Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
The Predictive Effect of the 8th AJCC Pathological Prognostic Staging on the Benefit of Postmastectomy Radiotherapy in N2/N3 Breast Cancer
Authors Yang SP, Zhou P, Lian CL, He ZY , Wu SG
Received 18 February 2022
Accepted for publication 5 May 2022
Published 13 May 2022 Volume 2022:14 Pages 133—144
DOI https://doi.org/10.2147/BCTT.S362355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pranela Rameshwar
Shi-Ping Yang,1,* Ping Zhou,2,* Chen-Lu Lian,2 Zhen-Yu He,3 San-Gang Wu2
1Department of Radiation Oncology, Hainan General Hospital (Hainan Affiliated Hospital of Hainan Medical University), Haikou, 570311, People’s Republic of China; 2Department of Radiation Oncology, Xiamen Cancer Center, Xiamen Key Laboratory of Radiation Oncology, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, 361003, People’s Republic of China; 3Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, 510060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhen-Yu He, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China, Tel +86 20 87343543, Fax +86 20 87343392, Email [email protected] San-Gang Wu, Department of Radiation Oncology, Xiamen Cancer Center, Xiamen Key Laboratory of Radiation Oncology, The First Affiliated Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, 361003, People’s Republic of China, Tel +86 592 2139531, Fax +86 592 2137222, Email [email protected]
Background: The role of the 8th American Joint Committee on Cancer (AJCC) pathological prognostic staging (PPS) on treatment-decision making of breast cancer (BC) remains unclear. This study aimed to investigate the predictive effect of the 8th AJCC PPS on the benefit of postmastectomy radiotherapy (PMRT) in N2/N3 BC.
Methods: We included women with stage N2/3 BC diagnosed between 2010 and 2018 from the Surveillance, Epidemiology, and End Results database. The effect of PMRT on breast cancer-specific survival (BCSS) was evaluated using the multivariate Cox proportional-hazards models.
Results: A total of 13,445 patients were identified, including 10,547 (78.4%) patients treated with PMRT. All patients had reassigned stages based on the 8th AJCC PPS. There were 7102 patients (52.8%) that had stage changed, including 1160 patients (8.6%) were upstaged and 5942 patients (44.2%) were downstaged from the 7th AJCC anatomical staging (AS) to the 8th AJCC PPS. Regarding the 7th AJCC AS, 7603 (56.5%), 948 (7.1%), and 4895 (36.4%) were stage IIIA, IIIB, and IIIC diseases, respectively. Using the 8th AJCC PPS, 3525 (26.2%), 460 (3.4%), 1335 (9.9%), 3457 (25.7%), 2169 (19.1%), and 2100 (15.6%) patients were restaged as IB, IIA, IIB, IIIA, IIIB, and IIIC diseases, respectively. The PPS displayed increased prognostic accuracy and improved model fit with respect to BCSS compared to the 7th AS (C-index, 0.731 vs 0.605, P < 0.001; Akaike Information Criterion, 42141 vs 43118). Regarding the AS, the receipt of PMRT was associated with a better BCSS in those with stage IIIA (P = 0.004), IIIB (P = 0.003), and IIIC (P < 0.001) diseases. Using the PPS, the receipt of PMRT was not associated with a better BCSS among patients with stage IB (P = 0.446), IIA (P = 0.140), and IIB (P = 0.248) disease, while the receipt of PMRT was associated with a better BCSS for those with stage IIIA (P = 0.009), IIIB (P < 0.001), and IIIC (P < 0.001) disease.
Conclusion: The 8th AJCC staging provides superior risk stratification and a better tool to predict the benefit of PMRT in N2/3 BC.
Keywords: breast cancer, mastectomy, radiotherapy, AJCC staging, biologic markers
Introduction
Breast cancer (BC) is the most common malignancy in women. There are more than two million new BC cases diagnosed worldwide annually.1 With the progress of multimodal therapy in recent years, the 5-year survival rate of BC has reached 90%.2 However, BC is a heterogeneous disease with distinct biologic behavior, contributing to diverse clinical outcomes.3 Such heterogeneities depend, not only on the extent of disease but also on the biological heterogeneity of tumors.
