Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 13
The Potentiality of Human Umbilical Cord Isolated Mesenchymal Stem/Stromal Cells for Cardiomyocyte Generation
Authors Abou-ElNaga A, El-Chennawi F, Ibrahim kamel S, Mutawa G
Received 16 July 2020
Accepted for publication 1 September 2020
Published 9 November 2020 Volume 2020:13 Pages 91—101
DOI https://doi.org/10.2147/SCCAA.S253108
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Amoura Abou-ElNaga, 1 Farha El-Chennawi, 2 Samar Ibrahim kamel, 1 Ghada Mutawa 3
1Zoology Department, Faculty of Sciences, Mansoura University, Mansoura 35516, Egypt; 2Clinical Pathology Department, Faculty of Medicine, Mansoura University, Mansoura 35516, Egypt; 3Department of Basic Science, Faculty of Dentistry, Horus University in Egypt (HUE), New Damietta 34518, Egypt
Correspondence: Ghada Mutawa
Department of Basic Science, Faculty of Dentistry, Horus University in Egypt (HUE), International Coastal Road New, Damietta City, Kafr Saad, Damietta Governorate 34518, Egypt
Tel +201021888628
Fax +2-057-328611
Email [email protected]
Background: The new therapeutic strategy of managing cardiac diseases is based on cell therapy; it highly suggests the use of multipotent mesenchymal stem/stromal cells (MSCs). MSCs widely used in researches are known to be isolated from bone marrow. However, this research seeks to use a human umbilical cord (HUC) as an alternative source of MSCs. Since HUC Wharton’s jelly (WJ)-isolated MSCs originate as fetal tissue they are highly preferable for their potential advantages over other adult tissues.
Methods: The researchers used enzymatic digestion to establish a primary HUC-WJ-isolated MSC line. Then, flow cytometry was used to characterize MSCs and hematopoietic stem cells (HSCs) markers’ expression. In addition, the cardiac differentiation capacity of HUC-WJ-isolated MSCs in vitro was investigated by two protocols. Protocol-1 necessitates the dependence on merely 5-azacytidine (5-Aza), whereas in protocol-2, 5-Aza was supported by basic fibroblast growth factor (BFGF). The comparative study between the two protocols was applied by inspecting the ultrastructure of differentiated cells, measuring RT-PCR mRNA cardiac markers and the quantitative detection of cardiac proteins.
Results: HUC-WJ isolated MSCs were expressed by CD90+ve, CD105+ve, CD106+ve, CD45−ve, and CD146−ve. Remarkable TNNT1, NKX2.5, and Desmin mRNA expression and higher quantitative LDH and cTnI were detected by applying protocol-2. This same protocol-2 induced cardiac morphological features that were revealed by identifying cardiomyocyte-like cells and typical sarcomeres.
Conclusion: HUC-WJ is proved to be an ethical and effective source of MSCs induced cardiac differentiation, whereas BFGF supports 5-Aza in MSCs-cardiomyocytes differentiation.
Keywords: mesenchymal stem/stromal cells, human umbilical cord, Wharton’s jelly, cardiomyocytes, 5-azacytidine, fibroblast growth factor
Corrigendum for this paper has been published
Plain Language Summary
Stem cell therapy has the ability to improve the traditional treatment of cardiac diseases by inducing stem cell differentiation into cardiomyocytes to repair damaged cardiac tissues. Many previous studies depended on the easy way that isolates stem cells from adult tissues like bone marrow and adipose tissue; however, the limited potentiality of such isolated stem cells delayed the progress of those studies. However, improving the potentiality of stem cells requires the development of applied protocols. So, this study was based on the human umbilical cord (HUC) Wharton’s jelly (WJ) as a suitable source of mesenchymal stem/stromal cells (MSCs). HUC-WJ isolated MSCs have a differentiation potentiality into multiple cell types without any ethical concerns. The study also developed a new protocol by using 5-Azacytidine (5-Aza) and basic fibroblast growth factor (BFGF) to differentiate MSCs into cardiomyocytes. This protocol encourages cardiac diseases. The resulting HUC-WJ isolated MSCs showed significant cardiac differentiation by acquiring special microscopic cardiac features, and by the high expression of cardiac markers. Accordingly, HUC-WJ isolated MSCs proved to be a promising strategy of applying stem cell-based therapy on cardiac diseases.
