Back to Journals » International Journal of Nanomedicine » Volume 19
The Physiological Inorganic Polymers Biosilica and Polyphosphate as Key Drivers for Biomedical Materials in Regenerative Nanomedicine
Authors Müller WEG , Neufurth M , Wang S, Schröder HC , Wang X
Received 24 October 2023
Accepted for publication 18 January 2024
Published 8 February 2024 Volume 2024:19 Pages 1303—1337
DOI https://doi.org/10.2147/IJN.S446405
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Werner EG Müller, Meik Neufurth, Shunfeng Wang, Heinz C Schröder, Xiaohong Wang
ERC Advanced Investigator Grant Research Group at the Institute for Physiological Chemistry, University Medical Center of the Johannes Gutenberg University, Mainz, Germany
Correspondence: Werner EG Müller; Xiaohong Wang, ERC Advanced Investigator Grant Research Group at the Institute for Physiological Chemistry, University Medical Center of the Johannes Gutenberg University, Duesbergweg 6, Mainz, D-55128, Germany, Tel +49 6131 392 5791, Email [email protected]; [email protected]
Abstract: There is a need for novel nanomaterials with properties not yet exploited in regenerative nanomedicine. Based on lessons learned from the oldest metazoan phylum, sponges, it has been recognized that two previously ignored or insufficiently recognized principles play an essential role in tissue regeneration, including biomineral formation/repair and wound healing. Firstly, the dependence on enzymes as a driving force and secondly, the availability of metabolic energy. The discovery of enzymatic synthesis and regenerative activity of amorphous biosilica that builds the mineral skeleton of siliceous sponges formed the basis for the development of successful strategies for the treatment of osteochondral impairments in humans. In addition, the elucidation of the functional significance of a second regeneratively active inorganic material, namely inorganic polyphosphate (polyP) and its amorphous nanoparticles, present from sponges to humans, has pushed forward the development of innovative materials for both soft (skin, cartilage) and hard tissue (bone) repair. This energy-rich molecule exhibits a property not shown by any other biopolymer: the delivery of metabolic energy, even extracellularly, necessary for the ATP-dependent tissue regeneration. This review summarizes the latest developments in nanobiomaterials based on these two evolutionarily old, regeneratively active materials, amorphous silica and amorphous polyP, highlighting their specific, partly unique properties and mode of action, and discussing their possible applications in human therapy. The results of initial proof-of-concept studies on patients demonstrating complete healing of chronic wounds are outlined.
Keywords: biomaterials, nanoparticles, biosilica, polyphosphate, silicatein, regenerative medicine
Graphical Abstract:
Introduction
Over the course of its life, the integrity of any metazoan, from the simplest organisms to humans, is compromised to varying degrees by injury or chronic degenerative changes, leading to dysfunction. In humans, when the physiological repair capacity is insufficient, medical intervention is required to prevent a collapse of the fragile cellular homeostasis and the tuned organization of cells and tissues. There is a need for new concepts in regenerative therapy. Animals that have physiologically extensive regenerative abilities, such as sponges, are possible models for copying these concepts from nature. During the evolution of the metazoans from their basal phylum, the sponges (Porifera), with their ~40,000 predicted protein-coding loci,1 to the “crown taxa”, humans, with 20,000 to 25,000 genes,2 a gradual decrease in the genetic complexity is observed. This fact supports the view that the basal animals like sponges have a higher redundancy potential, combined with a variety of alternative metabolic circuits that provide them with a higher evolutionary stability. In turn, the regeneration capability of basal animals is more extensive compared to the “more advanced” taxa, including humans.
In animals, particularly in humans, some defects are not adequately repaired especially when physiological functional restoration is not supported by the inherent regeneration potential. If tissue/cell homeostasis is not maintained, the repair processes are insufficient with the result that the affected, damaged tissue is only replaced by tissue-like entities that lack physiological and biochemical functions. Or, as in present-day solutions, the defective parts are replaced or augmented by largely inert or only partially biological substituting materials. For example, the deficiencies of the bone-supporting implants can be overcome with tissue regenerating materials that restore not only the mechanical but also the biological and functional properties of the damaged tissue in the defect region. It is important to emphasize that any regeneration process requires an optimal physiological functioning of the cells, which depends on molecular and mechanical signals from the environment.3 Both the cells in the tissue surrounding the defect, and particularly the cells within the areas to be repaired, must be supplied with growth factors and nutrients that support the anabolic repair pathways and, importantly, with metabolic energy (ATP or another energy-carrying molecule).4 Not only intracellularly but also extracellularly, a sufficient supply of biochemically useful energy is important for the regeneration of the extracellular matrix (ECM). The number of cells in these regions is often low, for example in cartilage, which contains only ~10% chondrocytes.5 During regeneration, biochemical reactions in the ECM are driven by the cell-based synthesis of fibrous mats (collagen) or hydrogels (hydrogel-forming polypeptides). These reactions are anabolic reactions that require the conversion of chemical fuels like glucose into ATP (energy). In addition, enzymes are needed that break down larger precursor molecules in order to drive anabolic ones in bone, cartilage, and muscle regeneration.6 Accordingly, the two cornerstones, enzymes and energy, contribute significantly to the regeneration pathways. The initial basis for this gain came from studies from the basal animals, the sponges.
This review has a special focus on the regenerative abilities of two inorganic biomaterials in the formation and repair of both soft and hard tissues, amorphous silica (“biosilica”) and amorphous polyphosphate (polyP). Both biomaterials or biopolymers are regeneratively active only in their amorphous form and, in accordance with their physiological function, exhibit properties that the conventional organic biopolymers used in regenerative medicine do not have, such as in the case of polyP, the delivery of metabolic energy required for energy-dependent repair processes, as discussed in this review. In particular, the role that sponges, as the most basal phylum, have played in understanding the critical function of enzymes in the energy-dependent tissue regeneration/repair processes, including the formation of biomineralized structures, is discussed, while previous reviews on these biomaterials primarily focus on either only one these materials7,8 or on their physicochemical properties or more mechanistic aspects.9
Regeneration Capacity Across Animal Taxa
The basic principles of regeneration processes have been enigmatic for a long time. In 1606, it was stressed that bone formation and the regeneration of this organ depend on spiritual forces, virtus naturalis, based and localized in the lower body area, which is characterized by a “warm and humid” local environment.10 Later, especially by Trembley, first evidence-based experiments were performed, which revealed remarkable regenerative abilities especially in the more basal Metazoa such as the phylum Cnidaria.11 The sponges (Porifera), the basal phylum of Metazoa, traditionally served as a model system for regeneration.12 Animal taxa that evolved later from sponges have a reduced regeneration capacity mainly due to the presence of lower amounts of stem cells.13 Thomas Hunt Morgan introduced the perspective of functional genomics by studying the regeneration of segments in earthworms as a model.14
In Table 1, a summary of the regeneration capacity across various animal taxa is shown. The ability to regenerate damaged or lost tissue greatly varies among different species and can include the whole body, the primary body axis, or only certain structures or tissues.15 Whole-body regeneration is particularly found in the basal metazoans such as sponges, cnidarians and ctenophores.16,17 The regeneration of the sponge body or sponge tissue after an injury can either start from small body fragments or occur via aggregation of dissociated sponge cells.16 Sponges as sessile marine organisms are particularly prone to wounding, eg, by grazers or mechanical injury, which may lead to the occurrence of chronic wounds if repair fails and homeostasis is not retained. Investigations of the response of the sponge Aplysina aerophoba to damage caused mechanically or by a spongivorous opisthobranch using differential gene expression analysis based on RNA-sequence data revealed a repair mechanism involving enzymes/proteins such as metalloproteases, transglutaminases, and integrins, and signaling pathways (Wnt and mitogen-activated protein kinase – MAPK), similar to that involved in wound healing in higher animals, including humans.18
 |
Table 1 Regeneration Capacity Across Various Animal Taxa |
Taking Concepts from Nature as a Blueprint for Regeneration
Intense efforts over the past 40 years have uncovered, in a scientific, causal-analytical manner, the strategies of how living systems function with the aim of exploiting them to meet the urgent needs in engineering tissue replacement biomaterials. Biomimetic and bioinspired approaches based on these achievements have contributed considerably to a paradigm change in the field of tissue engineering.20–22 Studies on sponges, the evolutionarily oldest metazoans, showed that these animals produce a range of organic biomolecules that they effectively defend against bacteria and viruses.23–25 Then, the application of molecular biology techniques made it possible to show – what was not expected before – that these basal animals have in their protein toolkit, eg, building blocks even for basic immune defense systems that are successfully used by humans against foreign invaders. The existence of the Rhesus factor26 or the polymorphic immunoglobulin molecules,27 members of the acquired immune system, are mentioned here as examples. Even more, analyses of the formation of the skeletal system of the siliceous sponges, made of amorphous silica (“biosilica”), enabled the discovery of principles underlying tissue regeneration including biomineral formation, which were later found also in higher vertebrates including humans. In particular, it was discovered that biomineral formation is driven by enzymes, just like the biological formation of organic biomolecules and the functioning of metabolic pathways.28,29 Thus, sponges served as a model to progress advances in osteochondral regeneration.30 In addition, due to their excellent mechanical but also light transmitting properties,31 which are particularly evident in the siliceous sponge spicules, sponges can be considered and used as blueprints for the design of functional composite materials not only for nanobiomedical but also for technological applications.
In general, scaffolds formed from natural biopolymers provide an excellent matrix and niche for cell attachment, growth, migration, and differentiation, mimicking the ECM of native tissues (for a recent review, see Ref.32). In these properties, natural biomaterials differ from bioinert synthetic materials used in tissue engineering and regenerative medicine that do not show bioactivity. Only by modifying them according to the design of natural polymers can synthetic bioinspired materials with biological activities be obtained.33 Natural biomimetic biomaterials also have the advantage that they are non-toxic and do not exhibit genotoxic or teratogenic effects. In contrast to the natural biomaterials currently used, which are organic biopolymers such as polysaccharides (eg, hyaluronic acid, alginate, chitosan, and cellulose), polyesters (eg, polylactic acid and polyhydroxyalkanoates), and polypeptides/proteins (eg, collagen, gelatin, and fibroin),32 the two inorganic materials described here, biosilica and polyP, although occurring physiologically, are also readily available by chemical methods, thereby overcoming the problem of batch variability shown by many natural polymers.
Sponges (Porifera): Their Seminal Contributions Towards Understanding Biomineralization
Based on the lessons learned from sponges, the two inorganic polymeric materials amorphous biogenic biosilica (“biosilica”) and inorganic polyphosphate (polyP) have attracted increasing interest in regenerative medicine due to their unique properties. While biosilica forms a sponge skeleton, polyP is a ubiquitous polymer, found from bacteria to men, in sponges particularly in the environment of biosilica. Findings in sponges have been of crucial importance for an understanding of the biological mineralization processes taking place in organisms, as compared to abiotic mineralization.34,35 While abiotic mineralization solely relies on chemical processes, biomineralization is based on chemical reactions that take advantage of genuine biochemical conversion processes. Biochemical reactions are principally catalyzed by enzymes, following the rules of thermodynamics. They often link individual processes together, where endergonic, non-spontaneous processes can be driven by exergonic, energy-releasing reactions.
More specifically, two principles have been revealed in sponges as are outlined in this review: First, that the vast majority of biochemical reactions, including biomineralization reactions, are enzyme driven and dependent. From sponges, the first enzyme that mainly contributes to inorganic mineral formation was discovered: silicatein.28,36,37 While chemical reactions only strive to reach an equilibrium, biological processes proceed in open systems and are attuned to non-equilibria allowing both continuous and discontinuous flux of matter and energy.38
Biosilica, enzymatically synthesized in vivo in sponges (Figure 1), has become a paragon for a mineralic biomaterial. Later, it became increasingly aware that enzymes are also involved in the synthesis and degradation of the inorganic scaffold of other skeletal systems such as bone.39 Of note here is the alkaline phosphatase (ALP), a ubiquitous, membrane-bound tetrameric enzyme that is attached via glycosyl-phosphatidylinositol moieties to the outer cell surface and involved in osteoid formation and mineralization.40 The tartrate-resistant acid phosphatase and cathepsin K, an osteoclast-specific enzyme, are involved in bone resorption.41 Furthermore, it has been suggested that bone formation also involves a carbonic anhydrase that synthesizes amorphous Ca-carbonate bioseeds during the course of Ca-phosphate bone mineral deposition.42
In contrast to enzymatic, biotic biomineralization, abiotic mineralization, eg, geothermal mineralization, is driven by heat derived from, eg, cosmic rays/ionizing radiation (Figure 1). In addition, it was shown that, in addition to enzymes, bone biomineralization as well as the organization of the bone architecture also require energy in the form of ATP,43,44 without specifying the extent.45,46 In fact, the energy issue was largely ignored until approaches to regenerate or repair tissue through biochemically based substitution therapy were addressed. Both osteoblasts and osteoclasts release ATP into the extracellular space in amounts that depend on the proliferation and differentiation state of the cells.43 Despite a variety of ATP export channels into the extracellular space, the ATP concentration there is very low at ~10 nM, in contrast to the large intracellular pool at ~100 µM (see Ref.5). Since the level of ATP in human blood is also low at ~100 nM, an additional energy source for ATP generation had to be postulated.
Based on the therapeutic success with polyP, after application of this polymer for the repair in different organs, bones, and chronic wounds,30,47–49 it was proposed and then proved that polyP with its energy-rich acid anhydride linkages could serve as a source for metabolically useful energy in the form of ATP and/or ADP,50,51 reviewed in Ref.5 As reported later, the energy stored in polyP can be converted to ADP/ATP in a stepwise enzymatic reaction chain.
In addition to the two corner stones, (i) enzymes and (ii) metabolic energy, organic templates are usually required for the deposition of minerals during biomineralization. In bone, there are the collagen matrices built from collagen bundles that act as a platform for the apposition of mineral aggregates. Their post-translationally modified side chains, processed by hydroxylation and glycosylation, provide suitable deposition sites.52
Sponge Biosilica
The sponges (Porifera) share a common body plan with the other, more evolved metazoans.53 This fundamental finding, based on extensive studies of the (expressed) sponge genome, together with the discovery that these animals synthesize their mineral skeletons with the help of enzymes, as mentioned above,28,36,37 formed the reason why these animals have become metazoic model taxa for the development of biomimetic materials and processes applicable for both nanobiomedical and engineering purposes for the benefit of humans. Equally important was the discovery that biomineral formation is an energy-consuming process that requires an energy (ATP) source.
Based on their skeletons, the Porifera are subdivided into the classes of the siliceous Demospongiae and Hexactinellida and the calcareous sponges, the class Calcarea.54 The siliceous spicules of most Demospongiae remain individualized and the secreted cellular tissue units are formed around them. In these sponges, the spicules are linked together with organic molecules (collagen-related spongin and lectin) that form a bulky extracellular matrix.55 It is the siliceous scaffold that directs the particular, species-characteristic form of the sponge tissue. The siliceous skeleton of the hexactinellids is composed of discrete spicules, which often fuse to a basket-like scaffold as in the hexactinellid Euplectella aspergillum (Figure 2A).56 In particular, the siliceous skeleton of this class of sponges transmits light along the outer surface of the cage (Figure 2B), but also within the inner fan pocket (Figure 2C). Particularly impressive are the central giant spicules of the deep-sea sponge Monorhaphis chuni with a length of up to 3 m (diameter 1.7 cm), which represent the largest biosilica structures on Earth (Figure 2D).57 Light can pass efficiently along these giant spicules (Figure 2E). These spicules are composed of up to 800 lamellae, each 5–10 μm thick, which are arranged concentrically around the axial canal (Figure 2F). In the center, the axial canal harbors a 10 µm thick proteinaceous filament, the axial filament (Figure 2F).
Enzymes Involved in Sponge Biomineralization
It is a distinctive feature of both classes of siliceous sponges, the demosponges and the hexactinellids, that their skeletal elements, composed of amorphous biosilica, are synthesized by an enzyme, silicatein. With silicatein, the first enzyme was discovered that catalyzes the formation of an inorganic “polymeric” material, here biosilica, from an inorganic precursor, here monomeric silicic acid.37,58–60
Silicatein is an enzyme protein found exclusively in Porifera. Three isoforms of silicatein are found in the axial filaments.28,37 Molecular sequence analyses revealed that the silicatein family of proteins originates from the cathepsin family of proteases, more precisely from cathepsin L.61 Like the silicateins, the cathepsins are hydrolytic enzymes.62 Since sponges are suspension/filter feeders, our group had proposed and then identified this major catabolic enzyme, cathepsin L.