In BC clinical practice, American Joint Committee on Cancer (AJCC) staging is an important indicator for determining survival outcomes and guiding treatment decision-making. The traditional AJCC anatomic staging (AS), including primary tumor (T), regional lymph nodes involvement (N), and distant metastasis (M) into the staging system. However, the traditional AS system does not integrate the prognostic effect of tumor biology, which is deficient in disease prognosis. With the advances in the understanding of BC biology, several biological markers for prognostication and treatment decision-making, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) status, and tumor grade, which have been identified and validated.4–6 Therefore, the above biological markers and the conventional TNM variables, have been incorporated into the latest AJCC pathological prognostic staging (PPS) system.7
Several studies including ours have confirmed that the 8th AJCC PPS provides more accurate risk stratification compared to the traditional AJCC AS.8–13 However, the role of the new AJCC PPS on treatment decision-making remains unclear. According to the previously validated studies, there were more than 50% of patients have staged migration using the PPS criterion, and most of them were downstaged.8,14–17 Therefore, treatment de-escalation and escalation should be investigated for patients that have their stage changed.
In the current clinical practice, the standard treatment for N2/3 BC patients after mastectomy is systemic treatment and adjuvant radiotherapy, and endocrine therapy or anti-HER2 therapy for those who have corresponding therapeutic targets.18 Postmastectomy radiotherapy (PMRT) could decrease the risk of locoregional and distant recurrence, translating into improvements in BC mortality.19 However, a previous study from the National Cancer Data Base (NCDB) showed that the receipt of PMRT was not associated with a lower risk of mortality in N2/N3 BC.20 Several studies including ours have found that BC subtypes established based on ER, PR, and HER2 could predict the benefit of PMRT in stage N2/3 BC.21–23 Therefore, whether the PPS established by anatomic and biological factors could predict the benefit of PMRT deserves further research. In light of this, the present study aimed to investigate the effect of the PPS on predicting the survival benefit of PMRT among those with N2/3 BC.
Patients and Methods
Patients
We included women BC diagnosed between 2010 and 2018 from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER program captures the cancer data for approximately 30% of the United States population, including the demographic and clinicopathological characteristics, the first course of treatments, and survival outcomes.24 We included patients who met the following criteria in this study: 1) aged 18–64 years; 2) stage III BC according to 7th AJCC staging criteria; 3) receiving mastectomy with or without PMRT; 4) receiving chemotherapy after mastectomy; 5) available data regarding T stage, N stage, grade, ER, PR, and HER2 status. Patients with stage T0, receiving non-beam irradiation or preoperative radiotherapy were excluded. Because the SEER program is a de-identified database, the institutional review board of the First Affiliated Hospital of Xiamen University determined the current study to be waived from review.
Variables
We included the following data in this study: age, race, histology, T stage, N stage, grade, ER, PR, and HER2 status. The use of PMRT was also included. All patients had reassigned stages based on the 8th AJCC PPS.7 The primary endpoint was breast cancer-specific survival (BCSS) in this study, which was defined as the time from the BC diagnosis to the death from BC.
Statistical Analysis
The difference in categorical data was compared by the Chi-square test. The receiver operating characteristics (ROC) curve was used to compare the risk stratification abilities between the AS and PPS to predict BCSS. The Harrell concordance index (C-index) and the Akaike Information Criterion (AIC) were then employed to measure the discriminatory ability between the two staging systems.25,26 A higher C-index indicates a better predictive value and a lower AIC correlates with a superior model fit. Kaplan–Meier method and Log rank testing were used to calculate BCSS curves and compare the survival distributions according to AS and PPS groups. Multivariate Cox proportional-hazards models were used to estimate the association of PMRT with BCSS after adjustment for known covariates. Sensitivity analyses after stratification by AS and PPS groups were used to further identify the specific subgroups that benefited from PMRT. All statistical analyses were conducted using the SPSS version 22 (SPSS Inc., Chicago, IL, USA), R project (The R Foundation, Vienna, Austria), or MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium). P-value < 0.05 was considered as significant in statistics.