Introduction
Cardiac diseases have remained the leading cause of death all over the world. Over the past decades, the traditional treatment of cardiac diseases has resulted in a tragic rise in mortality rate as it just managed to temporarily delay the progress of the disease.1 Although remarkable evolution is noticed in the treatment of cardiac diseases, still there is a social and economic burden on health institutions.2 Therefore, the conventional treatment of cardiac diseases cannot provide an authentic cure.
The recent trend in regenerative medicine nowadays is using stem cells as recovering-cell therapy for cardiac diseases. Mesenchymal stem/stromal cells (MSCs) are the pioneer example of adult stem cells that can lead to the new regenerative strategy.3 MSCs are non-hematopoietic stem cells (HSCs), multipotent-adult stem cells as they can differentiate into all stromal connective linages like fat, cartilage, and bone cells. MSCs cannot be considered pluripotent-embryonic stem cells just as inner cell mass in blastula because they cannot differentiate into all human cell types.4 MSCs are characterized by specific markers called cluster of differentiation (CD), especially as CD90, CD105, and CD106.5 These markers trigger MSCs’ unique stemness, growth, and differentiation properties.5 Also, MSCs lack markers of HSCs such as CD146 and CD45.5 MSCs have typical fibroblastic-morphology and adherent property to plastic surfaces during in vitro culture. All of these properties support the position of MSCs on applying regenerative therapy to solve the clashes in the treatment of cardiac diseases.2
MSCs are able to act as a repair-cellular therapy that is stimulated by as many pathological causes as the cardiac diseases.2 In the treatment of the cardiac diseases, there are many roles of MSCs in the mechanism of action, whereas they act as immune-regulators, anti-apoptotic, and allogeneic utilizers. Particularly MSCs play an integral role in dealing with cardiac diseases since they regenerate damaged cardiac tissue by being differentiated into cardiomyocytes.6 MSCs-cardiomyocytes differentiation is stimulated through the alteration in the cellular pattern of genes expression mediated by early transcriptional regulators such as NKX2.5 and GATA4.7 The early transcriptional factors regulate differentiation-specific gene expression which program various aspects of cardiac cell; this is especially done by inducing the expression of the late myocardial markers such as lactate dehydrogenize (LDH) and cardiac troponin I (cTnI).7,8
The tissue source can determine the differentiation potential of MSCs that control their efficiency as cellular therapy of cardiac diseases.9 In the 1960s, MSCs were being isolated from bone marrow,10 that has now become the most common MSCs’ source, depended upon in clinical trials. Also, adipose tissue is a well-known source of MSCs; its easy isolation by liposuction has greatly attracted the attention of researchers and clinical trials.1 Although bone marrow and adipose tissue are accessible and enriched sources of MSCs, the capacity of their isolated MSCs to differentiate into cardiomyocytes is not satisfying11 since other tissues can be proved to introduce a higher differentiation potential of MSCs.
Despite the pluripotency of embryonic stem cells, the ethical concerns limit its use in cell therapy.12 Human umbilical cord (HUC) is a fetal tissue arising from the extra-embryonic mesoderm at day-13 of embryogenesis. So its isolated MSCs could possess higher differentiation and stemness properties than other traditional adult sources. The stromal part of the HUC is known as Wharton’s jelly (WJ) that is the least studied source of MSCs. HUC-WJ is a medical waste discarded after the birth of a baby, so it is free from any ethical issues.13 Beside their ethical isolation availability from HUC-WJ, MSCs can show a high cardiac differentiation potentiality.
Previously, studied protocols could neither introduce a sufficient way of isolating pure MSCs from HUM-WJ nor an effective way of in vitro MSCs differentiation into cardiomyocytes.14 Those studies depended on treating MSCs with specific growth factors through inhibiting DNA methyltransferase. 5-azacytidine (5-Aza) is a DNA methyltransferase inhibitor that has an important role in the epigenetic regulation of genome expression.15 Sufficient differentiation of MSCs requires a high dose of 5-Aza, but it becomes cytotoxic.16 Thus, we recommend a new protocol by supporting 5-Aza in the differentiation of MSCs without being toxic.