Among the siliceous sponges, the demosponge Suberites domuncula (Figure 2H) has been used in most cell biological and molecular studies, because this sponge species can be readily kept in aquaria and allowed the establishment of a cell culture, the primmorphs.63 In the demosponges, the axial filament is voluminous (Figure 2G and I). The silicatein filaments determine the morphology of the spicules, either rod-shaped (as in S. domuncula) or star-shaped (as in Geodia cydonium).
Cross fractures through the spicules revealed that not only the central silicatein rod but also the surface of the spicules reacts with antibodies against silicatein (Figure 2J). Interestingly, when cryosections through S. domuncula are studied, a regional distribution of ATP is measured (Figure 2K). The highest ATP levels are found in the vicinity of the spicules. The latter finding reflects the crucial role of ATP in the biomineralization processes and the dependence of the formation of skeletal elements on metabolic activity.64 Biomineralization requires not only an enzyme that lowers the activation energy of this process but also metabolic energy in the form of ATP.
The other class of sponges, the calcareous sponges, Calcarea, are stabilized by calcareous spicules that are formed of calcite.65 Following the general rule that any physiological crystalline structure is built from amorphous precursors, calcite formation starts with amorphous Ca-carbonate (ACC).66 Our group found that the Ca-carbonate-based spicules are synthesized enzymatically by a carbonic anhydrase,29 a finding that has been suggested earlier.67 Also of interest is the finding that the ACC precursor is stabilized by polyP.68 Consequently, incubation of ACC with the polyP-hydrolyzing enzyme ALP causes the transformation of the ACC phase into calcite.69
The Substrate and Mechanism of Silicatein Reaction
Sponges take up silicon from seawater after conversion of SiO2 to soluble, biological biosilica (SiO2•nH2O) and from there to readily soluble H4SiO4 (aq).70 Silicic acid is transported via a Si transporter into the sponge cells,71 where it serves as a substrate for silicatein for the formation of amorphous biosilica. The synthetic silicon compound tetraethyl orthosilicate (TEOS), as a silicic acid precursor, was used for the functional analyses.28,58,72
Silicatein catalyzes the (poly)condensation/polymerization of its physiological substrate, ortho-silicic acid, at low concentrations (<1 mM).59,73 Higher concentrations are required for chemical, non-enzymatic condensation reactions (>1 mM).73–75 In bioinspired fabrication processes, silicatein acts as a hydrolase (silicic acid esterase) that facilitates the hydrolysis of alkoxysilane compounds (cleavage of Si–O–C “ester” bonds), eg, of TEOS.28,37,76
Among demosponges, the first deduced amino acid sequences of silicateins were published for S. domuncula28,77 and T. aurantium.37 The catalytic triad (catalytic center) of silicatein consists of the amino acids Ser, His, and Asn. The cathepsins have a Cys residue instead of Ser. Another characteristic sequence of silicatein is a Ser-rich cluster.
The mechanism of biosilica formation with silicatein has been outlined.78 In the marine environment, the concentration of silicon is around ~10 mg mL‒1. Silicic acid is taken up by cells via a specific transporter where it acts as a substrate for silicatein (Figure 3).71 The reaction starts with nucleophilic attack of the catalytic triad Ser-OH group at a silicic acid substrate molecule, supported by hydrogen bridge formation to the His imidazole group in the catalytic center (Figure 3). The third amino acid of the catalytic triad of silicatein, the Asn residue (not shown), binds to the OH leaving group of the substrate, which is released as a water molecule. This step enables the formed Ser-bound silicic acid, again facilitated by H-bridge formation to His, to undergo two condensation reactions, resulting in the formation of an enzyme-bound disilicic acid and then a trisilicic acid species, which is then released from the enzyme after cyclization as cyclotrisilicic acid (cyclotrisiloxane). Next, a purely chemical condensation process takes place, initiated by preferential addition of further silicic acid species to this cyclic silicon compound. The subsequent steps of silica formation basically follow the Stöber synthesis, allowing a controlled growth of spherical silica particles to condensed silica deposits by hydrolysis of alkyl silicates with subsequent polycondensation of the produced silicic acid units.79
The biosilica synthesized by silicatein is deposited onto a structured organic template, the enzyme (Figure 3A and B) which also forms a mature siliceous spicule. Consequently, silicatein existing in the axial canal (the axial filament) predetermines the morphology of the spicules (Figure 3C and D). If the spicules are thorned, the surface of the silicatein axial filament shows thorny protrusions (Figure 3D). Using TEOS as a synthetic substrate, silicatein has been used to coat various surfaces/templates (Figure 3E), such as microspheres (Figure 3F and G).
Biosilica: Bioinspired Application as a Morphogenetic Polymer
The field of application of (bio)silica and the bioinspired/biomimetic materials and biomineral structures based on it is wide, ranging from the use in regenerative medicine, improved bacterial fermentation to optical fibers and even as a construction model for architectural buildings.
Biosilica has been considered as a paradigm for biological mineral morphogenesis and evolution not only in sponges80 but also in diatoms.81 In both systems, the morphology of the structures formed is driven by genetically controlled mineralization. The morphogenetic biosilica effect is attributed to its gene-inducing effect.82 In a series of applied experiments, it could be demonstrated that biosilica is a potent material for osteogenic stimulation and differentiation.
Biosilica: A Generic Template for Bone and Tooth Repair
The property of biosilica to be morphogenetically active and supports and likewise accelerates regeneration/repair processes in humans was proven in vitro and in animals (rabbits).80,83 In a first bioinspired approach, the sponge biosilica was applied in in vitro cell systems to evaluate and then to document an increased mineralization potential, first by measuring the expression of respective genes, like the structural proteins within the tooth matrix proteins, amelogenin, ameloblastin, and enamelin, which were strongly upregulated.80 The rationale for this line of study came from Carlisle in her report in 1972.84 Carlisle showed that chicks fed a silicon-rich diet grew and developed substantially faster than control animals. These data were confirmed later85 by demonstrating that the bone strength of broilers is strongly enhanced by dietary supplementation with bioavailable silicon. In continuation, in a series of in vitro studies, it was measured that both biosilica and silicatein induce the growth of mineralizing cells on silicatein/biosilica-coated matrices and cause under these conditions a strong increase in hydroxyapatite (HA) mineral formation;86 in the absence of biosilica, no HA mineral nodules were detected.
Consequently, animal studies were performed using New Zealand White rabbits by inserting 600-µm-large microspheres pressed into pellets (Figure 4A) and implanted into the anterior patella (between the medial and lateral femoral condyles). As an active ingredient, either silicatein (0.8 µg g‒1) or biosilica (100 µg g‒1) was added (Figure 4B). The biosilica pellets, added into PLGA (poly(
 |
Figure 4 Morphogenetic activity of biosilica in animal experiments (rabbits) - regeneration of bone in holes drilled into patellar grooves. (A) Microspheres embedded in pellets and (B) biosilica prepared by silicatein for the implant experiments. (C) The microspheres were fabricated together with biosilica. Staining of the mineral nodules formed in rabbits after insertion for 5 days. Staining with OsteoImage (fluorescence flashing). In the (D) femoral implant experiments, new bone formation was detected after in vivo staining with (E and F) oxytetracycline dihydrate under UV light. A striking difference was found between β-TCP controls (E) and biosilica-supplemented microspheres (F). Adapted from Bone, volume 67, Wang SF, Wang XH, Draenert FG, et al. Bioactive and biodegradable silica biomaterial for bone regeneration. 292–304. © 2014, with permissions from Elsevier Inc.83 |
In general, crystalline biominerals such as bone HA are formed from the amorphous precursors.66 The mechanism underlying the morphogenetic activity of biosilica is not fully understood. The release of orthosilicic acid from amorphous biominerals could contribute to the stimulating effect on the mineralization of bone-forming cells as in bioglass. The mechanism proposed for bioglass involves an exchange of Na+ and Ca2+ with H+ ions, leading to the formation of silanol groups and an increase in the surrounding concentration of OH– ions.89 The cleavage of Si–O–Si bonds by OH– then results in the release of orthosilicic acid and the formation/re-condensation of further silanol groups, which – similar to biosilica – leads to a hydrated, silica-rich layer on the glass surface, which is a suitable matrix for mixed carbonated HA deposition by invading Ca2+, PO43–, OH– and CO32– ions. It is known that low silicate concentrations (0.05–0.5 mM) promote HA nucleation.90 Silica has also been shown to stabilize and prevent the crystallization of ACC, the precursor of amorphous calcium phosphate (ACP), and of crystalline HA.91 In addition, modeling studies showed that the silanol groups of cyclic trisilicic acid motifs on the silica surface stereochemically mimic the Ca2+-binding HA nucleation site on bone sialoprotein (BSP).92–94
These data underscore that biosilica shows morphogenetic activity on bone-forming cells not only in vitro but also in vivo, in animal experiments.95
Inorganic Polyphosphate
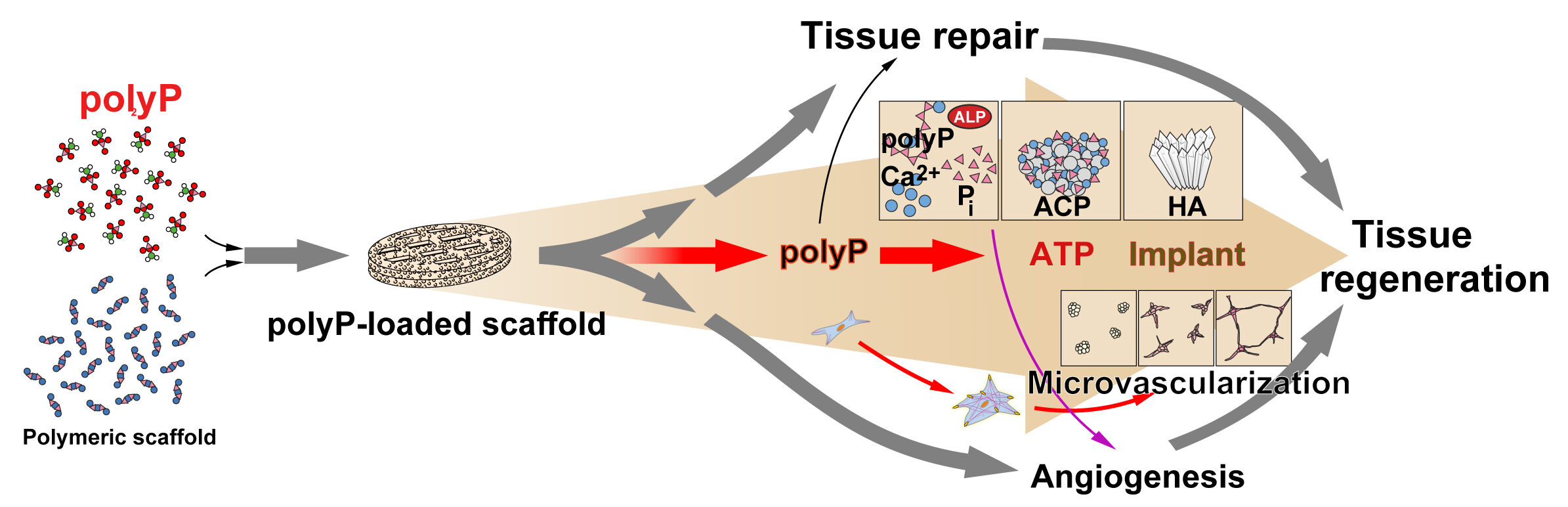
The second physiological inorganic polymer, identified in sponges,96 that has attracted increasing attention, is polyphosphate (polyP). Originally, polyP was identified in bacteria and yeast (for a review, see Ref.97), and later in animals, like in sponges96 and higher vertebrates/humans.98 This apparently ubiquitous polymer99,100 exhibits a unique property that no other inorganic material useful for human therapy possesses - the delivery of metabolic energy. PolyP (Figure 5A) has emerged as a prime example of a physiological polymer that not only fulfills the structural but also the energy-supplying requirements for a successful biomedical regeneration process.
 |
Figure 5 (A) Chemical structure of polyphosphate (polyP). (B) Intracellular synthesis of polyP. Glucose is the body’s main source of energy. After cellular uptake, glucose is metabolized to pyruvate during glycolysis, which is then channeled via the voltage-dependent anion channel (VDAC) to the mitochondrial intermembrane space and then to the matrix, where it undergoes oxidative metabolism. The generated reduced coenzymes (NADH and FADH2) drive redox reactions and the electron transport chain builds an electrochemical transmembrane proton gradient whose energy is converted to ATP. ATP is released into the cytoplasm via the adenine nucleotide translocator-2 (ANT2) and VDAC. Subsequently, ATP is channeled through the vacuolar transporter-chaperone complex (VTC) in the acidocalcisomal membrane, which functions in yeast as a polyP polymerase. From the acidocalcisomes in the megakaryocyte-platelets, polyP is released to the extracellular space either as soluble Na-polyP or as membrane-associated Ca-polyP nanoparticles (Ca-polyP-NP). Adapted from Müller WEG, Schröder HC, Wang XH. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix. Chem Rev. 2019;119:12337–12374. © 2019 American Chemical Society. Creative Commons.5 |
Physiologically, polyP is stored intracellularly in organelles, now termed acidocalcisomes, which have been intensively studied in trypanosomatids, protozoan parasites.101,102 Earlier they have been identified as metachromatic granules103 or volutin granules.104 In the blood platelets, polyP is accumulated in the dense granules, as identified by Ruiz et al.105 The discovery that ATP and polyP harbor metabolic energy came from Meyerhof and Lohmann (cited in Ref.106). The unequivocal identification of the chemical structure was described by Lohmann and Langen.107,108
Recently, as described later, the proof-of-concept of the therapeutic benefits in the clinic for polyP could even be successfully provided. As a hydrogel, the natural polymer not only provides a platform for cell proliferation, cell differentiation, and cell migration but also provides the metabolic energy required for maintaining the molecular and supramolecular organization of the extracellular matrix and cell function.48,49
Polyphosphate: Cell-Based Synthesis
The reaction chain by which polyP is synthesized in bacterial cells is fairly well understood (reviewed in Ref.109). Less is known about polyP synthesis in higher vertebrate cells.98,110 For the synthesis of polyP in mammals, ATP is required, which is generated intracellularly in the mitochondria (Figure 5B).111–113 There, in the respiratory chain, ATP formation is linked with complex V, the F1Fo-ATPase. For the chemical synthesis of the phosphoanhydride bonds in ATP, heating to >100°C for ~10 h is required.114 Accordingly, for an intracellular, physiological synthesis of ATP at 37°C, with its high-energy anhydride bonds, an activation energy of 110 kJ mol‒1 has to be expended, as determined using Arrhenius plot.115 Therefore, the reactions in the mitochondria must be mediated by enzymes. It is the ATP synthase, localized in the inner mitochondrial membrane, which catalyzes the synthesis of ATP from ADP. In turn, the biosynthetic pathway for the formation of polyP with its >30 high-energy anhydride bonds must involve enzymes as well. In mammalian systems, the genuine polyP-synthesizing enzyme has not yet been discovered. Experimental evidence in yeast suggests that polyP is formed enzymatically from ATP during import into the acidocalcisomes (Figure 5B).116,117 Indicative is the fact that, in the dense granules of the platelets that correspond to the yeast acidocalcisomes, the concentrations of ADP (600 mM), ATP (400 mM), and pyrophosphate (300 mM) are exceedingly high,118 while the polyP content is comparably low (130 mM; based on Pi).119 Therefore, it might be assumed that phosphatases or phosphotransferases present in platelets120,121 could be involved in polyP synthesis through backward reactions from ATP, driven by these enzymes. From the platelets, polyP is released in two forms, either in a soluble form, as a chain with Na+ as counterion, or in an “insoluble” form, as a NP with Ca2+ as counterion (Figure 5B).118,122 Since polyP compartmentalized into the acidic dense granules (pH 5.4) is released in a controlled manner,123,124 it is likely that the Ca2+ gradient (Ca2+ concentration in the dense granules is 2.2 M)119 determines the formation of the two forms of polyP as proposed.118
After full platelet activation, the concentration of polyP in the blood is relatively high with 0.5 to 3 μg mL‒1.118,125 There, the polymer has a physiological chain length of ~50 phosphate (Pi) units. In the particulate Ca2+ form, the polyP chains are longer with ~250 Pi units. However, this value is very variable since there are high levels of ALP in the blood, which hydrolyzes the polymer from the chain termini as an exopolyphosphatase.126
A biocompatible polymer can be particularly beneficial to patients when transported within the body to the target site for tissue repair. Here, too, polyP follows the route of a biomimetic medical compound. In vivo, under physiological conditions, polyP is efficiently distributed to the injured sites in the body with the blood circulation. There, the polymer is delivered by the blood platelets to the damaged tissue regions, where the polymer initiates the regeneration process.127 In fact, platelets with the stored polyP are hallmarks of regeneration.128 It was Julius Bizzozero who discovered platelets as small medicinal pellets of a size between 1 and 2 µm.129 He described them as the initiators of blood clotting. PolyP is a major constituent of platelets.105 Using these vehicles, polyP is distributed throughout the body in order to onset regeneration/repair. The distribution of polyP is flanked by macrophages, which bind or internalize polyP, independently of their signaling roles, via their P2Y1 and RAGE receptors.130 Besides acting on the clotting cascade and enhancing hemostasis, platelets are activated and release polyP into the extracellular space when certain growth factors, such as epidermal growth factor or platelet-derived growth factor, are released.122 In this environment, platelets bind directly to exposed collagen fibers, as well as to von Willebrand factor, fibronectin, and other adhesive proteins.131 This efficient distribution mechanism of polyP in the body contributes to the prominent position that polyP has achieved in regenerative medicine, as described later.
