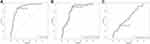Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
The Predictive Role of Aberrant Metabolic Parameters and Negative Automatic Thinking on the Cognitive Impairments Among Schizophrenia Patients with Metabolic Syndrome
Authors Zhang X , He C, Ju P, Xia Q, Gao J, Zhang L, Chen X, Yuan H, Gao H, Zhang Y, Yan J, Xie W, Zhu C
Received 22 March 2022
Accepted for publication 18 May 2022
Published 7 June 2022 Volume 2022:18 Pages 1087—1097
DOI https://doi.org/10.2147/NDT.S367392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xueying Zhang,1– 3,* Chen He,1– 3,* Peijun Ju,4,* Qingrong Xia,1– 3 Jianliang Gao,1– 3 Loufeng Zhang,1– 3 Xuequan Chen,1– 3 Hui Yuan,1– 3 Hua Gao,1– 3 Yang Zhang,1– 3 Junwei Yan,1– 3 Wen Xie,1– 3,* Cuizhen Zhu1– 3,*
1Affiliated Psychological Hospital of Anhui Medical University, Hefei, People’s Republic of China; 2Clinical Center for Psychiatry and Mental Health, Hefei Fourth People’s Hospital, Hefei, People’s Republic of China; 3Anhui Mental Health Center, Hefei, People’s Republic of China; 4Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai Key Laboratory of Psychotic Disorders, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wen Xie; Cuizhen Zhu, Affiliated Psychological Hospital of Anhui Medical University, No. 316 Huangshan Road, Shushan District, Hefei, Anhui, People’s Republic of China, Tel +86 13339102285 ; +86 13705535258, Fax +86 055163616000, Email [email protected]; [email protected]
Purpose: The study aimed to clarify the cognitive impairments of schizophrenia with metabolic syndrome while evaluating their potential as risk factors.
Patients and Methods: We recruited 153 participants and divided them into three groups according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria and the guideline standards for the prevention and treatment of dyslipidemia in Chinese adults in 2007 for metabolic syndrome, as follows: healthy control group (n = 47); nonmetabolic syndrome group (n = 58); and metabolic syndrome group (n = 48). Psychotic symptoms were evaluated using the Positive and Negative Syndrome Scale. Cognitive function and automatic thinking were estimated using the Montreal Cognitive Assessment Scale, Verbal Fluency Test, and Automatic Thoughts Questionnaire. Serum biochemical parameters were measured by automatic biochemistry analyzer.
Results: One-way ANOVA analysis revealed that differential cognition impairments in schizophrenia patients compared to controls. Furthermore, results of multiple comparisons showed that more serious barriers in orientation, language fluency, and negative automatic thinking existed in the metabolic syndrome group than in the healthy and non-metabolic syndrome groups. Spearman correlation and stepwise linear regression analyses showed that psychopathological symptoms, high waist circumference, and high triglyceride were the predictive factors for negative automatic thoughts, orientation, and language fluency. Those results collectively revealed that high waist circumference, high triglyceride and negative automatic thinking had validity and effectiveness in predicting the cognitive function impairments of the metabolic syndrome group.
Conclusion: The present findings strongly supported the notion that aberrant parameters of high waist circumference, high triglyceride and high negative automatic thoughts had validity and effectiveness predictive role for cognitive impairments in the schizophrenics with metabolic syndrome. The schizophrenia patients with metabolic syndrome should receive regular monitoring and adequate treatment for metabolic and psychological risk factors.
Keywords: second-generation antipsychotics, negative symptoms, diet, Montreal Cognitive Assessment Scale, Verbal Fluency Test
Introduction
Schizophrenia (SCZ) is a highly debilitating psychiatric disorder characterized by impairments of thinking, emotional and cognitive functions. Cognitive impairments are a group of core symptoms of SCZ. The prevalence of metabolic syndrome (MetS) caused by second-generation antipsychotics (SGAs) reached 33.4% in SCZ patients.1 SCZ has 20% less life expectancy than healthy individuals due to coronary heart disease (CHD), which is strongly related to MetS.2 Recent studies in North America and Europe showed that MetS could be associated with the cognitive performance impairments.3 Meanwhile, a cross-section study of MetS and cognitive performance was conducted among Chinese individuals who are more than 50 years old, the results showed that elderly Chinese people with diabetes have lower cognition function, furthermore, the level of dyslipidemia might be reversely associated with cognitive function.4 The latest meta-analysis reported that SCZ patients with MetS and diabetes had more serious cognitive deficits than those without metabolic disorders, especially, impairments in the domains of memory, attention, processing speed and execution.5 Hence, it is necessary to perform a comprehensive study between cognitive dysfunction, MetS components and automatic negative thoughts, so as to seek new treatment strategies and improve the clinical treatment effect.
