outcomes. However, there are several limitations for traditional infection biomarkers such as CRP, PCT, and WBC in the diagnosis and treatment of sepsis. Therefore, it is essential to explore new biological indicators with high diagnostic effectiveness for the efficacy observation and prognosis assessment of sepsis. HBP, a granular protein derived from neutrophils, has attracted much attention due to its antibacterial properties. After neutrophils are activated by chemokines, vesicles are rapidly secreted, releasing HBP. This leads to endothelial cytoskeleton rearrangement and cell contraction, thereby increasing endothelial permeability. As an inducer, HBP recruits more white blood cells to the infected sites, which further enhances immune response. Increased vascular endothelial permeability and over-activated immune response are important forms of pathogenesis in organ dysfunction and shock in patients with sepsis.19,20 According to a previous study,21 HBP has excellent predictive value for sepsis in the early stages of the disease and it is closely related to circulatory failure. In patients with sepsis, after inflammatory cells are activated, the coagulation system in the body is activated via various routes. Subsequently, anticoagulants are generated in the body to initiate the fibrinolytic system, resulting in microthrombosis in the blood vessels and generating large amounts of D-dimer.22 Additionally, disseminated intravascular coagulation (DIC) may occur in patients with severe conditions. In this study, one patient died as a result of DIC.
According to the findings of this study, the levels of HBP and D-dimer and the SOFA score in the survival group are significantly higher than those in the death group. According to the dispersion trends of both groups, the variability of these factors in the survival group is smaller than in the death group, which indicates that HBP and D-dimer are closely related to sepsis. This result is consistent with the findings of previous studies (Zhang23 and Lu24), which suggested that the expression of these factors in the death group was higher. As the diagnostic standard of sepsis, the SOFA score is calculated according to the severity of organ failure and it is the predictor with the highest correlation in patients with sepsis. The correlation analysis indicates that HBP and D-dimer levels are positively correlated with the SOFA score, while HBP is more effective than D-dimer. This suggests that HBP better reflects the severity of organ failure in patients with sepsis, which is consistent with the conclusions of a recently published multicenter study of patients in the emergency department.21 This may be because HBP is rapidly mobilized from neutrophils under migration to cope with bacterial infection when it occurs in the body. HBP induces vascular leakage and edema and has pro-inflammatory effects on several kinds of leukocytes and epithelial cells. However, D-dimer can only be produced after several steps, such as when the coagulation system is activated by inflammatory factors. Additionally, results from the ROC curve analysis indicate that both HBP and D-dimer have high diagnostic effectiveness and can be used to predict the outcome of patients with sepsis. Moreover, the AUC of HBP is larger than that of D-dimer, demonstrating that HBP is more effective in the diagnosis of this disease. This further confirms the value of using both HBP and D-dimer for predicting the prognosis of patients with sepsis. These results are generally consistent with the findings of Zhang23 and Lu.24 The results from the analysis and comparison in this study revealed that the AUC of the combined use of HBP and D-dimer in predicting the prognosis of patients with sepsis has the largest value (0.830). This indicates that combining HBP and D-dimer achieves higher predictive effectiveness for the prognosis of patients with sepsis. Thus, this combined method provides superior support for doctors in prognosis assessment and treatment guidance.
The dynamic changes in HBP and D-dimer levels in patients with sepsis reveal that the levels of both substances present a downward trend in the survival group, while they show an upward trend in the death group. This suggests that high-frequency dynamic monitoring of HBP and D-dimer can be used to continually evaluate treatment efficacy. Based on this, treatment plans can be optimized according to changes in HBP and D-dimer expression, thereby avoiding deterioration in the patient’s condition. In theory, the rate of change in HBP and D-dimer expression should correlate positively with the therapeutic effect. However, the patients in this study suffered from severe sepsis with complex conditions and the study was performed with a small sample size in a single center. As a result, we did not detect a relationship between the rate of change in HBP and D-dimer expression and the therapeutic effect. Also, the different half-lives and baseline values of HBP and D-dimer may explain this result. Although we confirmed that HBP and D-dimer can be applied to prognosis assessment and efficacy monitoring in sepsis, some limitations remain in this study. First, increases in HBP and D-dimer levels are also often detected in patients with non-infectious diseases in the ICU (eg, trauma, acute pancreatitis, respiratory and cardiac arrest, and other circulatory failure). This reduces the specificity of HBP and D-dimer as sepsis biomarkers. Additionally, the complex and diverse etiology and pathogenesis of sepsis also lead to poor sensitivity to HBP and D-dimer. Furthermore, the clinical value of this small sample study based on a single center needs to be verified through an exploration based on a larger sample size. In summary, HBP and D-dimer in combination with classical methods and new biomarkers can be employed to assist in the treatment of patients with severe sepsis.
Conclusions
As common clinical indicators for predicting sepsis, both HBP and D-dimer have high diagnostic effectiveness. Furthermore, the combined application of these two biomarkers can achieve favorable effects. In clinical practice, the combined application of these two biomarkers with the SOFA score and other indicators can predict the 28-day mortality and monitor the curative effect of treatment on patients with severe sepsis. Also, dynamic monitoring of HBP and D-dimer can quickly determine the prognosis and predict the treatment effect. These findings can be applied in clinical treatment, allowing clinicians to comprehensively evaluate the condition of patients and promptly improve therapeutic regimens, thereby improving the prognosis of patients with sepsis.
Data Sharing Statement
We declare that materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality. They are available from the first author on reasonable request ([email protected]).