Consequently, the current study established an effective strategy for the isolation of pure MSCs from HUM-WJ as a perfect source of stem cells. It also suggested a new protocol for the expansion and differentiation of HUM-WJ isolated MSCs into cardiomyocyte-like cells in vitro.
Materials and Methods
Chemicals
Culture media including DMEM, FBS, l-glutamine, penicillin-streptomycin, and 0.05% trypsin-EDTA were used in culturing MSCs. Cell culture medium and collagenase-A were obtained from Miltenyi Biotec, Bergisch Gladbach, Germany. Basic fibroblast growth factor (BFGF) and 5-Aza was purchased from Sigma-Aldrich, St. Louis, MO, USA. Deionized water was used throughout the experiments.
Sample Collection
A tissue sample isolated from a HUC was acquired from the Department of Gynecology and Obstetrics, Mansoura University, Egypt, from one pregnant female (30 years old), immediately after delivery. This is done under IRB approved protocol (MZ16001), which was following the Declaration of Helsinki. Accordingly, an informed written consent was obtained from the patient. HUC was collected in a sterile conical vial containing DMEM supported by 5% penicillin-streptomycin. The sample delivery to the laboratory as well as all manipulation were carried out under sterile conditions. The sample was delivered to the culture laboratory and stored at 4 °C for 2 hr before tissue processing.
Isolation and Establishment of the Primary HUC-WJ Isolated MSC Line
The whole HUC was washed in sterile phosphate buffer saline (PBS) triplicate to remove any red blood cells. A small piece about 2–3 cm from the HUC was placed in a petri dish, following by removing blood vessels then the WJ was carefully separated from the HUC membrane. WJ was cut into small fragments with sharp scissor scalpels. After WJ has been minced into 1–2 mm3 fragments, the fragments were treated with collagenase-A (3 mg/mL) in a 50 mL falcon tube for 3 hr at 37 °C in a shaking water bath (125 rpm). Then the slurry was centrifuged for 15 min at 1500 xg at 4 °C. The supernatant was subsequently discarded and the remaining slurry was collected to be treated with trypsin-EDTA for 30 minutes at 37 °C in 5% CO2 with a humidified atmosphere. The cells were filtered with sterile 70 µL sieve and were consequently re-suspended in DMEM, centrifuged at 1500 × g for 10 minutes. DMEM was aspirated, and the cell pellet was collected.
Cell Culture
The primary HUM-WJ isolated cell line was cultured by DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% l-glutamine; stored at 4 °C and warmed to 37 °C before using. This is followed by incubation in a 37 °C, 5% CO2 incubator. The cells were fed with fresh medium every 2–3 days after aspirating the old medium. A few days later, colonies of fibroblast cells were seen. After reaching 80% confluence, cells were trypsinized and sub-cultured in fresh supplemented DMEM into two new culture flasks to allow more space for further proliferation. Detached cells before each new passage were counted and tested for viability with trypan blue dye exclusion assay.
Characterization of MSCs
The mesenchymal characteristic of the cultured cells was analyzed by flow cytometry. At the 3rd and 4th passages, cells were detached and washed twice with PBS. Then, cells were mixed with fluorescein isothiocyanate (FITC) monoclonal-antibodies against human CD90, CD105, CD106, CD45, and CD146. All antibodies were purchased from BD Biosciences, San Jose, CA, USA. Mixed cells were incubated on ice in the dark for 15 minutes, washed with PBS, and suspended (5 × 105 cells) in 1 mL PBS. Expressions of these markers was measured with a flow cytometer (FACS Aria Cell Sorter; BD Biosciences, Billerica, MA, USA). An appropriate isotype-matched control was used in all analysis, and unstained MSCs were used as control.
Cardiac Induction
In the 5th passage, the 80% confluent cultured MSCs were divided into three cell groups. G1 (undifferentiated MSCs) was fed with complete medium and refreshed every 72 hr. This cell line was used as a control for the differentiated cells. G2 was fed with culture media supported only by 15 µM 5-Aza, while G3 was fed with culture media supported by 15 µM 5-Aza and 10 ng/mL BFGF. The differentiated cells (G2 and G3) were incubated with their differentiation media for 24 hr and were then washed by PBS twice. Then, the cardiac induction media was replaced with a complete medium that replaced every 72 hr during the differentiation period (three weeks).