Plenty of evidence revealed that the diabetes increases the risk of cognitive dysfunction, and abnormal blood glucose levels are major diagnostic criteria for MetS.6,7 Moreover, Fisher et al suggested that diabetes may be a stressor affecting the negative psychological state of patients.8 Depressive mood and poor emotional regulation are extremely common in cognitive impairments of patients with schizophrenia.9–11 Thus, it is important to understand whether negative thinking is significantly related to cognitive impairments among schizophrenia patients with metabolic syndrome. Poor emotional regulation is associated with negative self-evaluation, worry, and automatic negative thinking. Negative automatic thinking is considered as an important medium for suicide, the more depressed the patient is, the higher the frequency of negative automatic thinking will be.12,13 Depression is particularly obvious in the remission period of the disease and has become a stable and distinctive characteristic of SCZ.14 A multi-center study found that the decline of cognitive function in elderly patients with SCZ was also closely related to the depression that occurs after the alleviation of symptoms, and this result implied that post-schizophrenic depression may have effective predictive clues for cognitive dysfunction in SCZ.15,16 How the negative automatic thinking in SCZ patients with MetS mediates the cognitive dysfunction is worth exploring.17 So far, no studies focused on the links between negative automatic thinking and neurocognition in SCZ patients with MetS.
Psychosocial cognition and neurocognitive dimensions in MetS patients can be affected by interaction of negative thinking and biochemical metabolic, however, whether negative automatic thinking can aggravate cognition of patients in SCZ are controversial and inconclusive, moreover, these results are rarely reported. Meanwhile, negative automatic thinking mediates the impairments of cognitive function in SCZ with MetS caused by SGAs is still unknown. First, this study aims to determine whether MetS caused by SGAs is a risk factor of cognitive decline in patients with SCZ. Second, the possible correlation between MetS and overall psychosocial and neurocognitive cognitive performance dimensions in SCZ patients needs to be clarified. Thirdly, whether negative automatic thinking mediates the cognitive dysfunction and symptomatic severity of SCZ with MetS needs to be determined. Collectively, these findings would provide a theoretical basis to elucidate these issues and help improve cognitive performance in SCZ patients from the viewpoint of metabolism.
Patients and Methods
Procedure
This pilot study explored the relationship among negative automatic thinking, lifestyle, metabolic parameters and cognitive dysfunction in SCZ patients with MetS caused by SGAs. A total of 280 participants were initially selected. Of these, 85 participants did not meet the inclusion criteria, 22 individuals could not complete the scale assessment, and 20 individuals declined to sign the informed consent. Hence, 127 subjects who did not meet the experimental criteria were excluded from this experiment. Ultimately, the remaining 153 participants were included and divided into three groups according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),18 and the guideline standards for the prevention and treatment of dyslipidemia in Chinese adults in 2007 for MetS.19 The groups were healthy control group (n = 47), nonmetabolic syndrome (non-MetS) group (n = 58), and MetS group (n = 48). All patients were hospitalized at the Anhui Mental Health Center (AMHC) between April and December 2021. The healthy controls were recruited by the hospital physical examination center (Figure 1).
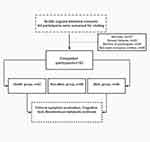 |
Figure 1 Flow chart. |
All participants were assessed using the Mini-International Neuropsychiatric Interview 6.0.0. (MINI6.0.0) According to the trial standards, the inclusion criteria for the patients in the MetS group were as follows: (1) age = 18–60 years; (2) patients treated with olanzapine, clozapine, and risperidone for more than 3 months; (3) fulfillment of the DSM-5 criteria for SCZ; (4) after taking the SGAs for three months, fulfillment of the guideline standards for the prevention and treatment of dyslipidemia in Chinese adults in 2007 for MetS. The inclusion criteria for the patients in the non-MetS group were as follows: (1) fulfillment of the first, second, and third criteria for inclusion in the MetS group; (2) after taking the SGAs for three months, non-fulfillment of the guideline standards for the prevention and treatment of dyslipidemia in Chinese adults in 2007. Healthy controls were recruited from the local community at the same time as other participants through advertising. The exclusion criteria were as follows: (1) people with MetS, endocrine and other serious physical diseases before entering the group; (2) patients with other mental diseases except SCZ and substance abuse; (3) patients with previous neurological diseases, such as epilepsy and dementia; (4) had received electroconvulsive therapy or transcranial magnetic stimulation within 6 months; (5) pregnant or lactating women. All subjects received face-to-face interviews. Basic sociodemographic data, clinical scale evaluation findings, and metabolic testing results were collected by trained professional psychiatrists.