Polyphosphate: Chemical Synthesis
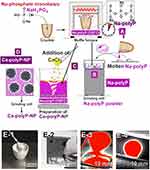
Chemically, polyP can be prepared in sufficiently large quantities.132 Na-polyP with its energy-rich phosphoanhydride bonds (Figure 6A) is obtained by melting of NaH2PO4 at temperatures up to 700°C (Figure 6B). For the fabrication of Ca-polyP, both an enzymatic/wet chemical approach133 and a calcination process have been introduced.134 By shortening the high-temperature processing protocol, it is possible to prepare both amorphous Na-polyP and Ca-polyP-NP almost in parallel (Figure 6B and C). The process runs at 700°C. At the end of the polycondensation reaction, polyP, with a chain length of ~50–100 Pi units, is supplemented with CaCl2 and heated for an additional period of time to obtain the amorphous Ca-polyP-NP with a diameter of ~100 nm (Figure 6D). When Na-polyP is synthesized, the material is ground to 50 µm powder (Figure 6B). Different steps of the procedure, starting from NaH2PO4, are summarized in Figure 6E-1–E-4.
Polyphosphate: A Biomimetic Molecule for Human Therapy
PolyP as a polyanion can be present in tissues as salt with various cations as counterions. The biological regeneration function of polyP differs depending on the counterion chosen. In addition, the functional activity of polyP depends on the form of the polyP salts, which can exist both in a soluble form, such as the sodium salt (Na-polyP), and in an insoluble nanoparticulate form that can be used as a storage (depot) form, such as salts with various divalent cations, eg, Ca2+ ions. The latter salts can also form a gel-like coacervate phase, a physiologically active form, as outlined later.
Cation-Specificity of Polyphosphate: A Smart Nano/Micro Biomaterial
Besides the thermal method described above, polyP can be fabricated in a biomimetic way as nanoparticles (NP) as in vivo in human cells using a wet chemical procedure.118,135 The biological activity of the polyP nanoparticles is determined, at least in part, by the counter-cations used for a particular scaffold (Figure 7). For cartilage repair, Ca2+- and also Mg2+-polyP complexes are more suitable,136 while for bone regeneration, the Ca-polyP forms might be favored due to their higher stability. In addition, the Sr2+-polyP complex shows strong regenerative activity both in vitro and in animal experiments.137 Drugs that are effective, eg, against bone tumors/metastases, such as the bisphosphonate zoledronic acid, can be conveniently integrated into the Ca-polyP-NP and used as implant particles.138 For another application target, chronic wound healing,48,49 a combination of Na-polyP and Ca-polyP-NP acts most efficiently on the regeneration process. In addition, polyP particles, when applied in an aerosol form, have a protective effect on the respiratory epithelium, after conversion into the coacervate form upon contact with mucin, the protein of the airway mucus.139 As a result, viral particles such as SARS-CoV-2 are entrapped and inactivated, in addition to a direct inhibitory effect on this virus.140,141
 |
Figure 7 PolyP is a genuine, smart nano/micro biomaterial whose properties and applications depend on the selected counterion. PolyP-NP, polyphosphate nanoparticle. Reproduced with permission from Wang XH, Schröder HC, Müller WEG. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: Towards a new paradigm in tissue engineering. J Mat Chem B. 2018;6:2385–2412. © 2018 The Royal Society of Chemistry. Creative Commons.142 |
Polyphosphate Coacervate Formation
It is an exceptional feature of polyP (as Na-polyP) to undergo coacervation in the presence of Ca2+ or other divalent cations, at physiological pH. In principle, polyP nanoparticles, including non-processed Ca-polyP-NP, are biologically inert. They have to be converted into a biologized form, a polyP-coacervate. During this phase transition, polyP provides its physiological functions. At pH 7, Ca2+ together with polyP forms a viscous aqueous phase, the coacervate (Figure 8A and C). These aggregates are formed through liquid–liquid phase separation, resulting in a denser phase and a dilute phase that are in thermodynamic equilibrium.143 In the coacervate phase, polyP shows morphogenetic activity as well as its function to generate ATP.144 In our approach, the two phases and their interconversion were studied using in silico simulation studies, as well as (physico)chemical analyses. The data showed that Ca-polyP coacervate formation occurs at pH 7 and is slower, compared to Ca-polyP-NP formation at pH 10 (Figure 8E and F). Interestingly, if CaCl2 is dropped into a Na-polyP solution at pH 7, the Ca-polyP coacervate initially forms and becomes then converted to NP at pH 10. Conversely, when Na-polyP is added to a CaCl2 solution, only the coacervate phase is obtained at both pH values.144
The coacervate is biocompatible and allows the infiltration of mesenchymal stem cells (MSC) into the gel matrix, where polyP promotes cell proliferation and differentiation;143 the MSC become completely embedded in the matrix (Figure 8D). When the coacervate (Ca2+-polyP) forms during the liquid–liquid phase separation, different local densities arise causing turbulences during which bacteria, such as E. coli, are enwrapped (Figure 8B) and then killed.49
PolyP particles are taken up by endocytosis, as determined by inhibition studies with trifluoperazine dihydrochloride.51 Intracellularly, the particles begin to transform into a coacervate. The free polyanionic polyP (not in a salt form) is not able to traverse cell membranes. Only after caging the polymer, eg, into a guanidinium/oligocarbonate vehicle, polyP can be channeled into the cells.145
Supramolecular Extracellular Matrix Organization: Importance of Polyphosphate Coacervation and Energy Requirement
Biomimetic materials used for soft or hard tissue regeneration can be classified according to the four tissue categories: covering tissue, connective/and supporting tissue, muscle tissue, and nervous tissue.146 The ECM of the different organs has a different percentage of cells, such as the liver with a high (>90%) and cartilage with a low cell density (<10%). A common feature of their ECMes is that the cells are embedded into a fibrillar, often collagenous, hydrogel, which allows architectural stability and simultaneous diffusion of nutrients as well as cell movement. To ensure biocompatibility, a physiological or close-to-physiological hydrogel is preferably used in regenerative medicine. Surely, such hydrogels can elicit signals stimulating tissue regeneration. Normally, hydrogels lack the ability to directly supply the cells with metabolic energy, preferably in the form of ATP.5
Supramolecular hydrogels have been introduced into regenerative medicine because of their unique dynamic properties for self-healing and injectability based on the non-covalent crosslinking organization of their macromolecules. Hydrogels based on this fabrication technology are held together by forces arising from non-covalent bonds such as electrostatic interactions, hydrogen bonds, metal coordination, aromatic stacking, and hydrophobic and van der Waals forces (Figure 9A).147,148 The individual molecules are synthesized by covalent bond formation (“Molecular chemistry”) and become subsequently organized into complex supramolecular chemistry-based systems. There (usually) more than one chemical unit interacts via non-covalent bonds, as in the case of receptors or enzymes. Consequently, ligands or substrates in the next higher hierarchy level typically associate with receptors in turn via noncovalent bonds and form an active state of the system. In this complex arrangement, signal transduction pathways (with the respective ligands) are initiated or enzymatic reactions follow, through which new covalent bond(s) are introduced into the reaction chain.148
Due to its ability to form a coacervate promoting immigration and embedding of cells in the presence of Ca2+ or other divalent cations, as well as its ability to induce stem cell growth and differentiation, polyP is an excellent material for mimicking the conditions in the ECM of human tissues. In particular, polyP has the unique property of not only exerting a morphogenic effect but also providing the metabolic energy needed for tissue regeneration (Figure 9B). Organic polymers used with polyP included negatively charged polysaccharides such as N,O-carboxymethyl chitosan (N,O-CMC), alginate,149 chondroitin sulfate,150 hyaluronic acid,136 and karaya gum,151 as well as collagen150 and poly(vinyl alcohol) (PVA).151 These in vitro results were confirmed by animal experiments (rat muscle) showing a replacement of the implants by granulation tissue already after an implantation period of 2 to 4 weeks.151 The most advanced polyP-based bio-ink, which provides metabolic energy in addition to morphogenetic activity, was developed using an N,O-CMC/alginate/gelatin hydrogel enriched with soluble Na-polyP- and Ca-polyP-NP (see below). This bio-ink enabled the successful 3D bioprinting of MSCs that remained functionally active and able to migrate, grow, and differentiate to mineralizing osteoblasts.152
In tissues, the organization of both the intra- and extracellular supramolecular structures requires an energy input.153,154 Self-assembly reactions are usually considered to be independent of energy supply; however, the subsequent downstream processes certainly require ATP, such as the organization of collagen fibers into bundles.155 Until recently, the source of metabolic energy in the ECM was uncertain. It should be mentioned that the concentration of ATP in the extracellular space is only ~10 nM, while intracellularly the ATP level is high, varying between 0.5 and 5 mM depending on the cell type.156,157 ATP is required for many functions in the ECM. There, ATP is involved in the cell-cell communication, particularly in the nervous system, but also for the activation of T cell receptor (TCR) signaling.158 ATP is also required, as a chemotactic agent, for endothelial cells to direct them to micro-vessels along an ATP gradient.51 In addition, ATP-consuming kinase reactions also run extracellularly and chaperones are also present (reviewed in Ref.5).
Physiological Polyphosphate: A Key in Regenerative Medicine
Autologous platelet concentrate, platelet-rich plasma, is rich in polyP and is used for the prevention and treatment of bone and cartilage defects and for wound closure, especially in small ulcers.159 Interestingly, fibrin (which contains platelets with their polyP) shows the same accelerating effect on regeneration processes.160
So far, the main focus of the polyP-correlated biomimetic application for human therapy is in the field of repair of osteochondral (bone and cartilage) and skin and soft tissue (chronic wounds). The common denominator for these targets are the MSC which promote both bone/cartilage161,162 and wound repair.163 Furthermore, in vitro studies have shown that polyP is a potent inducer of cell proliferation and differentiation164–166 as well as mineralization.167 There are two features that qualify the metabolizable polyP as a distinguished polymer in regenerative medicine; first, it is based on phosphate, an osteoconductive biomaterial required for bone cell growth,168 and second, the inherent function of polyP to dissipate metabolic activity.5,99
Principal Distinctive Feature of Polyphosphate: Generation of Metabolic Energy
The distinguished feature of polyP is that its orthophosphate units are condensed via energy-rich phosphoanhydride bonds, forming a linear polymer with a physiological chain length of ~50–100 Pi units.169 The energy (Gibbs free energy; ΔG0) stored in each bond and released during hydrolysis from the ends of the polymer is about −30 kJ mol‒1.170 The law of energy-constancy in a closed system implies that energy is converted into different forms, while the sum of energy remains constant. The energy simply converts from one form to another. In a biochemical reaction, a part of the energy is dissipated as heat and the rest is used as metabolic energy that drives biochemical reactions.171 In turn, ΔG0 for ADP hydrolysis is about −30 kJ mol‒1 and for ATP (both energy-rich bonds), it is about −60 kJ mol‒1 (Figure 10A). In contrast, polyP with a chain length of, eg, 40 Pi units (39 energy-rich bonds) comprises a ΔG0 of approximately −1170 kJ mol‒1. Experimental evidence from SaOS-2 cells, after incubation with polyP, showed that an increased amount of energy-rich nucleotides (ADP and ATP) accumulates extracellularly.50 During the incubation of the cells with polyP, vesicles with both ALP and adenylate kinase (ADK) activities are released. These two enzymes are plasma membrane associated.172,173 Inhibition studies indicate that initially ADP is formed (during degradation of polyP with ALP), which is further up-phosphorylated to ATP by ADK.51 So far, a quantitative evaluation of the yield of ATP production from polyP is missing.
Kinases: An Essential Transfer Element
The outlined generation of ATP from polyP would cease when an equilibrium defined by the equilibrium constants between polyP, AMP, ADP, and ATP is reached. Consequently, metabolic energy must flow out of this equilibrium to keep the system running (Figure 10B). At this point, kinases achieve a crucial function. Extracellularly, a number of proteins involved in mineralization are phosphorylated, in particular osteopontin, dentin matrix protein-1, and dentin sialophosphoprotein.174,175 As the only (major) enzyme that catalyzes the phosphorylation of these proteins, the Golgi-localized protein kinase Fam20C, a serine kinase, has been identified.176 Failure of the function of Fam20C often leads to lethal osteosclerotic bone dysplasia, known as the Raine syndrome.177
Repair of Osseous Hard Tissues by Polyphosphate
Already Lowenstam suggested that the calcareous invertebrate skeletons from the Early Cambrian ≈560 mya are made of ACC, a view that has been later corroborated by dissolution experiments.178,179 In sponges, the class of calcareous sponges (Calcarea) forms their hard skeletons of Ca-carbonate, which is individually composed of calcite formed enzymatically with the sponge-specific carbonic anhydrase.29 The inorganic biomineral of the otoliths in the human inner ear also completely consists of Ca-carbonate, which is present in the form of calcite.180 It is not yet clear which enzymatic processes are involved in Ca-carbonate biosynthesis in human systems. Experiments with human osteogenic SaOS-2 cells suggested that in this system the carbonic anhydrase CA-IX, a cytosolic enzyme localized on cell membranes, is upregulated after exposure of SaOS-2 cells to bicarbonate.142 HA/bone formation proceeds through the phases of hyaline cartilage (hyaline, translucent cartilage), “smooth” cartilage (smooth type of connective tissue), endochondral ossification (embryonic bone tissue formation), appositional growth (increase in diameter), and finally, maturation of the mineral deposits to crystalline HA (Figure 11A).181 Element mapping using SaOS-2 cells by scanning electron microscopy of the surfaces of mineral deposits showed that the Ca minerals show signals for phosphorus, as expected, but initially also for the element carbon (Figure 11B-1–B-4). These data corroborate the assumption that mineralization in human bone also passes through the Ca-carbonate stage before the conversion to Ca-phosphate can take place.42 Therefore, it was proposed that the initial step in bone formation starts with the deposition of Ca-carbonate, perhaps catalyzed by the carbonic anhydrase. The matrix onto which bone mineral deposition takes place is a collagen meshwork. Surely, the first mineral deposits are amorphous.66 The transformation of the enzymatically formed (carbonic anhydrase-mediated) amorphous Ca-carbonate (ACC) towards amorphous Ca-phosphate (ACP) proceeds as an exergonic reaction and is not enzyme-driven.182,183 After that, ACP transforms into crystalline hydroxyapatite (Figure 11A).