Results
Patient Characteristics
A total of 13,445 patients were identified. The patient selection flowchart is listed in Figure 1. Summary statistics on patient baseline characteristics are listed in Table 1. Of these patients, 10,547 received PMRT (78.4%) and the remaining 2899 patients (21.6%) did not receive PMRT. Most of patients were invasive ductal carcinoma (n=9989, 74.3%), moderately to poorly differentiated disease (n=12,346, 91.8%), ER-positive (n=10,463, 77.8%), PR-positive (n=8909, 66.3%), and HER2-negative (n=10,545, 78.4%). Race, histology, T stage, ER, PR, and HER2 status were factors associated with the compliance of PMRT (all P<0.05).
 |
Table 1 Patients’ Baseline Demographic and Clinicopathological Characteristics |
Staging Migration and Staging Model Fit
Regarding the 7th AJCC AS, 7603 (56.5%), 948 (7.1%), and 4895 (36.4%) were stage IIIA, IIIB, and IIIC diseases, respectively. Using the 8th AJCC PPS, 3525 (26.2%), 460 (3.4%), 1335 (9.9%), 3457 (25.7%), 2169 (19.1%), and 2100 (15.6%) patients were restaged as IB, IIA, IIB, IIIA, IIIB, and IIIC diseases, respectively. Of these patients, 7102 patients (52.8%) had stage changed, including 1160 patients (8.6%) were upstaged and 5942 patients (44.2%) were downstaged. The frequency of stage discrepancies among individual patients has shown in Table 2.
 |
Table 2 The Frequency of Stage Discrepancies Among Individual Patients |
The results of ROC curves between AS and PPS are shown in Figure 2. The C-index in the PPS was significantly higher than the AS (0.731 vs 0.605, P<0.001). Further supporting its improved performance, the PPS demonstrated a lower AIC (42141) compared to the AS (43118), indicating better model fit and accuracy.
Survival and Prognostic Analyses
The median follow-up was 44 months (range, 0–107 months). A total of 2890 death were observed, including 2437 patients (84.3%) who died from BC. The 5-year BCSS was 78.8 months. Regarding the AS, the 5-year BCSS was 84.6%, 68.6%, and 71.4% in those with stage IIIA, IIIB, and IIIC diseases, respectively (P<0.001) (Figure 3A). Patients with stage IIIC disease had better BCSS than those with stage IIIB disease (P=0.012). According to the PPS, the 5-year BCSS was 94.4%, 87.4%, 82.5%, 82.8%, 72.5%, 50.0% for those with stage IB, IIA, IIB, IIIA, IIIB, and IIIC diseases, respectively (P<0.001) (Figure 3B), but the survival difference between stage IIB and stage IIIA did not reach statistical significance (P=0.681)
 |
Figure 3 Kaplan–Meier survival curves of the 7th anatomic staging (A) and 8th pathological prognostic staging (B). Abbreviations: AJCC, American Joint Committee on Cancer. |
We conducted the first multivariate prognostic analyses which adjusted for age, race, histology, tumor grade, T stage, N stage, ER, PR, and HER2 status in addition to PMRT receipt (Table 3). The results indicated that tumor grade, T stage, N stage, ER, PR, and HER2 were the independent prognostic factors associated with BCSS, which supported the incorporation of biological factors into the risk stratification of BC. In addition, patients receiving PMRT had better BCSS than those without PMRT (hazard ratio [HR] 0.700, 95% confidence interval [CI] 0.640–0.766, P<0.001). We further conducted the second multivariate prognostic analysis which adjusted for age, race, histology, and PPS in addition to PMRT receipt (Table 4). The results indicated that the PPS was the independent prognostic factor associated with BCSS, which supported the PPS also hold true in this population. Moreover, patients receiving PMRT also had better BCSS than those without PMRT in the second multivariate prognostic model (HR 0.696, 95% CI 0.636–0.760, P<0.001).