Clinical Assessments
MINI 6.0.0
MINI 6.0.0 is a concise diagnostic interview for psychiatric disorders that was developed jointly by psychiatrists in the United States and Europe. The initial clinical diagnoses were validated by experienced psychiatrists using the MINI 6.0.0. Participants were screened to meet the inclusion criteria in the study.20
Positive and Negative Symptoms (PANSS)
PANSS is widely used to appraise severe symptoms in adult SCZ patients. It has been utilized for the assessment of positive and negative symptoms, as shown in Table 1. The Mandarin version model of the PANSS has good reliability and validity, it has a Cronbach’s alpha coefficient of 0.928 and an intra-class coefficient of 0.878.21
 |
Table 1 General Demographic Background, Blood Routine, and Biochemical Results |
Automatic Thoughts Questionnaire (ATQ)
ATQ is a scale used to evaluate the frequency of automatically occurring negative thoughts related to depression and includes four dimensions, namely, personal maladjustment and undesired for change, negative self-concept and negative expectations, low self-esteem, and giving up/helplessness.22 The total score of the scale is in the range of 30–150. The higher the score is, the heavier the negative thinking and depression are. In addition, ATQ shows good internal consistency among Chinese with mental disorders (Cronbach’s α = 0.96).23
Montreal Cognitive Assessment Scale (MOCA)
MOCA is a short tool used to examine cognitive function. It has a total of 30 items, including executive function, confrontation naming, attention, sentence repetition, verbal fluency, delayed verbal recall, and orientation. If the score is lower than 26, then the person is considered to have cognitive impairments; the score ranges from sensitive to mild and severe cognitive impairments.24 In mild cognitive impairments, the internal consistency of MOCA is reportedly very good, and it has the standardized program Cronbach’s alpha of 0.83.25
Verbal Fluency Test (VFT)
VFT is used to evaluate the language ability, semantic memory, and executive function of the subjects, which can be divided into semantic fluency, phonological fluency, and action verbal fluency. Subjects were required to list as many words of the same category as possible within 1 min.26
Laboratory Biochemical Evaluation
Samples were collected by trained nurses using a sample collection protocol. After an overnight fasting period, peripheral venous blood samples (5 mL) were collected from all subjects between 6:30 and 7:00 a.m. Samples were sent to the Department of Clinical Laboratory immediately for centrifugation, and the serum was separated. The blood routine analysis was performed using an automatic hematology analyzer (Mindray BC-2800, Shenzhen, China). Serum biochemical parameters were measured by an automatic biochemistry analyzer (AU480, Beckman Coulter, USA) using commercial kits (Roche, Switzerland). Blood pressure, which includes systolic pressure (DBP) and diastolic pressure (SBP), was measured using an automatic sphygmomanometer prior to blood collection. At the normal end of exhalation, the horizontal girth across the center of the umbilicus was measured as waist circumference. The diagnosis of MetS should meet the following three or more items: (1) abdominal obesity according to waist circumference (CM): male >90, female >85; (2) blood triglyceride (TG) >1.7 mmol/l; (3) blood high-density lipoprotein (HDL-C) <1.04 mmol/L; (4) blood pressure ≥130/85 mmHg; (5) fasting blood glucose (FPG) >6.1 mmol/l and/or postprandial 2-hour blood glucose (2H PG) is more than 7.8 mmol/l or with diabetes history.