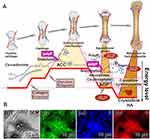 |
Figure 11 Ca-phosphate/bone formation. (A) Steps during bone formation: amorphous Ca-carbonate (ACC), amorphous Ca-phosphate (ACP) and finally crystalline Ca-phosphate/hydroxyapatite (HA). The different phases of bone initiation, growth and maturation are outlined in the upper trace. At least the final step, the transition from ACC to ACP, is driven enzymatically by ALP. There, the enzyme ALP hydrolyzes polyP and, by this, eliminates the stabilizing polymer polyP from the amorphous ACC and ACP phases. This process leads to the maturation of ACP to crystalline HA. During this process, the initial endergonic reactions are pulled by the subsequent exergonic processes. (B) Element mapping of the surfaces of Ca mineral deposits formed on human osteoblast-like SaOS-2 cells (B-1; SEM) for the elements oxygen (B-2), phosphorus (B-3) and carbon (B-4). Adapted with permission from Wang XH, Schröder HC, Müller WEG. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: Towards a new paradigm in tissue engineering. J Mat Chem B. 2018;6:2385–2412. © 2018 The Royal Society of Chemistry. Creative Commons.142 |
A huge amount of phosphate is required for bone formation. Based on in vitro data, it had been suggested that it is the organic phosphate, β-glycerophosphate, which, after hydrolysis by the ALP, provides the inorganic phosphate for bone formation.184 Later, when the crucial role of the platelets in bone synthesis and bone remodeling was revealed,185 it became evident that polyP is the main source of Pi.186 In this study, it was also reported that the gene expression of ALP,187 a marker enzyme for bone formation, is upregulated after polyP exposure.186 The enzyme-driven mineralization chain then proceeds to the ACP step and allows an appositional growth of the bone.188 In ACP, polyP acts as a stabilizing polymer of the amorphous phase during its transition to crystalline HA.167 After hydrolysis of polyP by the ALP, maturation from amorphous to crystalline Ca-phosphate can take place.69
Following these new scientific insights, polyP has been tested for its regenerative activity in vivo . Using the rat calvarial defect system as a model of bone repair, the polymer was integrated into PLGA microspheres. After eight weeks, the material was fully resorbed and the bone defect was completely restored, while β-TCP caused only limited repair. A comparative gene expression study revealed that polyP also significantly upregulates the expression of the collagen gene, in contrast to the β-TCP material.189
As mentioned above, crystalline biomaterials have only limited morphogenetic, regenerative activity, while amorphous materials exhibit regenerative potential.66 This differential effect can be demonstrated with the polyP-stabilized amorphous phase of Ca-phosphate, ACP. In the presence of small amounts of polyP (5% by weight), ACP still proceeds to the crystalline Ca-phosphate state (CCP). However, after increasing the polyP level to 15%, the ACP mineral remains frozen in the amorphous state.167 Therefore, a comparative animal study was performed with crystalline Ca-phosphate (containing 5% polyP), cCa(5)polyP, versus amorphous ACP/15% polyP particles (aCa(15)polyP).30 The Ca-phosphate formulations were integrated into PLGA microspheres. The starting material cCa(5)polyP shows a hexagonal columnar crystal morphology190 (Figure 12A). In the in vivo model (rabbit calvarial bone defect model), cCa(5)polyP only marginally repaired the bone defect after 6 weeks (Figure 12B). Interesting was the fact that the association (acceptance) of the microspheres to the tissue environment with cCa(5)polyP was very poor or even absent (Figure 12C).30 In comparison to crystalline ACP, the amorphous ACP (aCa(15)polyP) deposits appear as globular spheres (Figure 12D). Those ACP deposits accelerate the processes of migration, microvascularization, and mineralization in SaOS-2 cells. The repair process was dramatic; the newly formed tissue covered the regenerating zone and showed only remnants of the microspheres (Figure 12E). Even more, the microspheres in the regenerating area of the aCa(15)polyP-treated animal group readily attracted and interacted with the newly formed collagen bundles (Figure 12F). These data show that aCa(15)polyP combines osteoinductive activity with the morphogenetic potential to build the HA calvaria.
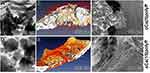 |
Figure 12 Bone healing with crystalline Ca-phosphate (cCa(5)polyP; containing 5% polyP). (A) Crystalline Ca-phosphate formed during aging in the presence of only 5% polyP. (B and C) Healing studies of encapsulated Ca-phosphate microspheres (MS) in vivo using the rabbit calvarial bone defect model. (B) After 6 weeks of insertion of MS filled with cCa(5)polyP, only a slightly repaired bone defect was seen in animals; (C) the interaction between the microspheres (crystalline phosphate implant) and the adjacent bone is poor. In contrast to the crystalline Ca-phosphate, containing only 5% polyP, (D) addition of 15% polyP to the starting substrates for the preparation of Ca-phosphate, [(NH4)2 HPO4 and CaCl2], freezes the product Ca-phosphate at the amorphous phase. (E) Those MS elicit a strong healing process, the MS fused with adjacent regenerating bone and (F) formed a tight collagen (co) around the MS. Adapted with permission from Acta Biomater. Volume 118, Müller WEG, Ackermann M, Al-Nawas B, etal. Amplified morphogenetic and bone forming activity of amorphous versus crystalline calcium phosphate/polyphosphate. 233–247, copyright 2020, with permission from Elsevier.30 |
Repair of Soft Tissue: Chronic Wounds
It is established that the supply of blood, and hence platelets, is crucial for bone tissue development and repair, and for healing of osteoporotic fractures.191–193 During these processes, platelets surround the affected site and become activated, and release growth factors and certainly also polyP. Even more obvious is the role of platelets in wound healing. It has been reported that platelet deficiency plays an adverse, critical role in skin healing and that its failure prevents healing of chronic wounds.194 In all phases of wound repair, hemostasis, followed by inflammatory reactions, up to the proliferative and remodeling phase, platelets decisively promote the regeneration process.195
Wound healing is energy-intensive. Most of the metabolic energy is consumed for positioning of new cells in the wound area.196 The epithelial cell motility, which is controlled by different members of the Rho family of GTPases, which act as molecular switches, consumes energy-rich nucleotides.197 In addition, wound healing is decisively dependent on new vessel formation, beginning with a polyP-induced microvascular network,198 which is required for optimal tissue perfusion and oxygenation. Finally, ATP acts as a stimulus for the endothelial cells to follow an ATP gradient and guide the cells via an autocrine sensing mechanism during the initial chemotactic formation of 100 µm circular endothelial cell rings.51,199,200 This ATP originates from glycolysis and not from the respiratory chain.201
Chronic wounds are becoming an increasingly common clinical problem, especially in patients with diabetes mellitus.202 In these patients, severe hyperglycemia due to an impaired insulin supply leads to reduced gene expression of enzymes involved in mitochondrial oxidative phosphorylation.203,204 In addition, an imbalance between angiogenic factors, angio-inhibitory factors, and abnormal apoptotic potential is observed, which reduces the proliferation of cells and their remodeling capacity. In diabetic wounds, the cells remain in a chronic inflammatory state and do not switch to the proliferation and remodeling phase. In turn, the use of drugs that generate metabolic energy in the wound is indicated. Besides ATP205 and a hydrogel supplemented with 0.02% adenine,206 it turned out that the physiological polymer polyP accelerates the healing rate of both experimental wounds in diabetic animals207 and chronic wounds in patients.48,49
In animal studies, polyP was applied into 8-mm wounds of diabetic mice and normal non-diabetic animals punched in the interscapular region down to the underlying fascia muscularis.207 In both animal groups, the polymer was applied once. While in normal mice (untreated), the physiological regeneration proceeded by 31% re-epithelialization during a 7-day healing period, the polyP-treated animals showed a strong acceleration of the regeneration potential to 72% during 7 days. In diabetic controls, re-epithelialization was even less, only about 20% by day 7, while with polyP-NP, healing was greatly accelerated and was complete after 14 days.
These very promising results prompted our group to conduct off-label studies in patients. Depending on the depth of the wound, either more superficial or deeper to the hypodermis along the muscle or down even to the bone, the active substance has to be integrated into a hydrogel or alternatively into a collagen-based mat.208 In the first human trial with chronic wounds, a hydrogel (with hydroxyethyl cellulose) containing 600 µg mL‒1 Na-polyP (to elicit immediate regenerative activity) together with 60 µg mL‒1 Ca-polyP-NP (acting as a depot) was prepared (Figure 13A).49 The Na-polyP in the gel formulation undergoes direct coacervation after application onto the wound, taking up the Ca2+ from the wound bed (Figure 13A-1). The Ca-polyP-NP ingredient (depot form) must form a coacervate before becoming active (Figure 13A-2 and A-3). A clinical example is illustrated in Figure 13A-4–A-6. The 55-year-old female patient was suffering from a ventral ulcer on the tibia for 3 months. This patient had been treated with cortisone over a long period due to a number of pre-existing illnesses. Treatment of the wound with the hydrogel started on 10.11. (Figure 13A-4). After 8 weeks of treatment, the wound area was reduced by 87% (Figure 13A-5). The patient was released from the clinics 10 weeks later (Figure 13A-6).
 |
Figure 13 Application of polyP for the treatment of chronic wounds. (A) Application of the polymer in (A-1) a hydrogel supplemented with Na-polyP and Ca-polyP-NP to cure more superficial wound. (A-2) Formation of a coacervate after contact with Ca2+ in the wound bed. (A-3) The presence of Ca-polyP-NP served as a depot for retarded polyP release. The chronic wound was treated with (A-4) the hydrogel. (A-5) After a 8 weeks of treatment, the wound dimension shrunk by 87%. (A-6) After a total of 18 weeks, the patient could go back home (B) For deeper wounds, (B-1) collagen-based mats were fabricated after compressing the collagen fibers. (B-2) Orientation of Ca-polyP-NP on the collagen bundles. (B-3) Every second day, a wetting solution composed of Na-polyP and Ca-polyP-NP was used and dropped onto the wound to keep it moist. There the Na-polyP underwent coacervation. Treatment success with the polyP-containing mats. (B-4) Initial state after debridement; (B-5) a strong reduction was observed after 6 weeks of treatment; (B-6) final state prior to patient discharge. (A) Adapted with permission from J Mat Sci Technol. Volume 135, Müller WEG, Schepler H, Neufurth M, et al. The physiological polyphosphate as a healing biomaterial for chronic wounds: Crucial roles of its antibacterial and unique metabolic energy supplying properties. 170–185. Copyright 2023. Creative Commons.49 (B) Adapted with permission from Schepler H, Neufurth M, Wang SF, et al. Acceleration of chronic wound healing by bio-inorganic polyphosphate: In vitro studies and first clinical applications. Theranostics. 2022;12:18–34. © 2022 Ivyspring. Creative Commons.48 |
Deep wounds can extend through the epidermis, dermis, hypodermis, and even to the cutaneous trunci muscle covering the skeletal muscle. These wounds require a wound dressing that elicits low antigenicity, suitable biocompatibility, and hemostatic properties to allow for cell proliferation and adhesion. One choice is collagen mats, which imitate the ECM, since collagen stabilizing the skin is present in the mats with an abundance of 60% to 70%. The collagen-based mats used here for application to deep wounds of patients48 were modified by compressing the collagen fibers in order to transfer the unstable collagen sheets to a strong wound-covering layer. For this purpose, bovine collagen samples were processed through a controlled pH shift series. The mats were then supplemented with 8 mg of Ca-polyP-NP per 1 mL collagen solution (10 mg mL‒1). The thickness of the mats was 1 to 1.3 mm (Figure 13B-1). The NP within the mats were oriented along the orientation of the collagen bundles (Figure 13B-2). Every second day, after application onto the wounds, the mats were moisturized with 1 mL of wetting solution composed of 300 μg g‒1 Na-polyP and 30 μg g‒1 (w/w final) Ca-polyP-NP to improve the healing process.48 After moisturizing the mats, a coacervate developed from the Na-polyP component (Figure 13B-3). As with the hydrogel treatment, the results of the off-label studies with the wound mats have been very encouraging. An example is shown in Figure 13B-4–B-6. The 79-year-old male patient suffered from a chronic wound resulting from surgical resection of a squamous cell carcinoma. The treatment of the wound, with a dimension of ~20 cm2, started in April 2021 (Figure 13B-4). After debridement by surgery and ultrasound, the wound was covered with the polyP-collagen mat. After 6 weeks of treatment, the wound diameter had shrunken to 37% (Figure 13B-5), and after a further 12 weeks, the patient could be released home (Figure 13B-6).
Repair of Soft Tissue: Respiratory Epithelium
The epithelium of the respiratory tract (from the trachea to the bronchi) has an important barrier function against inhaled pathogens. The mucus gel layer produced by the goblet cells of the respiratory epithelium plays a crucial role in this defense mechanism by the entrapment of respiratory bacteria and viruses such as the coronavirus SARS-CoV-2. This epithelium is prone to damage not only by these pathogenic agents but also by environmental factors such as pollution by particulate matter. PolyP was found to induce the expression of mucins, the main protein component of the mucus.139 In addition, it was found that polyP exhibits a strong antiviral activity against SARS-CoV-2.140,141 The finding that polyP elicits regenerative activity in the epithelial respiratory tract was not unexpected. The way it inhibits SARS-CoV-2 was surprising. Based on modeling studies, it has been demonstrated experimentally by in vitro studies and also supported by in vivo studies that polyP covers the tips of the coronavirus SARS-CoV-2 viral envelope spike proteins by binding to an unusually conserved cationic groove composed of basic amino acids (Arg, Lys, and His).139,140 Interestingly, the spacing of the basic amino acids on the spike protein perfectly matches the distances of 2.0 to 2.5 Å between the Pi units within the polyP molecule. In subsequent studies, a complete prevention of viral infection by polyP was experimentally proven. Due to this property, polyP can be classified as a component of the innate immune system and as a “smart” biomaterial (see Figure 7).209
Application of Biosilica and Polyphosphate in Emerging Tissue Engineering Techniques
The two biomaterials, silica and polyP, can also contribute significantly to the field of tissue regeneration through the application of new tissue manufacturing techniques such as 3D printing technology. Among the existing 3D fabrication techniques such as selective laser sintering, stereolithography, fusion deposition modeling, digital light processing, and 3D bioprinting (for a review, see Ref.210,211), the latter in particular is one of the most advanced techniques. The development of 3D bioprinting is undergoing rapid development. This extrusion-based additive manufacturing technique is not only suitable for the creation of customized bone implants but also the fabrication of even large-sized tissue constructs and complex organs.
Nevertheless, this technique still has a number of limitations that await novel solutions. These include, for example, the fabrication of hollow structures such as blood vessels that would collapse as a result of gravitational forces during the layer-by-layer printing process. This makes, among others, the production of vascularized tissues difficult. One way to circumvent this problem is to use embedded bioprinting,212 a gel-in-gel manufacturing technique in which the bio-ink is printed into a microgel or granular support bath. In the case of cortical bone implants, the printed material should have the required porosity and mechanical properties. Recapitulating the different mechanical properties and biological functions of the different parts of bone is a challenging task. Another requirement specifically for 3D cell printing (3D bioprinting) of tissues is the availability of a suitable bio-ink. This bio-ink, which usually consists of a hydrogel, certain other factors such as growth factors and the cells, must be able to protect the cells suspended in the hydrogel not only during the printing process but also afterwards, after curing (usually achieved by printing in a hardening solution containing Ca2+ ions), to maintain essential cell functions such as vitality, proliferation, migration, and differentiation abilities. Furthermore, the cells must be able to be printed in a density that corresponds to the high cell densities of native tissues and organs.
The introduction of the two biomaterials, silica and polyP, brought decisive progress, particularly with regard to the latter properties. Firstly, both amorphous biomaterials are regeneratively active themselves, they support cell vitality and promote cell proliferation/differentiation and do not require the addition of growth factors. The activity of such biodegradable growth factors/proteins is often difficult to control. Secondly, polyP in particular is able to provide the metabolic energy required for cell function, supporting cell migration and the synthesis, organization, and maintenance of the ECM structure. Cells can be printed at sufficiently high concentrations required for tissue regeneration/repair and formation of functional tissue constructs.