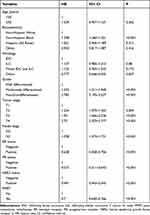 |
Table 3 Multivariate Prognostic Analysis in the Entire Cohort |
 |
Table 4 Multivariate Prognostic Analysis in the Entire Cohort, Including the 8th Pathological Prognostic Staging |
The Implication of the PPS for PMRT Decision Making
Finally, we performed sensitivity analyses to investigate the effect of PMRT in different AS and PPS groups (Table 5). Regarding the AS, the multivariate prognostic analyses showed that the use of PMRT was independently associated with a better BCSS in those with stage IIIA (HR 0.812, P=0.004), IIIB (HR 0.652, P=0.003), and IIIC (HR 0.627, P<0.001) diseases (Figure 4A–C), which indicated that the AS could not distinguish subgroups that PMRT could be safely omitted. Using the PPS, we found that the use of PMRT was not related to a better BCSS in those with stage IB (HR 0.882, P=0.446), IIA (HR 0.637, P=0.140), and IIB (HR 0.823, P=0.248) disease, but was independently associated with a better BCSS in those with stage IIIA (HR 0.767, P=0.009), IIIB (HR 0.645, P<0.001), and IIIC (HR 0.649, P<0.001) diseases (Figure 5A–E).
 |
Table 5 Sensitivity Analyses of Postmastectomy Radiotherapy Receipt on Breast Cancer-Specific Mortality Using the Cox Regression Models |
Discussion
In the present study, we used a population-based cohort to investigate the implication of the PPS for PMRT decision-making in N2/3 BC. Our results indicated that the PPS could provide better prognostic information and guide PMRT decision-making in this population.
Biological markers including grade, ER, PR, and HER2 status were useful for the selection of appropriate systemic therapies for BC.18 In this study, we were able to confirm the findings that the PPS provides a useful model for risk stratification and had more accurate prognostic information compared to the traditional AS.27 In previous large population-based cohort and single-institution cohort studies, there were 46.2–54.0% of non-metastatic BC patients changed the staging according to the new AJCC PPS, including 31.1–46.2% were downstaged and 7.5–21.2% were upstaged,8,14–17 which was similar to our study. The immediate clinical implications of the updated AJCC staging lie in the improved prognostic accuracy of BC. However, the updated PPS does not guide current treatment decision-making, which continues to base on T stage, N stage, ER, PR, and HER2 status. The biological classification of BC has allowed a better understanding of the predictive behavior of the disease.
PMRT remains the definitive indication for patients with stage N2/3 disease after mastectomy. The treatment guidelines from the American Society of Clinical Oncology,28 National Comprehensive Cancer Network,18 and European Society for Medical Oncology29 have recommended PMRT for patients with N2/3 BC after mastectomy. There were approximately 30–35% of stage N2/3 patients did not receive PMRT from the NCDB.20,30 In our study, only 21.6% of patients were not received PMRT, which was significantly lower than the above NCDB studies. Several reasons may contribute to the difference in PMRT compliance. First, all patients included in our study received chemotherapy, while 13.5% of patients were not received chemotherapy in the NCBD study.30 Therefore, it is a hypothesis that patients receiving chemotherapy may be more likely to receive the recommended PMRT. Second, we only included patients aged <65 years, while there were 31.2% of patients >65 years in another NCDB study.20 Differences in age distribution may also cause differences in compliance with PMRT. Finally, Underascertainment of PMRT receipt in the SEER database also leads to differences in the probability of PMRT use.31
Several previous studies including ours have tried to answer the role of biological factors in predicting the effect of PMRT in N2/3 BC.21–23 In 2008, the study from the Danish Breast Cancer Cooperative Group showed that PMRT was only associated with better survival outcomes in luminal A and luminal B BC, but was not in HER2 + and triple-negative BC,21 which was confirmed by several studies including ours.22,23 However, only limited patients in the above studies received anti-HER2 treatment, so it is difficult to accurately assess the benefit of PMRT for stage N2/3 patients in the era of multidisciplinary therapy. The 8th AJCC PPS manual was determined based on the BC population using the data from the NCDB that were offered and mostly treated with appropriate multidisciplinary treatment.32 However, the current studies based on the new staging mainly focus on the prognostic analyses, and their role in treatment decision-making remains unclear. In our previous study, regarding T2N1 BC, we have found that PMRT was independently related to a better BCSS in those with stage III disease according to the PPS criterion, but not in those with stage IA-IIB diseases.13 In T3N0 BC, we also found that the use of PMRT was independently associated with a better BCSS in those with stage IIB disease according to the PPS criterion, but not in those with stage IA, IB, IIA, and IIIA diseases.12 However, it is still unknown whether the PPS will affect the PMRT decision of N2/3 patients. In fact, the extent of disease rather than biological markers is widely used for current PMRT decisions. In our study, significantly more patients were downstaged than upstaged, and enhanced knowledge regarding biology in BC has potentially affected significant changes in the treatment decision-making involving adjuvant radiotherapy.