Statistical Analysis
Chi-square test and one-way ANOVA were used to compare demographic characteristics differences among the three groups, and Fisher’s least significant difference (LSD) was used for multiple comparisons. Spearman correlation analysis was used to test the relationship between possible correlation factors and cognitive scale scores. Stepwise linear regression analysis was used to explore the relationship and interaction between metabolic factors, psychiatric symptoms, and cognitive function in patients with MetS. The area under the receiver-operating characteristic curve was used to assess the metabolic indicators and clinical translational value factors to evaluate the impairments of cognitive function. Other performance metrics, including sensitivity and specificity, were also obtained at the optimal cutoff value of 0.5 as defined by the receiver-operating characteristic curve (ROC). All statistical tests were two-tailed tests, and the statistical significance was set as α < 0.05. All analyses were conducted using the SPSS version 22.0 (IBM Corp).
Results
General Features of the Sample
Overall, no significant difference was found in demographic background, such as age, gender, years of education, marriage, occupation, all dietary characteristics, blood routine, SBP, the levels of urea nitrogen (BUN) and glutamic-pyruvic transaminase (ALT) among the three groups (P ≥ 0.05). Furthermore, no difference was found in medicine usage, total course of disease and features, and clinical symptomatic severity between non-MetS and MetS group (P ≥ 0.05). Additionally, one-way ANOVA analysis found that body mass index (BMI), waist circumference, and the levels of TG, FBG, cholesterol (CHOL), apolipoprotein A (Apo-A), apolipoprotein B (Apo-B), and aspartate aminotransferase (AST) were higher in the MetS and non-MetS groups than in the healthy control group (P < 0.05). In particular, the biochemical indicators of FBG, CHOL, TG, Apo-A, Apo-B, and AST were higher in the MetS group than in the non-MetS group (P < 0.05). The level of HDL-C was lowest in the MetS group (P < 0.05). Results are shown in Table 1.
Discrepancy of Psychological Parameters
To evaluate the differential impairment conditions of cognitive function, language executive function, and negative automatic thinking, we used MOCA, ATQ, and VFT, respectively, among the subjects in the healthy, non-MetS, and MetS groups. First, we found differences in ATQ scores among the three groups (F = 12.40, P < 0.001). The MetS group had significantly more ATQ scores than the non-MetS and healthy groups (P = 0.029, P < 0.001). Similarly, the results of orientation had differential impairment conditions among the three groups (F = 8.32, P < 0.001). The MetS group had severe directional defect compared with the non-MetS and healthy groups (P = 0.013, P < 0.001). Moreover, in the VFT, there were more serious verbal fluency barriers at the beginning of the word “self” in the MetS group (F = 5.24, P = 0.006). The word “self” in the VFT in the MetS group was significantly lower than that in the non-MetS and healthy groups (P = 0.036, P = 0.002). These impairments existed on average in the MetS and non-MetS groups compared with the healthy group and were worst in the MetS group. The neurocognitive function deficits of SCZ with MetS may be caused by treatments with the antipsychotic drugs, thereby indicating the need to clarify the mechanisms underlying these conditions. Results are shown in Table 2.
 |
Table 2 Results of Cognitive Function, Automatic Thinking Among the Three Groups |
Predictive Risk Factors
To address the issues of predictive risk factors for the impairments of cognitive function and negative automatic thinking among the three groups, we used the correlation analysis to assess whether the abnormal biochemical markers, psychotic symptoms, and BMI were related. The results of BMI, body weight, waist circumference, FBG, TG, and negative symptom scores of PANSS were negatively correlated with orientation (r = −0.199, P = 0.014; r = −0.184, P = 0.023; r = −0.230, P = 0.004; r = −0.187, P = 0.021; r = −0.181, P = 0.025; r = −0.193, P = 0.048). When considering the function of verbal fluency, patients whose BMI, body weight, waist circumference, TG, PANSS total score, negative symptom score, and general psychopathological score of PANSS were negatively correlated with verbal fluency of words beginning with “self” needed to be identified (r = −0.174, P = 0.031; r = −0.162, P = 0.045; r = −0.260, P < 0.001; r = −0.179, P = 0.027; r = −0.385 P < 0.001; r = −0.392, P < 0.001; r = −0.368, P < 0.001). Furthermore, waist circumference, TG, total PANSS score, negative symptom score, and general psychopathological score of PANSS were found to be positively correlated with negative automatic thinking (r = 0.212, P = 0.009; r = 0.195, P = 0.015; r = 0.287, P = 0.003; r = 0.253, P = 0.009; r = 0.337, P < 0.001). Interestingly, the level of HDL-C was positively correlated with orientation and verbal fluency (r = 0.312, P < 0.001; r = 0.245, P = 0.002) and negatively correlated with ATQ score (r = −0.267, P < 0.001). Results are shown in Table 3.