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of the First People’s Hospital of Linping District, Hangzhou (No. 2018003). This study was conducted following the declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for Publication
All participants signed a document of informed consent.
Acknowledgments
We would like to acknowledge the dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
No external funding was received to conduct this study.
Disclosure
The authors declare that they have no competing interests.
References
1. McVeigh SE. Sepsis management in the emergency department. Nurs Clin North Am. 2020;55(1):71–79. doi:10.1016/j.cnur.2019.10.009
2. von Cube M, Schumacher M, Timsit JF. Sepsis. Lancet. 2020;396(10265):1804. doi:10.1016/S0140-6736(20)31609-3
3. Opal SM, Wittebole X. Biomarkers of infection and sepsis. Crit Care Clin. 2020;36(1):11–22. doi:10.1016/j.ccc.2019.08.002
4. Minderhoud TC, Azijli K, Nanayakkara PWB. Sepsis. Lancet. 2020;396(10265):1804. doi:10.1016/S0140-6736(20)32401-6
5. Kataria Y, Remick D. Sepsis biomarkers. Methods Mol Biol. 2021;2321:177–189. doi:10.1007/978-1-0716-1488-4_16
6. Agnello L, Giglio RV, Bivona G, et al. The value of a Complete Blood Count (CBC) for sepsis diagnosis and prognosis. Diagnostics. 2021;11(10):1881. doi:10.3390/diagnostics11101881
7. Chen H, Li T, Yan S, et al. Pentraxin-3 is a strong biomarker of sepsis severity identification and predictor of 90-day mortality in intensive care units via sepsis 3.0 definitions. Diagnostics. 2021;11(10):1906. doi:10.3390/diagnostics11101906
8. Yagmur E, Abu Jhaisha S, Buendgens L, et al. Clusterin plasma concentrations are decreased in sepsis and inversely correlated with established markers of inflammation. Diagnostics. 2022;12(12):3010. doi:10.3390/diagnostics12123010
9. Kyriakoudi A, Rovina N, Koltsida O, et al. Weaning failure in critically ill patients is related to the persistence of sepsis inflammation. Diagnostics. 2021;12(1):92. doi:10.3390/diagnostics12010092
10. Sari MI, Ilyas S. The expression levels and concentrations of PD-1 and PD-L1 proteins in septic patients: a systematic review. Diagnostics. 2022;12(8):2004. doi:10.3390/diagnostics12082004
11. Xiang Z, Zhang L, Hu R, et al. Establishment of heparin-binding protein time-resolved immunoassay and some potential clinical applications. Anal Biochem. 2021;631:114359. doi:10.1016/j.ab.2021.114359
12. Tverring J, Nielsen N, Dankiewicz J, Linder A, Kahn F, Åkesson P. Repeated measures of Heparin-binding protein (HBP) and procalcitonin during septic shock: biomarker kinetics and association with cardiovascular organ dysfunction. Intensive Care Med Exp. 2020;8(1):51. doi:10.1186/s40635-020-00338-8
13. O’Brien M. The reciprocal relationship between inflammation and coagulation. Top Companion Anim Med. 2012;27(2):46–52. doi:10.1053/j.tcam.2012.06.003
14. Chae BR, Kim YJ, Lee YS. Prognostic accuracy of the sequential organ failure assessment (SOFA) and quick SOFA for mortality in cancer patients with sepsis defined by systemic inflammatory response syndrome (SIRS). Support Care Cancer. 2020;28(2):653–659. doi:10.1007/s00520-019-04869-z
15. Li W, Wang M, Zhu B, Zhu Y, Xi X. Prediction of median survival time in sepsis patients by the SOFA score combined with different predictors. Burns Trauma. 2020;8:tkz006. doi:10.1093/burnst/tkz006
16. Liu C, Suo S, Luo L, Chen X, Ling C, Cao S. SOFA score in relation to sepsis: clinical implications in diagnosis, treatment, and prognostic assessment. Comput Math Methods Med. 2022;2022:7870434. doi:10.1155/2022/7870434
17. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
18. Global sepsis alliance - sepsis alliance. Available from: https://www.sepsis.org/sepsis-basics/what-is-sepsis/.
19. Cheng B, Hoeft AH, Book M, Shu Q, Pastores SM. Sepsis: pathogenesis, biomarkers, and treatment. Biomed Res Int. 2015;2015:846935. doi:10.1155/2015/846935
20. Grondman I, Pirvu A, Riza A, Ioana M, Netea MG. Biomarkers of inflammation and the etiology of sepsis. Biochem Soc Trans. 2020;48(1):1–14. doi:10.1042/BST20190029
21. Kahn F, Tverring J, Mellhammar L, et al. Heparin-binding protein as a prognostic biomarker of sepsis and disease severity at the emergency department. Shock. 2019;52(6):e135–e145. doi:10.1097/SHK.0000000000001332
22. Xiaobo LEI, Xiujie WANG. D-dimer detection and its clinical application progress. Med Recapitulate. 2020;26 (22):4521–4527. doi:10.3969/j.issn.1006-2084.2020.22.029
23. Zhongwei Z, Yimin Z, Yan C, et al. Predictive value of heparin binding protein for sepsis. Chin Crit Care Med. 2021;33(06):654–658. doi:10.3760/cma.j.cn121430-20210424-00605
24. Lu Y, Liu J. Value of D-dimer combined with SOFA score in predicting prognosis for patients with sepsis. Chi J Emerg Resusc Disaster Med. 2021;16(11):50–1153.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.