Characterization of Cardiac Differentiation Efficiency
Ultra-Structural Analysis
After the period of cardiac differentiation induction, ultra-structural analysis with transmission electron microscopy (TEM) was performed on the tested cell groups. The collecting cell pellet was centrifuged at 1200 rpm for 10 min and was then washed in 2% sodium phosphate buffer osmium tetroxide (pH = 7.4) triplicate for 30 min. The initial fixation was done in sodium phosphate buffer containing glutaraldehyde and 2% paraformaldehyde. The dehydration was done with 50% ethanol for 9 min. This is followed by washing the cells with distilled water twice for 15 min. The cells were later embedded in epoxy resin. Ultrathin sections were cut and observed at 80 kV using a JEOL 2100 TEM (JEOL GmbH, Eching, Germany).
Quantification Assays for Cardiac Markers
After the period of cardiac differentiation, the cell culture supernatant was collected from differentiated and undifferentiated cells. Any debris was removed from the supernatant media by centrifuging for 10 min at 2000 xg. The clear supernatant was used to analyze two cardiac markers: LDH and cTnI, triplicate for each cell group. According to the manufacturer’s instructions, LDH and cTnI were measured with a commercial kit (Elitech, France) and spectrophotometer.
Gene Expression Analysis
The traditional PCR and the real-time qPCR (RT-qPCR) analysis were applied to RNA that was isolated from the differentiated and undifferentiated cells by using the TRIzol reagent and purified with GeneJET™ RNA Purification Kit (Fermentas Thermo Fisher Scientific). The RNA sample was diluted in DEPC water before it was quantified by spectrophotometry at 260 nm. RNA (2 μg) from each cell group was applied for reverse transcription using the Maxima® First Strand cDNA Synthesis Kit (Fermentas Thermo Fisher Scientific). RT-qPCR was applied with the Maxima® SYBR Green qPCR Master Mix (Fermentas Thermo Fisher Scientific). Reactions were done in a 20μL volume. The primers for the TNNT1, NKX2.5, and DES genes are shown in the Supplementary material S1, with GAPDH as a control. qPCR was performed in triplicate for each sample with a RT-PCR detection system (Agilent Technologies) with an initial denaturation at 95ᵒC for 10 min, followed by 40 cycles for 15 s at 95ᵒC, the 30 s at melting temperature, 5ᵒC, and 30 s at 72ᵒC. Specificity was detected with melting curve analysis. RT-qPCR products were electrophoresed on (1–2)% agarose gels (according to the size of the amplification products). The gene expression levels of differentiated and undifferentiated cells were investigated with the comparative CT method. The observations were performed with the Real-Time PCR System (Applied Biosystems).
Statistical Analysis
All data were expressed as means ± S.E. statistical significance and were evaluated by one-way analysis of variance (ANOVA) using SPSS, 18.0 software, 2011, the individual comparisons were obtained by Duncan’s multiple range test (DMRT). Values were considered statistically significant when P ≤ 0.05.
Results
Morphological Characteristics of HUM-WJ Isolated MSCs
Freshly isolated HUM-WJ isolated MSCs appeared as heterogeneous sphere-shaped cells in suspended media under an inverted microscope (Figure 1A). After 72 hr in primary culture, MSCs adhered to the plastic surface forming small clusters of swirl-like cells and spreading on the plate (Figure 1B). During early culture, MSCs proliferated gradually and rapidly until they covered the culture plate, and their morphology changed to be atypical aggregated fibroblasts. In the 3rd passage, MSCs had a clear slender, elongated, spindle shape with extended processes (Figure 1C).
 |
Figure 1 Morphological characteristics of HUM-WJ isolated MSCs. |
Flow Cytometry Analysis
Flow cytometric analysis was performed on HUM-WJ isolated MSCs using antibodies against CD106, CD105, CD90, CD45, and CD146 cell surface markers. The result showed that the tested cells were positive for MSCs markers, such as CD106 (Figure 2A), CD105 (Figure 2B), and CD90 (Figure 2C), which were highly expressed by 87.8%, 60.8%, and 47.6%, respectively in tested cells. On the other hand, tested cells were negative for HSCs markers, such as CD45 (Figure 2D) and CD146 (Figure 2E), which had a low expression of 1.8% and 2.2%, respectively.