 |
Table 3 Correlation Analysis of Cognitive Function, Automatic Thinking-Related Factors Analysis Results |
To our knowledge, there is heterogeneity in the risk factors of cognitive impairments in MetS research. Hence, to determine the predictive factors for cognitive decline in the MetS group, we performed a stepwise linear regression analysis. The negative symptom of PANSS (β = 0.228, t = 2.469, P = 0.015), TG (β = 0.200, t = 2.165, P = 0.033), and times of meat intake (β = −0.214, t = −2.319, P = 0.022) were the influencing factors of ATQ. The negative symptom of PANSS (β = −0.289, t = −3.188, P = 0.002) and abdominal circumference (β = −0.257, t = −2.836, P = 0.006) were the predictive factors for impairment of orientation. Finally, the total score of PANSS (β = −0.371, t = −4.264, P < 0.001) and waist circumference (β = −0.262, t = −3.011, P = 0.003) were found to be the influencing factors for the “self” of verbal fluency. The results are shown in Table 4.
 |
Table 4 Stepwise Linear Regression Analysis of Cognitive Function, Automatic Thinking |
Validity and Effectiveness of Parameters
ROC curve analysis was used to predict the cognitive function impairments of MetS groups by using dysbiosis of MetS indexes and clinical symptoms. TG was able to predict the cognitive function impairments of MetS groups at a cutoff level of 1.740 mmol/l, with a sensitivity of 0.780 and a specificity of 0.894 (Figure 2A). Waist circumference can predict the cognitive function impairments of MetS groups at a cutoff level of 85.750 cm, with a sensitivity of 0.661 and a specificity of 0.936 (Figure 2B). ATQ may predict the cognitive function impairments of MetS groups at a cutoff level of 73.000 scores, with a sensitivity of 0.780 and a specificity of 0.383 (Figure 2C). Predictive analysis results revealed that the dysbiosis parameters of TG, waist circumference and ATQ were associated with the prediction of cognitive function impairment risk in the MetS group’s progression.
Discussion
This study aimed to explore the related risk factors of cognitive impairments in SCZ patients with MetS. The main findings of this study are as follows. First, compared with the healthy control group, the overall cognition function of SCZ patients in both MetS and non-MetS groups decreased significantly; meanwhile, cognitive function of the MetS group was the worst among three groups. Patients in the MetS group had more negative automatic thinking than those in non-MetS and healthy control groups. Furthermore, patients in the MetS group with high BMI, high waist circumference, high lipid level, low level of HDL-C, and negative symptoms were severely impaired in orientation and verbal fluency and showed increased negative automatic thinking. These results suggested that high waist circumference, TG and ATQ are high risk predictive factors for patients with psychological and neurocognitive impairments in the MetS group. Hence, a study to understand these variable findings is needed.
Previous studies on cognitive function of SCZ have demonstrated that the existence of extensive impairments in executive function, working memory, verbal fluency, attention, and social cognition.27 The prevalence of MetS in patients with SCZ treated with SGAs increased significantly, and the cognitive impairments were more obvious than in non-MetS patients.28 In this study, compared with healthy people, SCZ patients in the MetS and non-MetS groups had cognitive impairments, and the results are consistent with previous studies.29 Goughari found that SCZ patients with hypertension had cognitive deficiency in verbal memory and fluency.30 Likewise, Gonzalez found that verbal fluency damage was significantly under average in patients with MetS than in patients without MetS.31 These results are consistent with those obtained in the present study. The fluency function of verbal expression with the prefix “self” was significantly impaired in the MetS group than in the other two groups. Clinical research data showed that the prevalence of age-related orientation disorder was about 25% in hospitalized patients with SCZ.32 Moreover, the test of date and timelapse had less validity in patients with age orientation disorder than patients without age orientation disorder. These chronic SCZ patients could not reliably tell his or her age and had a static interpretation of time. The accumulating and increasing time orientation disorders may be closely related to psychotic clinical symptoms. In the present study, MetS patients were disoriented in terms of character, time, and space. These impairments were related to the negative symptoms of psychosis. To comprehensively improve the living ability of patients with SCZ in the future, it is necessary to enhance the intervention of negative symptoms. In the past, the cognitive impairments related to SCZ with MetS mostly focused on the impairments of executive functions, such as attention, memory, and response flexibility.33 However, in this study, no significant differences were found in attention and memory between MetS and non-MetS groups. This heterogeneity may be caused by differences in race, diagnostic criteria, and evaluation tools, this discrepancy needs to be further confirmed in future research.