Taking advantage of these unique properties of silica and polyP/polyP-NP, the first bioprintable biosilica and polyP-containing hydrogels were developed. Embedding SaOS-2 cells in an alginate hydrogel resulted in a 3D printable material that showed increased HA formation and expression of bone morphogenetic protein-2 (BMP-2) and osteoprotegerin (OPG) genes.213
The first cell-free, polyP-enriched bio-ink suitable for 3D printing consisted of Ca-polyP-NP embedded in a poly(ε-caprolactone) (PCL) matrix. The scaffolds printed with this bio-ink supported the ingrowth of SaOS-2 cells, accompanied by increased expression of the cell-attracting chemokine stromal cell-derived factor-1 (SDF-1).214 The first cell-containing polyP-bio-ink consisted of Na-polyP, gelatin, and alginate complexed by Ca2+ ions. The cells (SaOS-2) embedded in this ink remain alive and grow efficiently in the 3D printed construct.215 The best results were achieved – as described above – with a bio-ink based on N,O-CMC/alginate/gelatin containing both soluble polyP (Na-polyP) and Ca-polyP-NP. MSC contained in this matrix remained functionally active and showed increased cell migration and propensity to grow and differentiate into mineralizing osteoblasts.152
As a further method, the electrospinning method has been used to fabricate advanced biosilica- or polyP-containing scaffold materials. Electrospun nanofiber mats prepared from poly(
Preferred Fields of Application
Both biomaterials, biosilica and polyP, have a wide range of potential applications. The most important ones identified are summarized in Table 2. Although there are some potential applications in the technical field, the preferred application of both materials is in tissue engineering and regenerative medicine. For the latter applications, the amorphous nature of the material is necessary. As shown in animal studies (rabbits; see Figure 4), silica encapsulated in PLGA microspheres alone or together with silicatein strongly stimulates bone regeneration after implantation in the femur compared to β-TCP controls.83 As stated, a similar mineralization-promoting effect was also found with polyP, both with soluble polyP189 and with polyP-NP, but also with polyP-stabilized ACC68,218 and polyP-stabilized ACP.30,69,167 The effect of the polyP-NP is significantly influenced by the countercation of the polyanionic polyP. While Ca, Sr, and Mg are the preferred counterions in inducing bone regeneration,135,137,142,189 the Mg- and Ca-salts of the polymer are more suitable for cartilage regeneration.136 Using the calvarial bone defect model, polyP-stabilized ACC218 and polyP-stabilized ACP30 encapsulated in PLGA and embedded in cranial defects of rats or rabbits significantly increased the formation of new bone mineral. β-TCP and crystalline Ca-phosphate, used as a controls, were much less active.
 |
Table 2 Preferred Applications of Amorphous Biosilica and Amorphous polyP |
Instead of encapsulation in microspheres, silica and polyP can also be administered after integration into a hydrogel-forming organic polymer matrix that can be hardened in the presence of divalent cations. In this way, biohybrid materials are created that combine the regenerative properties of the inorganic biomaterials/biopolymers with the advantageous mechanical and water-binding properties of the organic polymers. SaOS-2 cells embedded in an alginate-based hydrogel matrix containing biosilica formed by silicatein213 or grown on 3D printed biosilica-alginate scaffolds219 showed increased growth, mineralization, and gene expression of BMP-2, collagen type 1, and OPG, but not of RANKL (receptor activator of NF-κB ligand). Increased osteogenic activity was also found with enzymatically formed biosilica on a chitosan-graft-polycaprolactone matrix.234 Organic polymers used together with polyP mainly included negatively charged polysaccharides, as well as collagen and PVA. In the presence of divalent metal cations such as Ca2+ (used for bone implants) or Mg2+ ions (for cartilage), these materials can be cured by forming metal bridges to produce materials with suitable hardness and viscoelastic properties for potential use in bone and cartilage regeneration/repair. PVA is hardened by intermolecular cross-linking by freeze-thawing.151 The addition of Ca2+ to the polyP-containing matrices can also lead to the in situ formation of Ca-polyP-NP,150,151 which upon contact with the body fluid are converted into a biologically active coacervate, as confirmed in animal studies.151 Furthermore, due to their regenerative activity, biosilica and polyP can also be used in dentistry for tooth restoration/repair,226 but also as dental sealants.80,227 Other identified potential applications of both polymeric biomaterials include their use in drug delivery, biologization of inert materials and protection against biofouling, including caries prevention (Table 2).
The most advanced application of polyP and polyP-NP in regenerative medicine is in the field of wound healing, specifically for the treatment of chronic wounds, where this polymer has already been successfully used in initial human studies, as described here. The material can be in the form of a wound gel49 or incorporated into a collagen-based wound mat.48,217 In addition, the use of the remarkable antiviral activity of polyP,125 especially against SARS-CoV-2,139,140 also proven in vivo, 141 in the form of a nasal spray is promising.
Conclusion and Outlook
At first glance, both the soft tissues, such as cartilage or fibrous connective tissue such as the dermal layer of the skin, and the bones or teeth, the hard tissue, are largely composed of non-cellular ECM structures that regulate almost all cellular functions. Especially during regeneration, these tissues are subject to continuous remodeling mediated by enzymes such as the catabolic-acting matrix-degrading enzymes235 or the anabolic-acting ALP.236 The dysregulation of the energy metabolism in cells of the ECM, such as fibroblasts (dermis layer of the skin), chondrocytes (cartilage), or bone cells, such as osteoblasts, osteocytes, and osteoclasts, disrupts the balance between various growth factors, proteoglycans, mineralizing cells, and enzymes.237 Focusing on cells of the mineralized tissue, the central role of enzymes was first discovered in sponges, as summarized in this review. Furthermore, the connection of the metabolism of the ECM and the skeletal elements with bioenergetics was first disclosed in these evolutionary oldest animals. The mineralized elements in sponges are formed in an ATP-rich environment. A suitable biomaterial, in turn, must provide these two landmarks, enzymes and metabolic energy, to enable regeneration, morphogenesis, and repair of damaged tissue (Figure 14). Here again, the sponges offered a solution, directing the bioinspired/biomimetic studies towards an inorganic biopolymer, polyP, which from then on became a distinguished biomaterial for engineering scaffolds and supplements for successful repair of damaged tissue with graded structural, mechanical, and compositional properties. The anionic polyP, in combination with cationic ions, acts as a structural and reinforcing biomaterial that tends to transform into a hydrogel-like coacervate in the presence of peptides.143 In this water-rich phase, polyP undergoes enzymatic degradation and processing into ADP and ATP.5
Also, wound healing is an energy-intensive process. Sponges are already provided with basal mechanisms involved in wound healing, including enzymes and signaling pathways that are found in higher animals and humans.18 Sponges as sessile animals are sensitive to injury and tend to develop chronic wounds when repair is compromised. However, they can survive even in adverse conditions with low oxygen levels, possibly by using polyP accumulated in these animals.238
Although biomineral formation in sponges and human bone shares some similarities in terms of enzyme involvement and energy requirements, it should be noted that both the chemical nature and the steps in which enzymes are involved are different. The sponge biosilica consists of covalently linked SiO4 tetrahedra that share an edge with each other (some edges of the tetrahedra remain unlinked as free silanol Si-OH groups) and is an amorphous material, while HA is a crystalline material composed of PO43‒, OH‒, CO32‒, and Ca2+ units linked together by ionic forces. In nature, in living organisms, both are composites, with silica as silica spicules consisting of multiple silica lamellae surrounding a central axial filament of silicatein and separated from each other by thin layers made of silicatein and some other proteins.239 Bone, on the other hand, consists mainly of HA and collagen, which forms the matrix for the deposition of the HA crystals, but also of some other proteins involved in the regulation of the biomineralization process. The steps in which enzymes are involved in mineral deposition and the nature of the protein framework for biomineral deposition are also different. In the sponge spicules, the protein scaffold around which silica is deposited, itself carries the enzymatic, silica-forming activity, while in bone, the proteinaceous collagen scaffold is enzymatically inactive and enzymes (ALP) are only involved in providing the required energy and phosphate (released from polyP or some other sources, eg, ATP), as well as in the formation of the ACC precursor of ACP and crystalline HA (carbonic anhydrase). As a regeneratively active biomineral for human tissue regeneration/repair, silica consists only of the inorganic amorphous material.
Biosilica derived from sponges has a wide range of bioinspired applications, ranging from orthopedic implants, as summarized in this review, to dental sealants80 and light transmission systems.31 The exploitation in human therapy is remarkable and based on the morphogenetic and regenerative activity of the material. Closely related to biosilica are bioactive glasses, also an amorphous material that was introduced in 1971.240,241 However, the chemical composition of biosilica is different from the CaO-SiO2-P2O5 composition of bioactive glasses prepared by sol–gel methods. Biosilica formed enzymatically by silicatein consists largely of a (water-rich) network of covalently linked SiO2 units, while 45S5 bioglass, for example, consists of 24.5 wt% CaO, 45.0 wt% SiO2, 6.0 wt% P2O5, and 24.5 wt% Na2O.242,243 As shown here, the morphogenetic activity of biosilica can be exploited in implants as demonstrated by the differential expression of various genes required for cell growth and differentiation in response to the biomineral.80 In addition, from the natural biosilica, the blueprint, the fabrication of distinctive hierarchical porous structures could be learned, which even enabled the production of fibers with specific optical properties.244
PolyP has a unique property to act both as a structural and degradable biomaterial for bone regeneration in orthopedics and simultaneously as a generator for extracellular metabolic energy. The application of polyP is even more advanced in the area of wound healing, especially the healing of chronic wounds. Healing of chronic wounds caused by metabolic diseases (diabetic foot ulcers), inadequate blood flow (venous leg ulcers), sustained pressure (bedsores), infections or cancer is still an unsolved medical challenge and a major cost driver in medical care. Initial applications in human patients with chronic wounds led to promising results, as described here. PolyP can be administered both in soluble form (as Na-polyP, immediately active) and as Ca-polyP-NP (depot form, prolonged effect), either as a wound creme or integrated into a collagen mat. Since the proof of concept for this biopolymer could be elaborated in human studies, its central position for physiological regeneration has been established.
Future tasks will also include using the two biomaterials silica and polyP to develop stimuli-responsive materials that are sensitive to specific signals from the tissue environment (pH, temperature, presence of certain biomolecules, etc.). Such materials may be endowed with the ability to decompose, swell or shrink, undergo sol–gel transition, or release certain drugs, growth factors, or other biomolecules in response to pH or other changes in the surrounding tissue.245 In particular, pH changes (acidosis) that occur, for example, in the course of wound healing,246 at tumor sites247 or during ischemia248 can be used to trigger the release of these molecules from the implanted or administered material. In recent years, a number of pH-dependent delivery systems have been developed, based, for example, on N,O-CMC,249 alginate-dialdehyde-gentamicin/chitosan,250 or poly(N-isopropylacrylamide-co-acrylic acid) hydrogels.251 PolyP, as described in this review, is particularly suitable for the development of such delivery systems because it can transform from the nanoparticulate state (polyP-NP) to the coacervate state in a pH-dependent manner. Using bisphosphonate zoledronic acid, Ca-polyP-NP carrying this drug for potential treatment of tumors/metastases has already been synthesized.138 A challenging task will be the development of silica- or polyP-based materials that carry multiple bioactive molecules or drugs, released at different times or on demand, and controlled by specific biochemical signals during multi-step repair processes such as the phases of wound healing (hemostasis, inflammation, proliferation, and maturation/remodeling) or the stages of bone regeneration (hematoma formation, inflammation, soft callus formation, hard callus formation, and remodeling). This could be realized by incorporating these biomolecules into various carrier systems such as core-shell particles, and nanoparticles (polyP-NP) with different counterions or nano/microspheres. Recently, a core-shell drug delivery system has been developed consisting of a polyP-NP core and a polyP-coacervate shell loaded with two different bioactive molecules (dexamethasone and ascorbic acid) that are released with different kinetics.228 Such systems would also be of interest for the long-term treatment of osteoporosis caused by a reduced ratio of expression of the two cytokines OPG and RANKL, leading to increased osteoclastogenesis/bone resorption, or rheumatoid arthritis, which is associated with overexpression of inflammatory cytokines such as tumor necrosis factor α or interleukins 1, 6, or 7 causing bone destruction. Both polyP and biosilica have been shown to increase OPG expression,9,213 and polyP to decrease proinflammatory cytokine levels.141 Finally, the beneficial effects of the regenerative activity of silica and polyP in other pathological conditions such as neurodegeneration need to be elucidated.
As summarized in this review, bioinspired/biomimetic thinking has the potential to reconcile the present-day demands of our comparatively very young species with its deficits. The human species (Homo sapiens) with an age of ~0.3 million years can benefit by learning from other metazoan taxa such as sponges (age 800 million years),252 and also from the world of microorganisms, which can be traced back to over 3465 million years of the Earth’s history.253 Keeping in mind that every organism on earth has a long, rich history driven by adaptation and survival of the fittest.254 The biosilica and polyP materials discussed here have the groundbreaking potential for further scientific, biomedical, and technical innovations. Surely, these two materials will push ahead sustainable progress of our society in science, medicine, and technology.
Acknowledgments
We thank our colleagues Prof. Dr W. Müller-Klieser and Prof. Dr S. Walenta (Institute of Pathophysiology; University Medical Center Mainz; Germany) for providing us with the cryosections through the sponge for the visualization of the ATP accumulation. W.E.G. M. is the holder of an ERC Advanced Investigator Grant (Grant No.: 268476). In addition, W.E.G. M. has obtained three ERC-PoC Grants (Si-Bone-PoC, Grant No.: 324564; MorphoVES-PoC, Grant No.: 662486; and ArthroDUR, Grant No.: 767234). In addition, this work was supported by Grants from the European Commission (Grant Nos.: 604036 and 311848), the International Human Frontier Science Program and the BiomaTiCS research initiative of the University Medical Center, Mainz. Further support came from the Grant of the BMBF (SKIN-ENERGY, Grant No.: 13GW0403A/B) and the Grant of the BMWi (Grant No.: ZF4294002AP9).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kenny NJ, Francis WR, Rivera-Vicéns RE, et al. Tracing animal genomic evolution with the chromosomal-level assembly of the freshwater sponge Ephydatia muelleri. Nat Commun. 2020;11(1):3676. doi:10.1038/s41467-020-17397-w
2. Pertea M, Salzberg SL. Between a chicken and a grape: estimating the number of human genes. Genome Biol. 2010;11(5):206. doi:10.1186/gb-2010-11-5-206
3. Williams D. The essential materials paradigms for regenerative medicine. JOM. 2011;63(4):51–55. doi:10.1007/s11837-011-0067-5
4. Chiang B, Essick E, Ehringer W, et al. Enhancing skin wound healing by direct delivery of intracellular adenosine triphosphate. Am J Surg. 2007;193(2):213–218. doi:10.1016/j.amjsurg.2006.08.069
5. Müller WEG, Schröder HC, Wang XH. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix. Chem Rev. 2019;119(24):12337–12374. doi:10.1021/acs.chemrev.9b00460
6. Trackman PC. Diverse biological functions of extracellular collagen processing enzymes. J Cell Biochem. 2005;96(5):927–937. doi:10.1002/jcb.20605
7. Wang XH, Schröder HC, Müller WEG. Enzyme-based biosilica and biocalcite: biomaterials for the future in regenerative medicine. Trends Biotechnol. 2014;32(9):441–447. doi:10.1016/j.tibtech.2014.05.004
8. Wang Y, Li M, Li P, et al. Progress and applications of polyphosphate in bone and cartilage regeneration. Biomed Res Int. 2019;2019:5141204. doi:10.1155/2019/5141204
9. Schröder HC, Wang XH, Neufurth M, Wang SF, Tan R, Müller WEG. Inorganic polymeric materials for injured tissue repair: biocatalytic formation and exploitation. Biomedicines. 2022;10(3):658. doi:10.3390/biomedicines10030658
10. Vallesius F. Controversiarum medicarum et philosophicarum. W.C. Hanoviae: Marnium; 1606.
11. Trembley A. Mémoires pour servir à l’histoire d’un genre de polypes d’eau douce à bra sen forme de cornes. Verbeek: Leide; 1744.