 |
Figure 2 Flow cytometry analysis of HUM-WJ isolated MSCs. |
Morphological Characteristics of Differentiated Cells
After 24 hr of incubation with differentiated media, the viability of HUM-WJ isolated MSCs in GII, that is affected by differentiated media, was visually observed (Figure 3A). On the other hand, compared with GII, differentiated media in GIII had a limited effect on cellular viability (Figure 3B). The viable MSCs in both groups proliferated and differentiated gradually throughout the days that followed induction. One week later, the morphology of GII cells changed by expanding and enlarging (Figure 3C), like the morphology of GIII cells. However, GIII cells showed more noticeable expansion (Figure 3D). Three weeks later, cells in GII became elongated and aligned forming a stick- like morphology with extensions linked to the surrounding cells (Figure 3E). However, cells in GIII, as visually observed, were typically differentiated with more regular uniformed morphology than those of GII (Figure 3F). These visual observations were detected by an inverted microscope by making a comparison with cells in GI, preserved with their original spindled fibroblastic morphology during culture, as a control.
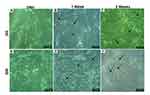 |
Figure 3 Morphological characteristics of differentiated cells. |
Ultrastructural Characterization of the Differentiated Cells
TEM observation of MSCs in GI was photographed to be a controlling factor for the differentiated cells, which were detected with the absence of any myofilaments (Figure 4A). TEM observation of cells in GII and GIII, after three weeks of induction, generally showed the differentiated cells with a centric nucleus and richer organelles in the cytoplasm, as rough endoplasmic reticulum, mitochondria, and free ribosomes. Specifically, cells in GII showed abundant myofilaments that aligned in a parallel shape without the appearance of striated sarcomeres (Figure 4B). Also, cells in GIII showed myofilaments but with extra typical striated sarcomeres and a denser look of granulated cytoplasm (Figure 4C).
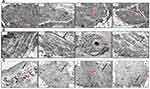 |
Figure 4 Ultrastructural characterization of the differentiated cells. |
Quantification Assays for Cardiac Markers
The protein expressions of cardiac markers (LDH and cTnI) were measured in media and they revealed the functionality of the differentiated cells’ dependent manner. The results showed that differentiated cells of both GII and GIII expressed significantly higher levels of LDH (Figure 5A) and cTnI (Figure 5B) proteins, compared to their corresponding undifferentiated cells in GI (P<0.05). However, these expressions increased by the time of induction. Limited expression of cardiac markers was specifically detected in cells of GII compared to cells of GIII that showed a remarkable higher expression of these markers.
 |
Figure 5 Quantification expressions for cardiac markers. |
Molecular Analysis
The traditional PCR analysis (Figure 6A) and the RT-qPCR analysis (Figure 6B) were carried out to measure the fold changes in the expression of NKX2.5, TNNT1, and Desmin by differentiated cells in GII and GIII, in comparison with cells of GI as a control. Cells of GII and GIII actively expressed NKX2.5, especially after one week with 10.97-fold and 4.40–fold, respectively, which are significantly higher than GI (P<0.05) (Figure 6B.i). Also, TNN1 gene was highly expressed by cells of GII and GIII, especially after three weeks with 3.54–fold and 2.64–fold, respectively, which are significantly higher than cells of GI (P<0.05) (Figure 6B.ii). Moreover, cells of GIII highly expressed Desmin, especially after three weeks with 2.36–fold, which is significantly higher than the other differentiated cells in GII and control cells in GI (P<0.05) (Figure 6B.iii).
 |
Figure 6 Expression of NKX2.5, TNNT1 and Desmin mRNA. |
Discussion
Recently, regenerative medicine based on MSCs becomes a promising therapeutic strategy for many diseases such as cardiac diseases.18 Many previous studies focused on well-known tissues as sources for MSCs such as; bone marrow and adipose tissue.19 Although bone marrow isolated extracted MSCs proved their efficiency in the treatment of many diseases, such as orthopedic disorders, this is related to their expression of genes associated with the osteogenetic habit.20 Thus, bone marrow isolated MSCs are not the best choice in the treatment of other diseases such as cardiac diseases. This study tested the hypothesis that: HUC-WJ is an unusual MSCs source. Not only for its ethically legal, but also for its higher differentiation potential. Therefore, we focused on HUC-WJ as a challenging ethical fetal tissue that is better than other conventional adult tissues as a competitive source for MSCs with cardiac differentiation potency.