Interestingly, we found that SCZ patients with MetS had more negative automatic thinking than those in the non-MetS group. High waist circumference and high blood lipid were positively correlated with negative automatic thinking. In addition, serious psychiatric symptoms including higher scores for negative and general pathological symptoms were more likely to be associated with more negative automatic thinking. We found that BMI, high weight, and high waist circumference were negatively correlated with “self” prefixed verbal fluency and orientation. In a longitudinal study on the cognitive function of people with MetS who are over 65 years old in three French cities, the verbal fluency of MetS patients was significantly lower than that of patients without MetS at the initial stage of the survey, if subjects suffered from diabetes, then the level of HDL-C reduction will increase the risk of impairment in verbal fluency within 4 years.34 This result is consistent with our results on impaired verbal fluency in patients of SCZ with MetS. In the latest large-sample cohort study on the cognitive function of MetS patients who were diagnosed with MetS at baseline, the patients were at risk of impaired directional accumulation and had increased risk of dementia in later follow-up studies. When the metabolic state returned to normal, the risk of cognitive decline was still not restored. Therefore, to minimize the damage, early detection and management of potential risk factors of MetS should be encouraged.35
Our study also found that the total score, negative symptoms, and general psychopathological score of PANSS were negatively correlated with the verbal expression ability and orientation of patients, whereas these aberrant parameters were positively correlated with negative automatic thinking. These findings revealed that the severity of SCZ is closely correlated with the decline of cognitive function and negative automatic thinking. Regression analysis showed that general psychiatric scores and TG are potential risk factors for negative automatic thinking, severe negative symptoms and high abdominal weight are the risk factors of orientation, as well as high PANSS total score and high waist circumference are risk factors for verbal fluency.36 Furthermore, we found the aberrant parameters of high waist circumference and high TG had high predictive validity and effectiveness for cognitive impairments in the MetS group, meanwhile, we discovered the high ATQ has moderate predictive sensitivity to cognitive impairments among MetS patients. Besides these aberrant metabolic indicators, we should consider the role of psychological factors in the cognitive impairments among MetS patients in the future.
Several potential limitations were present in this study. Our study was a short-term cross-sectional design, limiting the extent that causal relationships between MetS and cognition can be determined. In the future, longitudinal research is necessary to confirm the causal relationship between MetS and impaired cognitive function in SCZ patients. Then, there would be sufficient time for accurately assessing other potential risk factors, such as hyperglycaemia. Another limitation of this study is that three drugs were included; therefore, a stratified study with a larger sample size is needed to clarify the different conditions of cognitive impairments caused by different drugs. The MetS associated with antipsychotic meditation in this study was almost observed in chronic patients. However, it may be also observed among antipsychotic naïve individuals with first-episode patients, hence other potential factors for associating MetS with cognitive ability could be identified.
Conclusion
In conclusion, compared with the healthy controls, the cognitive function of SCZ patients in both MetS and non-MetS group decreased significantly. Worst results were found in the MetS group among three groups. Patients with high BMI, weight gain, high waist circumference and high blood lipid level had impaired verbal fluency and were prone to negative automatic thinking. Serious negative symptoms of psychosis are connected to more serious impairments of cognitive function and higher tendency to demonstrate negative automatic thinking. These results suggested that high TG, high waist circumference and ATQ are the predictors of cognitive impairments among SCZ patients with MetS. These predictors are important adjustment and intervenable risk factors for MetS caused by SGAs.
Ethics Approval and Consent to Participate
The study was approved by the Medical Ethics Committee of the Anhui Mental Health Centre (AMHC). The trial clinical registration number was ChiCTR2100045240. Thus, the effect of this human study has been approved by the appropriate ethics committee and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided written consent prior to participation.