12. Galtsoff PS. The amoeboid movement of dissociated sponge cells. Biol Bull. 1923;45(3):153–161. doi:10.2307/1536625
13. Müller WEG. The stem cell concept in sponges (Porifera): metazoan traits. Semin Cell Dev Biol. 2006;17(4):481–491. doi:10.1016/j.semcdb.2006.05.006
14. Morgan TH. Regeneration. New York/London: Macmillan; 1901.
15. Bely AE, Nyberg KG. Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol. 2010;25(3):161–170. doi:10.1016/j.tree.2009.08.005
16. Ereskovsky A, Borisenko IE, Bolshakov FV, Lavrov AI. Whole-body regeneration in sponges: diversity, fine mechanisms, and future prospects. Genes. 2021;12(4):506. doi:10.3390/genes12040506
17. Ricci L, Srivastava M. Wound-induced cell proliferation during animal regeneration. Wiley Interdiscip Rev Dev Biol. 2018;7(5):e321. doi:10.1002/wdev.321
18. Wu YC, Franzenburg S, Ribes M, Pita L. Wounding response in Porifera (sponges) activates ancestral signaling cascades involved in animal healing, regeneration, and cancer. Sci Rep. 2022;12(1):1307. doi:10.1038/s41598-022-05230-x
19. Cary GA, Wolff A, Zueva O, Pattinato J, Hinman VF. Analysis of sea star larval regeneration reveals conserved processes of whole-body regeneration across the metazoa. BMC Biol. 2019;17(1):16. doi:10.1186/s12915-019-0633-9
20. Rybicka-Jasińska K, Derr JB, Vullev VI. What defines biomimetic and bioinspired science and engineering?. Pure Appl Chem. 2021;93(11):1275–1292. doi:10.1515/pac-2021-0323
21. Gough A, Soto-Gutierrez A, Vernetti L, Ebrahimkhani MR, Stern AM, Taylor DL. Human biomimetic liver microphysiology systems in drug development and precision medicine. Nat Rev Gastroenterol Hepatol. 2021;18(4):252–268. doi:10.1038/s41575-020-00386-1
22. Benyus JM. Biomimicry: Innovation Inspired by Nature. Perennial, New York: HaperCollins Pub; 2002.
23. Furth JJ, Cohen SS. Inhibition of mammalian DNA polymerase by the 5’-triphosphate of 1-β-D-arabinofuranosylcytosine and the 5’-triphosphate of 9-β-D-arabinofuranoxyladenine. Cancer Res. 1968;28(10):2061–2067.
24. Schabel FM. The antiviral activity of 9-β-D-arabinofuranosyladenine (ARA-A). Chemotherapy. 1968;13(6):321–338. doi:10.1159/000220567
25. Müller WEG, Zahn RK, Bittlingmaier K, Falke D. Inhibition of herpesvirus DNA-synthesis by 9-ß-D-arabinofuranosyladenosine in vitro and in vivo. Ann New York Acad Sci. 1977;284(1):34–48. doi:10.1111/j.1749-6632.1977.tb21935.x
26. Seack J, Pancer Z, Müller IM, Müller WEG. Molecular cloning and primary structure of a Rhesus (Rh)-like protein from the marine sponge Geodia cydonium. Immunogenetics. 1997;46(6):493–498. doi:10.1007/s002510050310
27. Müller WEG, Blumbach B, Müller IM. Evolution of the innate and adaptive immune systems: relationships between potential immune molecules in the lowest metazoan phylum [Porifera] and those in vertebrates. Transplantation. 1999;68(9):1215–1227. doi:10.1097/00007890-199911150-00001
28. Krasko A, Lorenz B, Batel R, Schröder HC, Müller IM, Müller WEG. Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur J Biochem. 2000;267(15):4878–4887. doi:10.1046/j.1432-1327.2000.01547.x
29. Müller WEG, Wang XH, Grebenjuk V, et al. Common genetic denominators for Ca++-based skeleton in metazoan: role of osteoclast stimulating factor and carbonic anhydrase in a calcareous sponge. PLoS One. 2012;7(4):e34617. doi:10.1371/journal.pone.0034617
30. Müller WEG, Ackermann M, Al-Nawas B, et al. Amplified morphogenetic and bone forming activity of amorphous versus crystalline calcium phosphate/polyphosphate. Acta Biomater. 2020;118:233–247. doi:10.1016/j.actbio.2020.10.023
31. Müller WEG, Wendt K, Geppert C, Wiens M, Reiber A, Schröder HC. Novel photoreception system in sponges? Unique transmission properties of the stalk spicules from the hexactinellid Hyalonema sieboldi. Biosens Bioelectron. 2006;21(7):1149–1155. doi:10.1016/j.bios.2005.04.017
32. Liu S, Yu JM, Gan YC, et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Mil Med Res. 2023;10(1):16. doi:10.1186/s40779-023-00448-w
33. Omidian H, Wilson RL, Babanejad N. Bioinspired polymers: transformative applications in biomedicine and regenerative medicine. Life. 2023;13:1673.
34. Weiner S, Dove PM. An overview of biomineralization processes and the problem of the vital effect. Rev Mineral Geochem. 2003;54(1):1–29. doi:10.2113/0540001
35. Westbroek P. Biological metal accumulation and biomineralization in a geological perspective. In: Westbroek P, de Jong EW, editors. Biomineralization and Biological Metal Accumulation. Dordrecht, Holland: D. Reidel Publishing Co; 1983:1–11.
36. Krasko A, Gamulin V, Seack J, Steffen R, Schröder HC, Müller WEG. Cathepsin, a major protease of the marine sponge Geodia cydonium: purification of the enzyme and molecular cloning of cDNA. Molec Marine Biol Biotechnol. 1997;6:296–307.
37. Shimizu K, Cha J, Stucky GD, Morse DE. Silicatein α: cathepsin L-like protein in sponge biosilica. Proc Natl Acad Sci USA. 1998;95(11):6234–6238. doi:10.1073/pnas.95.11.6234
38. Mărgineanu DG. Equilibrium and non-equilibrium approaches in biomembrane thermodynamics. Arch Internat Physiol Biochim. 1987;95:381–422.
39. Seibel MJ. Biochemical markers of bone turnover. Part I Biochemistry and variability. Clin Biochem Rev. 2005;26(4):97–122.
40. Harris H. The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta. 1990;186(2):133–150. doi:10.1016/0009-8981(90)90031-M
41. Schini M, Vilaca T, Gossiel F, Salam S, Eastell R. Bone turnover markers: basic biology to clinical applications. Endocr Rev. 2023;44:417–473.
42. Müller WEG, Schröder HC, Schloßmacher U, Grebenjuk VA, Ushijima H, Wang XH. Induction of carbonic anhydrase in SaOS-2 cells, exposed to bicarbonate and consequences for calcium phosphate crystal formation. Biomaterials. 2013;34(34):8671–8680. doi:10.1016/j.biomaterials.2013.07.096
43. Andrilli LHS, Sebinelli HG, Favarin BZ, et al. NPP1 and TNAP hydrolyze ATP synergistically during biomineralization. Purinergic Signal. 2023;19:353–366.
44. Morrison MS, Turin L, King BF, Burnstock G, Arnett TR. ATP is a potent stimulator of the activation and formation of rodent osteoclasts. J Physiol. 1998;511(2):495–500. doi:10.1111/j.1469-7793.1998.495bh.x
45. de Paula FJ, Rosen CJ. Bone remodeling and energy metabolism: new perspectives. Bone Res. 2013;1:72–84.
46. Motyl KJ, Guntur AR, Carvalho AL, Rosen CJ. Energy metabolism of bone. Toxicol Pathol. 2017;45(7):887–893. doi:10.1177/0192623317737065
47. Müller WEG, Wang SF, Ackermann M, et al. Biologization of allogeneic bone grafts with polyphosphate: a route to a biomimetic periosteum. Adv Funct Mater. 2019;29(44):1905220. doi:10.1002/adfm.201905220
48. Schepler H, Neufurth M, Wang SF, .et al. Acceleration of chronic wound healing by bio-inorganic polyphosphate: in vitro studies and first clinical applications. Theranostics. 2022;12(1):18–34. doi:10.7150/thno.67148
49. Müller WEG, Schepler H, Neufurth M, et al. The physiological polyphosphate as a healing biomaterial for chronic wounds: crucial roles of its antibacterial and unique metabolic energy supplying properties. J Mat Sci Technol. 2023;135:170–185.
50. Müller WEG, Wang SF, Neufurth M, Kokkinopoulou M, Feng Q, Schröder HC. Polyphosphate as a donor of high-energy phosphate for the synthesis of ADP and ATP. J Cell Sci. 2017;130:2747–2756.
51. Müller WEG, Ackermann M, Tolba E, et al. Role of ATP during the initiation of microvascularization. Acceleration of an autocrine sensing mechanism facilitating chemotaxis by inorganic polyphosphate. Biochem J. 2018;475(20):3255–3273. doi:10.1042/BCJ20180535
52. Michaëlsson E, Malmström V, Reis S, Engström A, Burkhardt H, Holmdahl R. T cell recognition of carbohydrates on type II collagen. J Exp Med. 1994;180:745–749.
53. Müller WEG, Wiens M, Adell T, Gamulin V, Schröder HC, Müller IM. Bauplan of Urmetazoa: basis for genetic complexity of Metazoa. Intern Rev Cytol. 2004;235:53–92.
54. Uriz MJ, Turon X, Becerro MA, Agell G. Siliceous spicules and skeleton frameworks in sponges: origin, diversity, ultrastructural patterns, and biological functions. Microsc Res Tech. 2003;62:279–299.
55. Müller WEG, Blumbach B, Wagner-Hülsmann C, Lessel U. Galectins in the phylogenetically oldest metazoa, the sponges [Porifera]. Trends Glycosci Glycotechnol. 1997;9(45):123–130. doi:10.4052/tigg.9.123
56. Weaver JC, Aizenberg J, Fantner GE, et al. Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum. J Struct Biol. 2007;158(1):93–106. doi:10.1016/j.jsb.2006.10.027
57. Wang XH, Schröder HC, Müller WEG. Giant siliceous spicules from the deep-sea glass sponge Monorhaphis chuni: morphology, biochemistry and molecular biology. Int Rev Cell Mol Biol. 2009;273:69–115.
58. Zhou Y, Shimizu K, Cha JN, Stucky GD, Morse DE. Efficient catalysis of polysiloxane synthesis by silicatein α requires specific hydroxy and imidazole functionalities. Angew Chem Int Ed Engl. 1999;38:779–782.
59. Schröder HC, Borejko A, Krasko A, Reiber A, Schwertner H, Müller WEG. Mineralization of SaOS-2 cells on enzymatically (Silicatein) modified bioactive osteoblast-stimulating surfaces. J Biomed Mat Res Part B Appl Biomater. 2005;75B:387–392.
60. Schloßmacher U, Wiens M, Schröder HC, Wang XH, Jochum KP, Müller WEG. Silintaphin-1: interaction with silicatein during structure-guiding biosilica formation. FEBS J. 2011;278(7):1145–1155. doi:10.1111/j.1742-4658.2011.08040.x
61. Müller WEG, Boreiko A, Wang XH, et al. Silicateins, the major biosilica forming enzymes present in demosponges: protein analysis and phylogenetic relationship. Gene. 2007;395(1–2):62–71. doi:10.1016/j.gene.2007.02.014
62. Tabatabaei Dakhili SY, Caslin SA, Faponle AS, Quayle P, de Visser SP, Wong LS. Recombinant silicateins as model biocatalysts in organosiloxane chemistry. Proc Natl Acad Sci USA. 2017;114:E5285–E5291.
63. Müller WEG, Wiens M, Batel R, et al. Establishment of a primary cell culture from a sponge: primmorphs from Suberites domuncula. Mar Ecol Progr Ser. 1999;178:205–219. doi:10.3354/meps178205
64. Walenta S, Dötsch J, Mueller-Klieser W. ATP concentrations in multicellular tumor spheroids assessed by single photon imaging and quantitative bioluminescence. Eur J Cell Biol. 1990;52(2):389–393.
65. Boury-Esnault N, Rützler K. Thesaurus of sponge morphology. Smithson Contrib Zool. 1997;596(596):1–55. doi:10.5479/si.00810282.596
66. Weiner S, Mahamid J, Politi Y, Ma Y, Addadi L. Overview of the amorphous precursor phase strategy in biomineralization. Frontiers Mat Sci China. 2009;3:104–108.
67. Jackson DJ, Macis L, Reitner J, Degnan BM, Wörheide G. Sponge paleogenomics reveals an ancient role for carbonic anhydrase in skeletogenesis. Science. 2007;316(5833):1893–1895. doi:10.1126/science.1141560
68. Tolba E, Müller WEG, El-Hady BMA, et al. High biocompatibility and improved osteogenic potential of amorphous calcium carbonate/vaterite. J Mat Chem B. 2016;4(3):376–386. doi:10.1039/C5TB02228B
69. Müller WEG, Neufurth M, Ushijima H, et al. Molecular and biochemical approach for understanding the transition of amorphous to crystalline calcium phosphate deposits in human teeth. Dental Mater. 2022;38:2014–2029.
70. Crundwell FK. On the mechanism of the dissolution of quartz and silica in aqueous solutions. ACS Omega. 2017;2(3):1116–1127. doi:10.1021/acsomega.7b00019
71. Schröder HC, Perović-Ottstadt S, Rothenberger M, et al. Silica transport in the demosponge Suberites domuncula: fluorescence emission analysis using the PDMPO probe and cloning of a potential transporter. Biochem J. 2004;381(3):665–673. doi:10.1042/BJ20040463
72. Müller WEG, Schloßmacher U, Wang XH, et al. Poly(silicate)-metabolizing silicatein in siliceous spicules and silicasomes of demosponges comprises dual enzymatic activities (silica-polymerase and silica-esterase). FEBS J. 2008;275:362–370.
73. Wang XH, Schröder HC, Brandt D, et al. Sponge biosilica formation involves syneresis following polycondensation in vivo. Chembiochem. 2011;12(15):2316–2324. doi:10.1002/cbic.201100345
74. Belton DJ, Deschaume O, Patwardhan SV, Perry CC. A solution study of silica condensation and speciation with relevance to in vitro investigations of biosilicification. J Phys Chem B. 2010;114(31):9947–9955. doi:10.1021/jp101347q
75. Belton DJ, Deschaume O, Perry CC. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012;279(10):1710–1720. doi:10.1111/j.1742-4658.2012.08531.x
76. Cha JN, Shimizu K, Zhou Y, et al. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc Natl Acad Sci USA. 1999;96:361–365.
77. Müller WEG, Belikov SI, Tremel W, et al. Siliceous spicules in marine demosponges (example Suberites domuncula). Micron. 2006;37(2):107–120. doi:10.1016/j.micron.2005.09.003
78. Schröder HC, Wang XH, Manfrin A, et al. Silicatein: acquisition of structure-guiding and structure-forming properties during maturation from the pro-silicatein to the silicatein form. J Biol Chem. 2012;287(26):22196–22205. doi:10.1074/jbc.M112.351486
79. Stöber W, Fink A, Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci. 1968;26(1):62–69. doi:10.1016/0021-9797(68)90272-5
80. Müller WEG, Boreiko A, Wang XH, et al. Morphogenetic activity of silica and bio-silica on the expression of genes, controlling biomineralization using SaOS-2 cells. Calcif Tissue Int. 2007;81(5):382–393. doi:10.1007/s00223-007-9075-4
81. Görlich S, Pawolski D, Zlotnikov I, Kröger N. Control of biosilica morphology and mechanical performance by the conserved diatom gene Silicanin-1. Commun Biol. 2019;2(1):245. doi:10.1038/s42003-019-0436-0
82. Ege D, Zheng K, Boccaccini AR. Borate Bioactive Glasses (BBG): bone regeneration, wound healing applications, and future directions. ACS Appl Bio Mater. 2022;5(8):3608–3622. doi:10.1021/acsabm.2c00384
83. Wang SF, Wang XH, Draenert FG, et al. Bioactive and biodegradable silica biomaterial for bone regeneration. Bone. 2014;67:292–304. doi:10.1016/j.bone.2014.07.025
84. Carlisle EM. Silicon: an essential element for the chick. Science. 1972;178(4061):619–621. doi:10.1126/science.178.4061.619
85. Burton EJ, Scholey DV, Belton DJ, Bedford MR, Perry CC. Efficacy and stability of a novel silica supplement for improving bone development in broilers. Br Poult Sci. 2020;61(6):719–724. doi:10.1080/00071668.2020.1799328
86. Wiens M, Wang XH, Schloßmacher U, et al. Osteogenic potential of bio-silica on human osteoblast-like (SaOS-2) cells. Calcif Tissue Int. 2010;87(6):513–524. doi:10.1007/s00223-010-9408-6
87. Pettersson LF, Kingham PJ, Wiberg M, Kelk P. In vitro osteogenic differentiation of human mesenchymal stem cells from jawbone compared with dental tissue. Tissue Eng Regen Med. 2017;14:763–774.