In the present study, primary HUC-WJ isolated MSCs line was established by the formulated simple method depending upon enzymatic digestion. It does not perform only by collagenase, but also by the effect of trypsin to ensure a sufficient count of MSCs.21 The purity of primary cultured adherent cells with their microscopic spindle appearance is confirmed by flow cytometry, depending on previous studies. It is estimated that MSCs were expressed by specific markers such as CD106, CD105, and CD90.22 CD106 is a member of the immunoglobulin superfamily and it was highly expressed in these cells (87.8%). It is also known as vascular cell adhesion molecule-1 (VCAM-1), because it has an essential role in adhesion and homing.23 CD105 or endoglin was expressed in 60.8% of tested cells whereas it is necessary for cell division and cellular differentiation.24 An extra MSCs specific marker, CD90 or Thy-1 is a cell surface anchored glycoprotein, and it is essential for cell mobility and migration.25 It was positively expressed in 47.6% of the tested cells. Many previous studies estimated that MSCs were negative for the hematopoietic marker (CD45) and endothelial marker (CD146).5 Since CD45 is a transmembrane protein tyrosine phosphatase that controls the motility of hematopoietic progenitor cells and the maturation of leukocytes as lymphocytes.26 Besides, CD146 or M-CAM regulates the migration and motility of endothelial cells.27 In the present study, CD45−ve and CD146−ve (1.8% and 2.2%; respectively) proved the purity of the mesenchymal nature of tested cells and the lacking of contamination with other cell types.
The study also tested the differentiation of HUC-WJ isolated MSCs into cardiomyocytes that were enhanced, not only by a 5-Aza factor but also by 5-Aza with BFGF. We hypothesized the combination of 5-Aza and BFGF as having a synergic effect in the differentiation of MSCs into cardiomyocytes. Previous studies concluded that many growth factors can stimulate higher myocardial differentiation of MSCs and induce the gene expression related to cardiomyocytes under proper condition.28,29 Although 5-Aza is known as a differential factor for MSCs into cardiomyocytes cells,30,31 it could not be regarded as safe and sufficient for inducing the cardiomyogenic fate.32,33 BFGF is considered a potential co-inducer required to support the effect of 5-Aza in forcing the differentiation of MSCs into cardiomyocytes.34 In the current study, the morphological changes of MSCs confirmed this hypothesis: 5-Aza affect harmfully the viability of cells that were handled by BFGF. The differentiation of MSCs was improved by BFGF that supported the effect of 5-Aza through the remarkable morphological expansion of differentiated cells. The existence of primitive myofilaments was the sharp morphological sign that emphasized the higher differentiation induction effect of the 5-Aza and BFGF combination.
The present study showed the morphological changes in MSCs resulted from the expression of cardiomyogenic proteins. Whereas, cell culture supernatant showed a high level of LDH and cTnI significantly after cellular differentiation. LDH is mainly on the filaments of cardiac muscle fibers that have immune-function. cTnI is a part of the contractile mechanism of the cardiac muscle.35 The synergic effect of both myocardial differential inducers (5-Aza and BFGF) of MSCs is confirmed through the up-regulation of these cardiac-specific biomarkers by time. This result confirmed that cardiogenic differentiation is performed not only by 5-Aza36 but also by the help of BFGF in regulating the myocardial differentiation.37
5-Aza induced MSCs were previously proved to be able to express cardiac-specific genes such as NKX2.5, TNNT1, and Desmin,38 while every gene has its specific cardiogenic role. The study also highlighted NKX2.5 or homeobox gene as the earliest-specific marker of cardiac lineage during embryogenesis.39 It also shows how TNNT1 gene encodes the slow skeletal muscle troponin.40 Desmin is an essential gene in maintaining the structure of sarcomeres as it has a necessary role in cardiac muscle tone.41 Thus, this study detected the progress of myocardial differentiation of MSCs through the expression of these genes at the RNA level to determine the best differential protocol in a time-dependent manner (one and three weeks). Both protocols of differentiation confirmed a significantly higher expression of these cardiac-specific genes in comparison with undifferentiated cells. TNNT1 and Desmin expression increased gradually by time, while NKX2.5 is an early marker that elevated in the first week and peaked after three weeks from the starting of induction in both protocols. Particularly the significantly high level of Desmin was detected after differential induction by extra help of BFGF. The expression of these cardiac genes encoded the morphological and functional properties of differentiated cells.42 So high expression of TNNT1, Desmin, and NKX2.5 was driving the cardiomyocytes’ features and the expression of functional proteins. The results showed the cardio-differential potency of HUC isolated MSCs through the morphological, the functional, and the genetic levels.