Acknowledgments
This study was supported by funding of Hospital project of Hefei Fourth People’s Hospital (grant number: 2019023), Fund Project of Anhui Medical University (grant number: 2019xkj206), Key research and development program of Anhui Province (grant number:1804h08020292), Shanghai Key Laboratory of Psychotic Disorders Open Grant (grant number: 13dz2260500), Natural science research projects in Anhui Universities (grant number: KJ2020A0218), Applied medicine research project of Hefei Health Committee (grant number: Hwk2020zd0016) and Applied medicine research project of Anhui Health Committee (grant number: AHWJ2021a036). The funding sources had no involvement in study design, collection, analysis, writing of this paper and publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347. doi:10.1002/wps.20252
2. Mohamud WN, Ismail AA, Sharifuddin A, et al. Prevalence of metabolic syndrome and its risk factors in adult Malaysians: results of a nationwide survey. Diabetes Res Clin Pract. 2011;91(2):239–245. doi:10.1016/j.diabres.2010.11.025
3. Lee AMH, Ng CG, Koh OH, Gill JS, Aziz SA. Metabolic syndrome in first episode schizophrenia, based on the National Mental Health Registry of Schizophrenia (NMHR) in a general hospital in Malaysia: a 10-year retrospective cohort study. Int J Environ Res Public Health. 2018;15:933. doi:10.3390/ijerph15050933
4. Chen B, Jin X, Guo R, et al. Metabolic syndrome and cognitive performance among Chinese≥50 years: a cross-sectional study with 3988 participants. Metab Syndr Relat Disord. 2016;14(4):222–227. doi:10.1089/met.2015.0094
5. Hagi K, Nosaka T, Dickinson D, et al. Association between cardiovascular risk factors and cognitive impairment in people with schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(5):510–518. doi:10.1001/jamapsychiatry.2021.0015
6. Li W, Huang E, Gao S. Type 1 Diabetes mellitus and cognitive impairments: a systematic review. J Alzheimers Dis. 2017;57(1):29–36. doi:10.3233/JAD-161250
7. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. doi:10.1038/s41574-018-0048-7
8. Fisher L, Hessler D, Polonsky W, Strycker L, Bowyer V, Masharani U. Toward effective interventions to reduce diabetes distress among adults with type 1 diabetes: enhancing emotion regulation and cognitive skills. Patient Educ Couns. 2019;102(8):1499–1505. doi:10.1016/j.pec.2019.03.021
9. Vesco AT, Howard KR, Anderson LM, Papadakis JL, Hood KK, Weissberg-Benchell J. Examining indirect effects of anxiety on glycated hemoglobin via automatic negative thinking and diabetes-specific distress in adolescents with type 1 diabetes. Can J Diabetes. 2021;45(5):473–480. doi:10.1016/j.jcjd.2021.05.002
10. Shimada H, Park H, Makizako H, Doi T, Lee S, Suzuki T. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149–156. doi:10.1016/j.jpsychires.2014.06.004
11. Yang X, Qi S, Wang M, et al. Subtypes of depression characterized by different cognitive decline and brain activity alterations. J Psychiatr Res. 2021;138:413–419. doi:10.1016/j.jpsychires.2021.04.023
12. Choon MW, Abu Talib M, Yaacob SN, et al. Negative automatic thoughts as a mediator of the relationship between depression and suicidal behaviour in an at-risk sample of Malaysian adolescents. Child Adolesc Ment Health. 2015;20(2):89–93. doi:10.1111/camh.12075
13. Mohammadkhani P, Bagheri M, Dobson KS, et al. Negative thoughts in depression: a study in Iran. Int J Psychol. 2020;55(1):83–89. doi:10.1002/ijop.12541
14. Subodh BN, Grover S. Depression in schizophrenia: prevalence and its impact on quality of life, disability, and functioning. Asian J Psychiatr. 2020;54:102425. doi:10.1016/j.ajp.2020.102425
15. Pascal de Raykeer R, Hoertel N, Blanco C, et al. Effects of depression and cognitive impairment on quality of life in older adults with schizophrenia spectrum disorder: results from a multicenter study. J Affect Disord. 2019;256:164–175. doi:10.1016/j.jad.2019.05.063
16. Cruz BF, Resende CB, Carvalhaes CF, et al. Interview-based assessment of cognition is a strong predictor of quality of life in patients with schizophrenia and severe negative symptoms. Braz J Psychiatry. 2016;38(3):216–221. doi:10.1590/1516-4446-2015-1776
17. Takeda T, Nakataki M, Ohta M, et al. Negative and positive self-thoughts predict subjective quality of life in people with schizophrenia. Neuropsychiatr Dis Treat. 2019;15:293–301. doi:10.2147/NDT.S190381
18. First MB. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. 2013;201(9):727–729. doi:10.1097/NMD.0b013e3182a2168a
19. Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(5):390–419. Chinese. PMID: 17711682.