88. Pautke C, Vogt S, Kreutzer K, et al. Characterization of eight different tetracyclines: advances in fluorescence bone labeling. J Anat. 2010;217(1):76–82. doi:10.1111/j.1469-7580.2010.01237.x
89. Hench LL. Bioceramics. J Am Ceram Soc. 1998;81(7):1705–1728. doi:10.1111/j.1151-2916.1998.tb02540.x
90. Wang Y, Jiang S, Pan H, Tang R. Less is more: silicate in the crystallization of hydroxyapatite in simulated body fluids. CrystEngComm. 2016;18(3):379–383. doi:10.1039/C5CE01861G
91. Kellermeier M, Melero-García E, Glaab F, et al. Stabilization of amorphous calcium carbonate in inorganic silica-rich environments. J Am Chem Soc. 2010;132(50):17859–17866. doi:10.1021/ja106959p
92. Sahai N. Modeling apatite nucleation in the human body and in the geochemical environment. Am J Sci. 2000;305(6–8):661–672. doi:10.2475/ajs.305.6-8.661
93. Sahai N, Tossell JA. Molecular orbital study of apatite (Ca5(PO4)3OH) nucleation at silica bioceramic surfaces. J Phys Chem B. 2000;104(18):4321–4322. doi:10.1021/jp9935889
94. Salih E, Flückiger R. Complete topographical distribution of both the in vivo and in vitro phosphorylation sites of bone sialoprotein and their biological significance. J Biol Chem. 2004;279(19):19808–19815. doi:10.1074/jbc.M310299200
95. Wysokowski M, Jesionowski T, Ehrlich H. Biosilica as a source for inspiration in biological materials science. Amer Mineral. 2018;103:665–691.
96. Lorenz B, Batel R, Bachinski N, Müller WEG, Schröder HC. Purification and characterization of two exopolyphosphatases from the marine sponge Tethya lyncurium. Biochim Biophys Acta. 1995;1245(1):17–28. doi:10.1016/0304-4165(95)00067-L
97. Kulaev IS. Biochemistry of inorganic polyphosphates. Rev Physiol Biochem Pharmacol. 1975;73:131–158.
98. Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270(11):5818–5822. doi:10.1074/jbc.270.11.5818
99. Achbergerová L, Nahálka J. Polyphosphate--an ancient energy source and active metabolic regulator. Microb Cell Fact. 2011;10(1):63. doi:10.1186/1475-2859-10-63
100. Desfougères Y, Saiardi A, Azevedo C. Inorganic polyphosphate in mammals: where’s Wally?. Biochem Soc Trans. 2020;48(1):95–101. doi:10.1042/BST20190328
101. Docampo R, Huang G. Acidocalcisomes of eukaryotes. Curr Opin Cell Biol. 2016;41:66–72. doi:10.1016/j.ceb.2016.04.007
102. Lander N, Cordeiro C, Huang G, Docampo R. Polyphosphate and acidocalcisomes. Biochem Soc Trans. 2016;44(1):1–6. doi:10.1042/BST20150193
103. Babes V. Beobachtungen über die metachromatischen Körperchen, Sporenbildung, Verzweigung, Kolben- und Kapselbildung pathogener Bakterien. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1895;20:412–420.
104. Meyer A. Orientierende Untersuchungen über Verbreitung, Morphologie, und Chemie des Volutins. Bot Zeit. 1904;62:113–152.
105. Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279(43):44250–44257. doi:10.1074/jbc.M406261200
106. Langen P, Hucho F. Karl Lohmann and the discovery of ATP. Angew Chem Int Ed Engl. 2008;47(10):1824–1827. doi:10.1002/anie.200702929
107. Langen P, Liss E, Lohmann K. Art, Bildung und Umsatz der Polyphosphate der Hefe [Formation and turnover of polyphosphates in yeast]. In: Jacquinot P, editor. Acides Ribonucléiques Et Polyphosphates: Structure, Synthèse Et Fonctions. CNRS No 105. Paris: Centre National de la Recherche Scientifique; 1962:604–614.
108. Lohmann K. Über das Vorkommen, und den Umsatz von Pyrophosphat in Zellen. I. Mitteilung: nachweis und Isolierung des Pyrophosphates. Biochem Z. 1928;202:466–493.
109. Bowlin MQ, Gray MJ. Inorganic polyphosphate in host and microbe biology. Trends Microbiol. 2021;29(11):1013–1023. doi:10.1016/j.tim.2021.02.002
110. Hooley P, Whitehead MP, Brown MR. Eukaryote polyphosphate kinases: is the ‘Kornberg’ complex ubiquitous?. Trends Biochem Sci. 2008;33(12):577–582. doi:10.1016/j.tibs.2008.09.007
111. Pavlov E, Zakharian E, Bladen C, et al. A large, voltage-dependent channel, isolated from mitochondria by water-free chloroform extraction. Biophys J. 2005;88(4):2614–2625. doi:10.1529/biophysj.104.057281
112. Pavlov E, Aschar-Sobbi R, Campanella M, Turner RJ, Gomez-Garcia MR, Abramov AY. Inorganic polyphosphate and energy metabolism in mammalian cells. J Biol Chem. 2010;285(13):9420–9428. doi:10.1074/jbc.M109.013011
113. Abramov AY, Fraley C, Diao CT, et al. Targeted polyphosphatase expression alters mitochondrial metabolism and inhibits calcium-dependent cell death. Proc Natl Acad Sci USA. 2007;104(46):18091–18096. doi:10.1073/pnas.0708959104
114. Budavari S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals.
115. Soga N, Kinosita K, Yoshida M, Suzuki T. Efficient ATP synthesis by thermophilic Bacillus Fo F1-ATP synthase. FEBS J. 2011;278(15):2647–2654. doi:10.1111/j.1742-4658.2011.08191.x
116. Hothorn M, Neumann H, Lenherr ED, et al. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science. 2009;324(5926):513–516. doi:10.1126/science.1168120
117. Guan Z, Chen J, Liu R, et al. The cytoplasmic synthesis and coupled membrane translocation of eukaryotic polyphosphate by signal-activated VTC complex. Nat Commun. 2023;14(1):718. doi:10.1038/s41467-023-36466-4
118. Verhoef JJ, Barendrecht AD, Nickel KF, et al. Polyphosphate nanoparticles on the platelet surface trigger contact system activation. Blood. 2017;129(12):1707–1717. doi:10.1182/blood-2016-08-734988
119. Morrissey JH, Smith SA. Polyphosphate as modulator of hemostasis, thrombosis, and inflammation. J Thromb Haemost. 2015;13(Suppl 1):S92–S97. doi:10.1111/jth.12896
120. Polasek J. Platelet lysosomal acid phosphatase enzyme activity as a marker of platelet procoagulant activity. Blood Transfus. 2009;7(2):155–156. doi:10.2450/2008.0053-08
121. Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the nucleotide axis hypothesis: ATP release and metabolism by coronary endothelium. Am J Physiol Heart Circ Physiol. 2001;281(4):H1657–H1666. doi:10.1152/ajpheart.2001.281.4.H1657
122. Weitz JI, Fredenburgh JC. Platelet polyphosphate: the long and the short of it. Blood. 2017;129(12):1574–1575. doi:10.1182/blood-2017-01-761593
123. Sharda A, Flaumenhaft R. The life cycle of platelet granules. F1000Res. 2018;7:236. doi:10.12688/f1000research.13283.1
124. Tyagi T, Jain K, Gu SX, et al. A guide to molecular and functional investigations of platelets to bridge basic and clinical sciences. Nat Cardiovasc Res. 2022;1(3):223–237. doi:10.1038/s44161-022-00021-z
125. Lorenz B, Leuck J, Köhl D, Müller WEG, Schröder HC. Anti-HIV-1 activity of inorganic polyphosphates. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(2):110–118. doi:10.1097/00042560-199702010-00003
126. Lorenz B, Schröder HC. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim Biophys Acta. 2001;1547(2):254–261. doi:10.1016/S0167-4838(01)00193-5
127. Ho-Tin-Noé B, Boulaftali Y, Camerer E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood. 2018;131(3):277–288. doi:10.1182/blood-2017-06-742676
128. Harrison P, Alsousou J, Andia I; Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1895–1900. doi:10.1111/jth.14223
129. Bizzozero J. Ueber einen neuen Formbestandtheil des Blutes und dessen Rolle bei der Thrombose und der Blutgerinnung. Virchow’s Arch Pathol Anat Physiol Klin Med. 1882;90(2):261–332. doi:10.1007/BF01931360
130. Roewe J, Stavrides G, Strueve M, et al. Bacterial polyphosphates interfere with the innate host defense to infection. Nat Commun. 2020;11(1):4035. doi:10.1038/s41467-020-17639-x
131. Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100(12):1673–1685. doi:10.1161/01.RES.0000267878.97021.ab
132. Jackson LE, Kariuki BM, Smith ME, Barralet JE, Wright AJ. Synthesis and structure of a calcium polyphosphate with an unique criss-cross arrangement of helical phosphate chains. Chem Mater. 2005;17(18):4642–4646. doi:10.1021/cm050984x
133. Shiba T, Nishimura D, Kawazoe Y, et al. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J Biol Chem. 2003;278(29):26788–26792. doi:10.1074/jbc.M303468200
134. Lorenz B, Marmé S, Müller WEG, Unger K, Schröder HC. Preparation and use of polyphosphate-modified zirconia for purification of nucleic acids and proteins. Anal Biochem. 1994;16(1):118–126. doi:10.1006/abio.1994.1015
135. Müller WEG, Tolba E, Schröder HC, et al. A new polyphosphate calcium material with morphogenetic activity. Mater Lett. 2015;148:166. doi:10.1016/j.matlet.2015.02.070
136. Wang XH, Ackermann M, Tolba E, et al. Artificial cartilage bio-matrix formed of hyaluronic acid and Mg2+-polyphosphate. Eur Cell Mater. 2016;32:271–283. doi:10.22203/eCM.v032a18
137. Müller WEG, Tolba E, Ackermann M, et al. Fabrication of amorphous strontium polyphosphate microparticles that induce mineralization of bone cells in vitro and in vivo. Acta Biomater. 2017;50:89–101. doi:10.1016/j.actbio.2016.12.045
138. Müller WEG, Neufurth M, Wang SF, et al. Amorphous, smart, and bioinspired polyphosphate nano/microparticles: a biomaterial for regeneration and repair of osteo-articular impairments in-situ. Int J Mol Sci. 2018;19(2):427. doi:10.3390/ijms19020427
139. Müller WEG, Schröder HC, Neufurth M, Wang XH. An unexpected biomaterial against SARS-CoV-2: bio-polyphosphate blocks binding of the viral spike to the cell receptor. Mater Today. 2021;51:504–524. doi:10.1016/j.mattod.2021.07.029
140. Neufurth M, Wang XH, Tolba E, et al. The inorganic polymer, polyphosphate, blocks binding of SARS-CoV-2 spike protein to ACE2 receptor at physiological concentrations. Biochem Pharmacol. 2020;182:114215. doi:10.1016/j.bcp.2020.114215
141. Ferrucci V, Kong DY, Asadzadeh F, et al. Long-chain polyphosphates impair SARS-CoV-2 infection and replication. Sci Signal. 2021;14(690):eabe5040. doi:10.1126/scisignal.abe5040
142. Wang XH, Schröder HC, Müller WEG. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: towards a new paradigm in tissue engineering. J Mat Chem B. 2018;6(16):2385–2412. doi:10.1039/C8TB00241J
143. Müller WEG, Wang SF, Tolba E, et al. Transformation of amorphous polyphosphate nanoparticles into coacervate complexes: an approach for the encapsulation of mesenchymal stem cells. Small. 2018;14(27):1801170. doi:10.1002/smll.201801170
144. Müller WEG, Neufurth M, Lieberwirth I, Wang SF, Schröder HC, Wang XH. Functional importance of coacervation to convert calcium polyphosphate nanoparticles into the physiologically active state. Mater Today Bio. 2022;16:100404. doi:10.1016/j.mtbio.2022.100404
145. Fernandes-Cunha GM, McKinlay CJ, Vargas JR, Jessen HJ, Waymouth RM, Wender PA. Delivery of inorganic polyphosphate into cells using amphipathic oligocarbonate transporters. ACS Cent Sci. 2018;4(10):1394–1402. doi:10.1021/acscentsci.8b00470
146. Barbeck M, Alkildani S, Jung O. Editorial of the Special Issue: “Soft and Hard Tissue Regeneration”. Biomedicines. 2022;10(2):356. doi:10.3390/biomedicines10020356
147. Lehn JM. Supramolecular chemistry: receptors, catalysts, and carriers. Science. 1985;227(4689):849–856. doi:10.1126/science.227.4689.849
148. Fasting C, Schalley CA, Weber M, et al. Multivalency as a chemical organization and action principle. Angew Chem Int Ed Engl. 2012;51(42):10472–10498. doi:10.1002/anie.201201114
149. Müller WEG, Neufurth M, Wang S, Tolba E, Schröder HC, Wang XH. Morphogenetically active scaffold for osteochondral repair (polyphosphate/alginate/N,O-carboxymethyl chitosan). Eur Cell Mater. 2016;31:174–190. doi:10.22203/eCM.v031a12
150. Müller WEG, Neufurth M, Ackermann M, et al. Fabrication of a new physiological macroporous hybrid biomaterial/bioscaffold material based on polyphosphate and collagen by freeze-extraction. J Mat Chem B. 2017;5(21):3823–3835. doi:10.1039/C7TB00306D
151. Tolba E, Wang XH, Ackermann M, et al. In-situ polyphosphate nanoparticle formation in hybrid poly(vinyl alcohol)/karaya gum-hydrogels: a porous scaffold inducing infiltration of mesenchymal stem cells. Adv Sci. 2018;2018:1801452.