Collectively, the results of this study recommend using HUC-WJ as a competitive source of MSCs, that has a promising regenerative therapeutic effect on cardiac diseases. Significant differentiation of HUC-WJ isolated MSCs into cardiomyocyte-like cells has been detected in vitro by combining 5-Aza with BFGF. In addition, in vivo experimental studies and functional tests are requested for the myocardial differentiation of HUC-WJ isolated MSCs in order to be applicable in the clinical trials.
Conclusion
In brief, this study concluded applying for regenerative medicine by MSCs may represent a therapeutic strategy for achieving better treatment of cardiac diseases. The study presents HUM-WJ as a competitive source for seeding MSCs, and an efficient mechanism mediated by 5-Aza and BFGF to enhance the HUC-WJ isolated MSCs with myocardial features.
Acknowledgments
We thank “MERC” of Mansoura Faculty of Medicine for their support. We appreciate the excellent English editorial assistance of Nermine A. Gomaa, associate professor of English literature at Horus University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116(8):1413–1430.
2. Roura S, Gálvez-Montón C, Mirabel C, Vives J, Bayes-Genis A. Mesenchymal stem cells for cardiac repair: are the actors ready for the clinical scenario? Stem Cell Res Ther. 2017;8(1):238. doi:10.1186/s13287-017-0695-y
3. Majka M, Sułkowski M, Badyra B, Musiałek P. Concise review: mesenchymal stem cells in cardiovascular regeneration: emerging research directions and clinical applications. Stem Cells Transl Med. 2017;6(10):1859–1867. doi:10.1002/sctm.16-0484
4. Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35(2):e00191. doi:10.1042/BSR20150025
5. Maleki M, Ghanbarvand F, Reza Behvarz M, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118–126. doi:10.15283/ijsc.2014.7.2.118
6. Alrefai MT, Murali D, Paul A, Connell JM, Shum-Tim D. Cardiac tissue engineering and regeneration using cell-based therapy. Stem Cells Cloning. 2015;8:81–101.
7. Oka T, Xu J, Molkentin JD. Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18(1):117–131. doi:10.1016/j.semcdb.2006.11.012
8. Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (20102015). Stem Cell Res Ther. 2016;7(1):82.
9. Xu L, Liu Y, Sun Y, et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther. 2017;8(1):275. doi:10.1186/s13287-017-0716-x
10. Drzewiecki BA, Thomas JC, Tanaka ST. Bone marrow-derived mesenchymal stem cells: current and Future applications in the urinary bladder. Stem Cells Int. 2011;2010:765167.
11. Sun Q, Zhang Z, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1(1):113–119.
12. Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30(3):204–213. doi:10.1210/er.2008-0031
13. Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14(6):11692–11712. doi:10.3390/ijms140611692
14. Meng M, Liu Y, Wang W, et al. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am J Transl Res. 2018;10(1):212–223.
15. Secco M, Zucconi E, Vieira NM, et al. Multipotent stem cells from umbilical cord: cord is richer than blood. Stem Cells. 2008;26(1):146–150. doi:10.1634/stemcells.2007-0381
16. Lee S, Kim HS, Roh KH, et al. DNA methyltransferase inhibition accelerates the immunomodulation and migration of human mesenchymal stem cells. Sci Rep. 2015;5:8020. doi:10.1038/srep08020
17. Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi:10.1634/stemcells.2004-0013
18. Weiss ML, Medicetty S, Bledsoe AR, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. doi:10.1634/stemcells.2005-0330
19. Abbas OL, Özatik O, Gönen ZB, et al. Comparative analysis of mesenchymal stem cells from bone marrow, adipose tissue, and dental pulp as sources of cell therapy for zone of stasis burns. J Invest Surg. 2018;1–14.