Despite the current guidelines recommending PMRT for N2/3 BC,18,28,29 a study from the NCDB showed that PMRT was not a significant predictor for survival.20 In our study, we found that PMRT was independently related to a better BCSS regardless of the AS, which was similar to the recommendation from the current guidelines. However, we found that PMRT improved the BCSS of patients with stage IIIA, IIIB, and IIIC diseases based on the PPS criterion, while no survival benefits of PMRT were found in those with stage IB, IIA, and IIB diseases. Our findings suggest that the PPS system not only has better prognostic value but also has a critical role in predicting the benefit of PMRT for those with N2/3 BC.
In this study, 13,446 patients were identified, and there were 3525, 460, and 1335 were reassigned as IB, IIA, and IIB according to the PPS criterion, respectively. Our findings suggest that the use of the PPS for PMRT decision-making may enable approximately 40% of patients to avoid PMRT. PMRT will not only increase the economic burden on patients and society but also have potential toxicity to the heart, lungs as well as thyroid.33–36 Therefore, de-escalation of PMRT has not only an economic effect but also a protective effect in this population, although the cost-effectiveness and cost-benefit analyses require more long-term and large-scale studies. Moreover, more studies are required to use individualized patient profiling and patient stratification, and to apply multi-level diagnostics to advance clinically relevant prediction on the effect of the new staging system for PMRT-decision making.37–40
This study had several limitations. First, the nature of the retrospective analyses may induce selection bias. However, no randomized controlled trials are currently undergone to investigate the PPS on treatment decision-making, and a large randomized trial is not likely to be feasible in the current clinical practice. Therefore, observational analysis based on a large population cohort may provide the best level of evidence. Second, the chemotherapy regimen, chemotherapy completion rate, target volume, dose, and technique of PMRT, as well as the information regarding anti-HER2 therapy and endocrine therapy were not included in the SEER database. However, the patients included in the present study were in the era of multidisciplinary treatment, and the survival outcomes in our study were similar to the results from the BC population that determined the new AJCC staging.32 Therefore, it is reasonable to assume that most of our patients receive appropriate multidisciplinary treatment. In addition, the patterns of failure after PMRT are not recorded in the SEER program. Moreover, this study is only applicable to patients receiving appropriate multidisciplinary treatment, and may not apply to some African and Asian populations as well as some underserved communities in the United States.41 Finally, the relatively short follow-up time limits our results for clinical practice, and a long-term follow-up is required to determine the effect of biological markers on predicting the survival benefit of PMRT in this population.
Conclusions
In conclusion, the current analyses provide initial evidence that the 8th AJCC staging has resulted in superior risk stratification and a better tool to predict the benefit of PMRT in N2/3 BC compared to the 7th AJCC AS staging. These results support that PMRT conferred the greatest improvement in survival of patients with the unfavorable prognostic group using the new staging system. More studies that prospective validation of this strategy are required before widespread adoption.
Acknowledgments
This work was partly supported by the Social Development Projects of Key R & D Programs in Hainan Province (No. ZDYF2022SHFZ130), the Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25), the Natural Science Foundation of Fujian Province (No. 2020J011240), and the Natural Science Foundation of Hainan Province (No. 819QN34).