20. Fang Y, Wang W, Zhu C, et al. Use of tobacco in schizophrenia: a double-edged sword. Brain Behav. 2019;9(11):e01433. doi:10.1002/brb3.1433
21. Wu BJ, Lan TH, Hu TM, Lee SM, Liou JY. Validation of a five-factor model of a Chinese mandarin version of the positive and negative syndrome scale (CMV-PANSS) in a sample of 813 schizophrenia patients. Schizophr Res. 2015;169(1–3):489–490. doi:10.1016/j.schres.2015.09.011
22. Dekker RL. Measurement of negative thinking in patients with heart failure: a critical review and analysis. J Cardiovasc Nurs. 2011;26(1):9–20. doi:10.1097/jcn.0b013e3181dfcbce
23. Wong DF, Chau P, Kwok A, Kwan J. Cognitive–behavioral treatment groups for people with chronic physical illness in Hong Kong: reflections on a culturally attuned model. Int J Group Psychother. 2007;57(3):367–385. doi:10.1521/ijgp.2007.57.3.367
24. Musso MW, Cohen AS, Auster TL, McGovern JE. Investigation of the Montreal Cognitive Assessment (MoCA) as a cognitive screener in severe mental illness. Psychiatry Res. 2014;220(1–2):664–668. doi:10.1016/j.psychres.2014.07.078
25. Rosca EC, Cornea A, Simu M. Montreal cognitive assessment for evaluating the cognitive impairment in patients with schizophrenia: a systematic review. Gen Hosp Psychiatry. 2020;65:64–73. doi:10.1016/j.genhosppsych.2020.05.011
26. Olabarrieta-Landa L, Torre EL, Lopez-Mugartza JC, Bialystok E, Arango-Lasprilla JC. Verbal fluency tests: developing a new model of administration and scoring for Spanish language. NeuroRehabilitation. 2017;41(2):539–565. doi:10.3233/NRE-162102
27. Bortolato B, Miskowiak KW, Kohler CA, Vieta E, Carvalho AF. Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatr Dis Treat. 2015;11:3111–3125. doi:10.2147/NDT.S76700
28. Boyer L, Richieri R, Dassa D, et al. Association of metabolic syndrome and inflammation with neurocognition in patients with schizophrenia. Psychiatry Res. 2013;210(2):381–386. doi:10.1016/j.psychres.2013.06.020
29. Kurtz MM, Gopal S, John S, Thara R. Cognition, social cognition and functional disability in early-stage schizophrenia: a study from southern India. Psychiatry Res. 2018;265:231–237. doi:10.1016/j.psychres.2018.03.091
30. Goughari AS, Mazhari S, Pourrahimi AM, Sadeghi MM, Nakhaee N. Associations between components of metabolic syndrome and cognition in patients with schizophrenia. J Psychiatr Pract. 2015;21(3):190–197. doi:10.1097/PRA.0000000000000065
31. Gonzalez HM, Tarraf W, Vasquez P, et al. Metabolic syndrome and neurocognition among diverse middle-aged and older Hispanics/Latinos: HCHS/SOL results. Diabetes Care. 2018;41(7):1501–1509. doi:10.2337/dc17-1896
32. Crow TJ, Stevens M. Age disorientation in chronic schizophrenia: the nature of the cognitive deficit. Br J Psychiatry. 1978;133:137–142. doi:10.1192/bjp.133.2.137
33. Boyer L, Testart J, Michel P, et al. Neurophysiological correlates of metabolic syndrome and cognitive impairment in schizophrenia: a structural equation modeling approach. Psychoneuroendocrinology. 2014;50:95–105. doi:10.1016/j.psyneuen.2014.07.019
34. Raffaitin C, Féart C, Le Goff M, et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76(6):518–525. doi:10.1212/WNL.0b013e31820b7656
35. Wu S, Chen WL. Longitudinal trajectories of metabolic syndrome on different neurocognitive domains: a cohort study from the Taiwan biobank. Aging. 2021;13(11):15400–15412. doi:10.18632/aging.203099
36. Peer M, Lyon R, Arzy S. Orientation and disorientation: lessons from patients with epilepsy. Epilepsy Behav. 2014;41:149–157. doi:10.1016/j.yebeh.2014.09.055
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.