20. Panepucci RA, Siufi JL, Silva WA, et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–1278. doi:10.1634/stemcells.2004-0024
21. Salehinejad P, Alitheen NB, Ali AM. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. In Vitro Cell Dev Biol Anim. 2012;48(2):75–83. doi:10.1007/s11626-011-9480-x
22. Rojewski MT, Weber BM, Schrezenmeier H. Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus Med Hemother. 2008;35(3):168–184. doi:10.1159/000129013
23. Yang ZX, Han ZB, Ji YR, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS One. 2013;8(3):e59354. doi:10.1371/journal.pone.0059354
24. Aslan H, Zilberman Y, Kandel L, et al. Osteogenic differentiation of noncultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006;24:1728–1737.
25. Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20:1045–1054. doi:10.1096/fj.05-5460rev
26. Shivtiel S, Kollet O, Lapid K, et al. CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med. 2008;205:2381–2395. doi:10.1084/jem.20080072
27. Ouhtit A, Gaur RL, Abd Elmageed ZY, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009;1795:130–136.
28. Muguruma Y, Reyes M, Nakamura Y, et al. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003;31:1323–1330. doi:10.1016/j.exphem.2003.09.003
29. Bartunek J, Croissant JD, Wijns W, et al. Pretreatment of adult bone marrow mesenchymal stem cells with cardiomyogenic growth factors and repair of the chronically infarcted myocardium. Am J Physiol Heart Circ Physiol. 2007;292:1095–1104. doi:10.1152/ajpheart.01009.2005
30. Nartprayut K, U-Pratya Y, Kheolamai P, et al. Cardiomyocyte differentiation of perinatally-derived mesenchymal stem cells. Mol Med Rep. 2013;7:1465–1469. doi:10.3892/mmr.2013.1356
31. Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int. 2013;132642.
32. Bae S, Shim SH, Park CW, et al. Combined omics analysis identifies transmembrane 4 L6 family member 1 as a surface protein marker specific to human mesenchymal stem cells. Stem Cells Dev. 2011;20:197–203. doi:10.1089/scd.2010.0127
33. Tomita S, Li RK, Weisel R, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(19):11247–11256. doi:10.1161/01.CIR.100.suppl_2.II-247
34. Heng BC, Haider HK, Sim EK, Cao T, Ng SC. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc Res. 2004;62:34–42. doi:10.1016/j.cardiores.2003.12.022
35. Brotto MA, Biesiadecki BJ, Brotto LS, Nosek TM, Jin JP. Coupled expression of troponin T and troponin I isoforms in single skeletal muscle fibers correlates with contractility. Am J Physiol Cell Physiol. 2006;290(2):567–576. doi:10.1152/ajpcell.00422.2005
36. Wan Safwani WK, Makpol S, Sathapan S, Chua KH. 5-Azacytidine is insufficient for cardiogenesis in human adipose-derived stem cells. J Negat Results Biomed. 2012;11:13. doi:10.1186/1477-5751-11-3
37. Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest. 2005;115(7):1724–1733. doi:10.1172/JCI23418
38. Qian Q, Qian H, Zhang X, et al. 5-Azacytidine induces cardiac differentiation of human umbilical cord derived mesenchymal stemcells by activating extracellular regulated kinase. Stem Cells Dev. 2012;21(1):67–75. doi:10.1089/scd.2010.0519
39. Lien CL, Wu C, Mercer B, et al. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development. 1999;126(1):75–84.
40. Kuroda T, Yasuda S, Nakashima H, et al. Identification of a gene encoding slow skeletal muscle troponin t as a novel marker for immortalization of retinal pigment epithelial cells. Sci Rep. 2017;7(1):8163. doi:10.1038/s41598-017-08014-w
41. Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res. 2004;301(1):1–7. doi:10.1016/j.yexcr.2004.08.004
42. Nomura S, Satoh M, Fujita T, et al. Cardiomyocyte gene programs encoding morphological and functional signatures in cardiac hypertrophy and failure. Nat Commun. 2018;9(1):4435. doi:10.1038/s41467-018-06639-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