Disclosure
The authors declare no conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
3. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. doi:10.1038/s41572-019-0111-2
4. Regan MM. Risk stratification according to stage and pathology. Breast. 2019;48(Suppl 1):S23–S25. doi:10.1016/S0960-9776(19)31117-8
5. Plichta JK, Campbell BM, Mittendorf EA, et al. Anatomy and breast cancer staging: is it still relevant? Surg Oncol Clin N Am. 2018;27(1):51–67. doi:10.1016/j.soc.2017.07.010
6. Weiss A, King TA, Hunt KK, et al. Incorporating biologic factors into the american joint committee on cancer breast cancer staging system: review of the supporting evidence. Surg Clin North Am. 2018;98(4):687–702. doi:10.1016/j.suc.2018.03.005
7. Giuliano AE, Connolly JL, Edge SB, et al. Breast cancer-major changes in the american joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(4):290–303. doi:10.3322/caac.21393
8. Kim EJ, Park HS, Kim JY, et al. Assessment of the prognostic staging system of american joint committee on cancer 8th edition for breast cancer: comparisons with the conventional anatomic staging system. J Breast Cancer. 2020;23(1):59–68. doi:10.4048/jbc.2020.23.e9
9. Wang M, Chen H, Wu K, et al. Evaluation of the prognostic stage in the 8th edition of the American Joint Committee on Cancer in locally advanced breast cancer: an analysis based on SEER 18 database. Breast. 2018;37:56–63. doi:10.1016/j.breast.2017.10.011
10. Kurundkar A, Gao X, Zhang K, et al. Comparison of AJCC anatomic and clinical prognostic stage groups in breast cancer: analysis of 3322 cases from a single institution. Clin Breast Cancer. 2018;18(6):e1347–e1352. doi:10.1016/j.clbc.2018.07.013
11. Plichta JK, Ren Y, Thomas SM, et al. Implications for breast cancer restaging based on the 8th edition ajcc staging manual. Ann Surg. 2020;271(1):169–176. doi:10.1097/SLA.0000000000003071
12. Wu SG, Wang J, Lei J, et al. Prognostic validation and therapeutic decision-making of the AJCC eighth pathological prognostic staging for T3N0 breast cancer after mastectomy. Clin Transl Med. 2020;10(1):125–136. doi:10.1002/ctm2.3
13. Wu SG, Wang J, Lian CL, et al. Evaluation of the 8th edition of the American joint committee on cancer’s pathological staging system in prognosis assessment and treatment decision making for stage T1-2N1 breast cancer after mastectomy. Breast. 2020;51:2–10. doi:10.1016/j.breast.2020.02.012
14. Hu J, Fung MW, Tsang JY, et al. Improved prognostication for the updated ajcc breast cancer pathological prognostic staging varied in higher-stage groups. Clin Breast Cancer. 2020;20(3):253–261.e7. doi:10.1016/j.clbc.2020.01.011
15. Savage P, Yu N, Dumitra S, et al. The effect of the American Joint Committee on cancer eighth edition on breast cancer staging and prognostication. Eur J Surg Oncol. 2019;45(10):1817–1820. doi:10.1016/j.ejso.2019.03.027
16. Cervera-Bonilla S, Rodríguez-Ossa P, Vallejo-Ortega M, et al. Evaluation of the AJCC eighth-edition prognostic staging system for breast cancer in a Latin American Cohort. Ann Surg Oncol. 2021. doi:10.1245/s10434-021-09907-x
17. Shao N, Xie C, Shi Y, et al. Comparison of the 7th and 8th edition of American Joint Committee on Cancer (AJCC) staging systems for breast cancer patients: a Surveillance, Epidemiology and End Results (SEER) Analysis. Cancer Manag Res. 2019;11:1433–1442. doi:10.2147/CMAR.S185212
18. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: breast. clinical practice guidelines in oncology: breast; 2021.
19. McGale P, Taylor C, Correa C,et al.; Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi:10.1016/S0140-6736(14)60488-8
20. Shi Z, Peddi P, Burton G, et al. Effect of postmastectomy radiation on survival of AJCC pN2/N3 breast cancer patients. Anticancer Res. 2016;36(1):261–269.
21. Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(9):1419–1426. doi:10.1200/JCO.2007.14.5565
22. Wu SG, He ZY, Li Q, et al. Predictive value of breast cancer molecular subtypes in Chinese patients with four or more positive nodes after postmastectomy radiotherapy. Breast. 2012;21(5):657–661. doi:10.1016/j.breast.2012.07.004
23. Wang SL, Li YX, Song YW, et al. Triple-negative or HER2-positive status predicts higher rates of locoregional recurrence in node-positive breast cancer patients after mastectomy. Int J Radiat Oncol Biol Phys. 2011;80(4):1095–1101. doi:10.1016/j.ijrobp.2010.03.038
24. Surveillance, Epidemiology, and End Results (SEER) program SEER*stat database: incidence - SEER research plus data, 18 registries, Nov 2020 sub (2000–2018) - linked to county attributes - total U.S., 1969–2019 counties, National Cancer Institute, DCCPS, surveillance research program, released April 2021, based on the November 2020 submission. Available from: www.seer.cancer.gov.
25. Akaike H. A new look at the statistical model identifcation. IEEE Trans Aut Control. 1974;19:716–723. doi:10.1109/TAC.1974.1100705
26. Kang L, Chen W, Petrick NA, et al. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34(4):685–703. doi:10.1002/sim.6370
27. Weiss A, Chavez-MacGregor M, Lichtensztajn DY, et al. Validation study of the American Joint Committee on cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4(2):203–209. doi:10.1001/jamaoncol.2017.4298
28. Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American society of clinical oncology, American society for radiation oncology, and society of surgical oncology focused guideline update. Pract Radiat Oncol. 2016;6(6):e219–e234. doi:10.1016/j.prro.2016.08.009
29. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi:10.1093/annonc/mdz173
30. Chu QD, Caldito G, Miller JK, et al. Postmastectomy radiation for N2/N3 breast cancer: factors associated with low compliance rate. J Am Coll Surg. 2015;220(4):659–669. doi:10.1016/j.jamcollsurg.2014.12.045
31. Jagsi R, Abrahamse P, Hawley ST, et al. Underascertainment of radiotherapy receipt in surveillance, epidemiology, and end results registry data. Cancer. 2012;118(2):333–341. doi:10.1002/cncr.26295
32. AJCC. Cancer Staging Manual.
33. Eaglehouse YL, Manjelievskaia J, Shao S, et al. Costs for breast cancer care in the military health system: an analysis by benefit type and care source. Mil Med. 2018;183(11–12):e500–e508. doi:10.1093/milmed/usy052
34. Darvish L, Ghorbani M, Teshnizi SH, et al. Evaluation of thyroid gland as an organ at risk after breast cancer radiotherapy: a systematic review and meta-analysis. Clin Transl Oncol. 2018;20(11):1430–1438. doi:10.1007/s12094-018-1875-7
35. Ng J, Shuryak I, Xu Y, et al. Predicting the risk of secondary lung malignancies associated with whole-breast radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83(4):1101–1106. doi:10.1016/j.ijrobp.2011.09.052
36. van den Bogaard VA, Ta BD, van der Schaaf A, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017;35(11):1171–1178. doi:10.1200/JCO.2016.69.8480
37. Golubnitschaja O, Liskova A, Koklesova L, et al. Caution, “normal” BMI: health risks associated with potentially masked individual underweight-EPMA position paper 2021. EPMA J. 2021;12(3):243–264. doi:10.1007/s13167-021-00251-4
38. Golubnitschaja O, Filep N, Yeghiazaryan K, et al. Multi-omic approach decodes paradoxes of the triple-negative breast cancer: lessons for predictive, preventive and personalised medicine. Amino Acids. 2018;50(3–4):383–395. doi:10.1007/s00726-017-2524-0
39. Crigna AT, Samec M, Koklesova L, et al. Cell-free nucleic acid patterns in disease prediction and monitoring-hype or hope? EPMA J. 2020;11(4):1–25. doi:10.1007/s13167-020-00226-x
40. Zhan X, Li J, Guo Y, et al. Mass spectrometry analysis of human tear fluid biomarkers specific for ocular and systemic diseases in the context of 3P medicine. EPMA J. 2021;12(4):449–475. doi:10.1007/s13167-021-00265-y
41. de Souza JA, Hunt B, Asirwa FC, et al. Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. J Clin Oncol. 2016;34(1):6–13. doi:10.1200/JCO.2015.62.2860
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