152. Neufurth M, Wang SF, Schröder HC, Al-Nawas B, Wang XH, Müller WEG. 3D bioprinting of tissue units with mesenchymal stem cells, retaining their proliferative and differentiating potential, in polyphosphate-containing bio-ink. Biofabrication. 2022;14(1):015016. doi:10.1088/1758-5090/ac3f29
153. Krieg E, Bastings MM, Besenius P, Rybtchinski B. Supramolecular polymers in aqueous media. Chem Rev. 2016;116(4):2414–2477. doi:10.1021/acs.chemrev.5b00369
154. Samperi M, Pérez-García L, Amabilino DB. Quantification of energy of activation to supramolecular nanofibre formation reveals enthalpic and entropic effects and morphological consequence. Chem Sci. 2019;10(44):10256–10266. doi:10.1039/C9SC03280K
155. Harris JR, Lewis RJ. The collagen type I segment long spacing (SLS) and fibrillar forms: formation by ATP and sulphonated diazo dyes. Micron. 2016;86:36–47. doi:10.1016/j.micron.2016.04.008
156. Trautmann A. Extracellular ATP in the immune system: more than just a “danger signal”. Sci Signal. 2009;2(56):pe6. doi:10.1126/scisignal.256pe6
157. Huang H, Zhang X, Li S, et al. Physiological levels of ATP negatively regulate proteasome function. Cell Res. 2010;20(12):1372–1385. doi:10.1038/cr.2010.123
158. Schenk U, Westendorf AM, Radaelli E, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1(39):ra6. doi:10.1126/scisignal.1160583
159. Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29(6):556–568. doi:10.1080/09537104.2018.1430357
160. Swieringa F, Spronk HMH, Heemskerk JWM, van der Meijden PEJ. Integrating platelet and coagulation activation in fibrin clot formation. Res Pract Thromb Haemost. 2018;2(3):450–460. doi:10.1002/rth2.12107
161. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi:10.1089/107632701300062859
162. Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi:10.1016/j.actbio.2017.01.036
163. Laverdet B, Micallef L, Lebreton C, et al. Use of mesenchymal stem cells for cutaneous repair and skin substitute elaboration. Pathol Biol. 2014;62(2):108–117. doi:10.1016/j.patbio.2014.01.002
164. Leyhausen G, Lorenz B, Zhu H, et al. Inorganic polyphosphate in human osteoblast-like cells. J Bone Mineral Res. 1998;13(5):803–812. doi:10.1359/jbmr.1998.13.5.803
165. Schröder HC, Kurz L, Müller WEG, Lorenz B. Polyphosphate in bone. Biochemistry. 2000;65(3):296–303.
166. Morimoto D, Tomita T, Kuroda S, et al. Inorganic polyphosphate differentiates human mesenchymal stem cells into osteoblastic cells. J Bone Miner Metab. 2010;28(4):418–423. doi:10.1007/s00774-010-0157-4
167. Müller WEG, Tolba E, Schröder HC, Muñoz-Espí R, Diehl-Seifert B, Wang XH. Amorphous polyphosphate-hydroxyapatite: a morphogenetically active substrate for bone-related SaOS-2 cells in vitro. Acta Biomater. 2016;31:358–367. doi:10.1016/j.actbio.2015.11.060
168. Bolander J, Ji W, Geris L, et al. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells. Eur Cell Mater. 2016;31:11–25. doi:10.22203/eCM.v031a02
169. Morrissey JH, Choi SH, Smith SA. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012;119(25):5972–5979. doi:10.1182/blood-2012-03-306605
170. Hill T, Morales M. On “High energy phosphate bonds” of biochemical interest 1. J Am Chem Soc. 1951;73(4):1656–1660. doi:10.1021/ja01148a072
171. Lipman F. Metabolic generation and utilization of phosphate bond energy. In: Nord FF, Werkman CH, editors. Advances in Enzymology and Related Subjects of Biochemistry. Vol. 1. New York: Interscience Publishers Inc; 1941:99–162.
172. Hessle L, Johnson KA, Anderson HC, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA. 2002;99(14):9445–9449. doi:10.1073/pnas.142063399
173. Klepinin A, Zhang S, Klepinina L, et al. Adenylate kinase and metabolic signaling in cancer cells. Front Oncol. 2020;10:660. doi:10.3389/fonc.2020.00660
174. Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD, Giniger E. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS One. 2012;7(8):e42988. doi:10.1371/journal.pone.0042988
175. Tagliabracci VS, Xiao J, Dixon JE. Phosphorylation of substrates destined for secretion by the Fam20 kinases. Biochem Soc Trans. 2013;41(4):1061–1065. doi:10.1042/BST20130059
176. Tagliabracci VS, Wiley SE, Guo X, et al. A single kinase generates the majority of the secreted phosphoproteome. Cell. 2015;161(7):1619–1632. doi:10.1016/j.cell.2015.05.028
177. Raine J, Winter RM, Davey A, Tucker SM. Unknown syndrome: microcephaly, hypoplastic nose, exophthalmos, gum hyperplasia, cleft palate, low set ears, and osteosclerosis. J Med Genet. 1989;26(12):786–788. doi:10.1136/jmg.26.12.786
178. Lowenstam HA. Minerals formed by organisms. Science. 1981;211(4487):1126–1131. doi:10.1126/science.7008198
179. Ruben JA, Bennett AA. The evolution of bone. Evolution. 1987;41(6):1187–1197. doi:10.2307/2409087
180. Mann S, Parker SB, Ross MD, Skarnulis AJ, Williams RJ. The ultrastructure of the calcium carbonate balance organs of the inner ear: an ultra-high resolution electron microscopy study. Proc R Soc Lond B Biol Sci. 1983;218:415–424.
181. Setiawati R, Rahardjo P. Bone development and growth. In: Yang HS, editor. Osteogenesis and Bone Regeneration. Intechopen; 2019. doi:10.5772/intechopen.82452
182. Müller WEG, Neufurth M, Huang J, et al. Nonenzymatic transformation of amorphous CaCO3 into calcium phosphate mineral after exposure to sodium phosphate in vitro: implications for in vivo hydroxyapatite bone formation. ChemBioChem. 2015;16(9):1323–1332. doi:10.1002/cbic.201500057
183. Tadier S, Rokidi S, Rey C, Combes C, Koutsoukos PG. Crystal growth of aragonite in the presence of phosphate. J Crystal Growth. 2017;458:44–52. doi:10.1016/j.jcrysgro.2016.10.046
184. Boskey AL, Guidon P, Doty SB, Stiner D, Leboy P, Binderman I. The mechanism of β-glycerophosphate action in mineralizing chick limb-bud mesenchymal cell cultures. J Bone Miner Res. 1996;11(11):1694–1702. doi:10.1002/jbmr.5650111113
185. Sharif PS, Abdollahi M. The role of platelets in bone remodeling. Inflamm Allergy Drug Targets. 2010;9(5):393–399. doi:10.2174/187152810793938044
186. Müller WEG, Wang XH, Diehl-Seifert B, et al. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011;7(6):2661–2671. doi:10.1016/j.actbio.2011.03.007
187. Khersonsky O, Tawfik DS. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu Rev Biochem. 2010;79(1):471–505. doi:10.1146/annurev-biochem-030409-143718
188. Lotsari A, Rajasekharan AK, Halvarsson M, Andersson M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat Commun. 2018;9(1):4170. doi:10.1038/s41467-018-06570-x
189. Wang XH, Wang SF, He F, et al. Polyphosphate as a bioactive and biodegradable implant material: induction of bone regeneration in rats. Adv Engin Mat. 2016;18(8):1406–1417. doi:10.1002/adem.201600057
190. Meldrum FC, Cölfen H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem Rev. 2008;108(11):4332–4432. doi:10.1021/cr8002856
191. Mayr-Wohlfart U, Waltenberger J, Hausser H, et al. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30(3):472–477. doi:10.1016/S8756-3282(01)00690-1
192. Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25(18):4489–4502. doi:10.1016/j.biomaterials.2003.11.013
193. Marenzana M, Arnett TR. The key role of the blood supply to bone. Bone Res. 2013;1(3):203–215. doi:10.4248/BR201303001
194. Bilgen F, Ural A, Bekerecioglu M. Platelet-rich fibrin: an effective chronic wound healing accelerator. J Tissue Viability. 2021;30(4):616–620. doi:10.1016/j.jtv.2021.04.009
195. Schultz GS, Chin GA, Moldawer L, Diegelmann RF. Principles of wound healing. In: Fitridge R, Thompson M, editors. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists, Chapter 23. Adelaide (AU): University of Adelaide Press; 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534261.
196. Rodriguez-Diaz A, Toyama Y, Abravanel DL, et al. Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. HFSP J. 2008;2(4):220–237. doi:10.2976/1.2955565
197. Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol. 2001;3(5):E117–E123. doi:10.1038/35074643
198. Santamaría R, González-álvarez M, Delgado R, Esteban S, Arroyo AG. Remodeling of the microvasculature: may the blood flow be with you. Front Physiol. 2020;11:586852. doi:10.3389/fphys.2020.586852
199. Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615(1–2):7–32. doi:10.1016/S0005-2736(03)00210-4
200. De Bock K, Georgiadou M, Schoors S, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154(3):651–663. doi:10.1016/j.cell.2013.06.037
201. Leung SWS, Shi Y. The glycolytic process in endothelial cells and its implications. Acta Pharmacol Sin. 2022;43(2):251–259. doi:10.1038/s41401-021-00647-y
202. Spampinato SF, Caruso GI, De Pasquale R, Sortino MA, Merlo S. The treatment of impaired wound healing in diabetes: looking among old drugs. Pharmaceuticals. 2020;13(4):60. doi:10.3390/ph13040060
203. Sreekumar R, Halvatsiotis P, Schimke JC, Nair KS. Gene expression profile in skeletal muscle of type 2 diabetes and the effect of insulin treatment. Diabetes. 2002;51(6):1913–1920. doi:10.2337/diabetes.51.6.1913
204. Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100(14):8466–8471. doi:10.1073/pnas.1032913100
205. Sarojini H, Bajorek A, Wan R, et al. Enhanced skin incisional wound healing with intracellular ATP delivery via macrophage proliferation and direct collagen production. Front Pharmacol. 2021;12:594586. doi:10.3389/fphar.2021.594586
206. Yang JY, Chen CC, Chang SC, et al. ENERGI-F703 gel, as a new topical treatment for diabetic foot and leg ulcers: a multicenter, randomized, double-blind, Phase II trial. EClinicalMedicine. 2022;51:101497. doi:10.1016/j.eclinm.2022.101497
207. Müller WEG, Relkovic D, Ackermann M, et al. Enhancement of wound healing in normal and diabetic mice by topical application of amorphous polyphosphate. Polymers. 2017;9(12):300. doi:10.3390/polym9070300
208. Stan D, Tanase C, Avram M, et al. Wound healing applications of creams and “smart” hydrogels. Exp Dermatol. 2021;30(9):1218–1232. doi:10.1111/exd.14396
209. Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. J Cell Physiol. 2005;203(3):465–470. doi:10.1002/jcp.20270
210. Budharaju H, Suresh S, Sekar MP, et al. Ceramic materials for 3D printing of biomimetic bone scaffolds – current state-of-The-art & future perspectives. Mater Des. 2023;231:112064. doi:10.1016/j.matdes.2023.112064
211. Ananth KP, Jayram ND. A comprehensive review of 3D printing techniques for biomaterial-based scaffold fabrication in bone tissue engineering. Ann 3D Print Med. 2024;13:100141. doi:10.1016/j.stlm.2023.100141
212. Budharaju H, Sundaramurthi D, Sethuraman S. Embedded 3D bioprinting – an emerging strategy to fabricate biomimetic & large vascularized tissue constructs. Bioact Mater. 2024;32:356–384. doi:10.1016/j.bioactmat.2023.10.012
213. Müller WEG, Schröder HC, Feng QL, Schlossmacher U, Link T, Wang XH. Development of a morphogenetically active scaffold for three-dimensional growth of bone cells: biosilica-alginate hydrogel for SaOS-2 cell cultivation. J Tissue Eng Regen Med. 2015;9(11):E39–E50. doi:10.1002/term.1745
214. Neufurth M, Wang XH, Wang SF, et al. 3D printing of hybrid biomaterials for bone tissue engineering: calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017;64:377–388. doi:10.1016/j.actbio.2017.09.031
215. Neufurth M, Wang XH, Schröder HC, et al. Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells. Biomaterials. 2014;35(31):8810–8819. doi:10.1016/j.biomaterials.2014.07.002
216. Müller WEG, Tolba E, Schröder HC, Diehl-Seifert B, Link T, Wang XH. Biosilica-loaded poly(ϵ-caprolactone) nanofibers mats provide a morphogenetically active surface scaffold for the growth and mineralization of the osteoclast-related SaOS-2 cells. Biotechnol J. 2014;9(10):1312–1321. doi:10.1002/biot.201400277
217. Müller WEG, Tolba E, Dorweiler B, Schröder HC, Diehl-Seifert B, Wang XH. Electrospun bioactive mats enriched with Ca-polyphosphate/retinol nanospheres as potential wound dressing. Biochem Biophys Rep. 2015;3:150–160. doi:10.1016/j.bbrep.2015.08.007
218. Wang XH, Ackermann M, Wang SF, et al. Amorphous polyphosphate/amorphous calcium carbonate implant material with enhanced bone healing efficacy in a critical-size defect in rats. Biomed Mater. 2016;11(3):035005. doi:10.1088/1748-6041/11/3/035005
219. Schloßmacher U, Schröder HC, Wang XH, et al. Alginate/silica composite hydrogel as a potential morphogenetically active scaffold for three-dimensional tissue engineering. RSC Adv. 2013;3(28):11185–11194. doi:10.1039/c3ra23341c
220. Braun S, Rappoport S, Zusman R, Avnir D, Ottolenghi M. Biochemically active sol-gel glasses: the trapping of enzymes. Mater Lett. 1990;10(1–2):1–5. doi:10.1016/0167-577X(90)90002-4
221. Nassif N, Roux C, Coradin T, Rager MN, Bouvet OMM, Livage J. A sol–gel matrix to preserve the viability of encapsulated bacteria. J Mat Chem. 2003;13(2):203–208. doi:10.1039/b210167j
222. Müller WEG, Engel S, Wang XH, et al. Bioencapsulation of living bacteria (Escherichia coli) with poly(silicate) after transformation with silicatein-α gene. Biomaterials. 2008;29(7):771–779. doi:10.1016/j.biomaterials.2007.10.038
223. Müller WEG, Wang XH, Proksch P, et al. Principles of biofouling protection in marine sponges: a model for the design of novel biomimetic and bio-inspired coatings in the marine environment?. Mar Biotechnol. 2013;15(4):375–398. doi:10.1007/s10126-013-9497-0
224. Müller WEG, Tolba E, Schröder HC, et al. A new printable and durable N,O-carboxymethyl chitosan-Ca2+ -polyphosphate complex with morphogenetic activity. J Mat Chem B. 2015;3(8):1722–1730. doi:10.1039/C4TB01586J
225. Wang SF, Neufurth M, Schepler H, et al. Acceleration of wound healing through amorphous calcium carbonate, stabilized with high-energy polyphosphate. Pharmaceutics. 2023;15(2):494. doi:10.3390/pharmaceutics15020494
226. Ackermann M, Tolba E, Neufurth M, et al. Biomimetic transformation of polyphosphate microparticles during restoration of damaged teeth. Dent Mater. 2019;35(2):244–256. doi:10.1016/j.dental.2018.11.014
227. Müller WEG, Neufurth M, Tolba E, et al. A biomimetic approach to ameliorate dental hypersensitivity by amorphous polyphosphate microparticles. Dent Mater. 2016;32(6):775–783. doi:10.1016/j.dental.2016.03.027
228. Müller WEG, Tolba E, Wang SF, et al. Nanoparticle-directed and ionically forced polyphosphate coacervation: a versatile and reversible core-shell system for drug delivery. Sci Rep. 2020;10(1):17147. doi:10.1038/s41598-020-73100-5
229. Neufurth M, Wang XH, Tolba E, et al. Modular small diameter vascular grafts with bioactive functionalities. PLoS One. 2015;10(7):e0133632. doi:10.1371/journal.pone.0133632
230. Ackermann M, Wang XH, Wang SF, et al. Collagen-inducing biologization of prosthetic material for hernia repair: polypropylene meshes coated with polyP/collagen. J Biomed Mater Res B. 2018;106(6):2109–2121. doi:10.1002/jbm.b.34016
231. Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6(10):1750–1756. doi:10.1111/j.1538-7836.2008.03104.x
232. Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci USA. 2006;103(4):903–908. doi:10.1073/pnas.0507195103
233. Moon JH, Park JH, Lee JY. Antibacterial Action of Polyphosphate on Porphyromonas gingivalis. Antimicrob Agents Chemother. 2011;55(2):806–812. doi:10.1128/AAC.01014-10
234. Wiens M, Elkhooly TA, Schröder HC, Mohamed TH, Müller WEG. Characterization and osteogenic activity of a silicatein/biosilica-coated chitosan-graft-polycaprolactone. Acta Biomater. 2014;10(10):4456–4464. doi:10.1016/j.actbio.2014.06.036
235. Phang JM, Liu W, Zabirnyk O. Proline metabolism and microenvironmental stress. Annu Rev Nutr. 2010;30(1):441–463. doi:10.1146/annurev.nutr.012809.104638
236. Zhou Z, Fan Y, Jiang Y, et al. Mineralized enzyme-based biomaterials with superior bioactivities for bone regeneration. ACS Appl Mater Interfaces. 2022;14(32):36315–36330. doi:10.1021/acsami.2c05794
237. Yang J, Ueharu H, Mishina Y. Energy metabolism: a newly emerging target of BMP signaling in bone homeostasis. Bone. 2020;138:115467. doi:10.1016/j.bone.2020.115467
238. Imsiecke G, Münkner J, Lorenz B, Bachinski N, Müller WEG, Schröder HC. Inorganic polyphosphates in the developing freshwater sponge Ephydatia muelleri: effect of stress by polluted waters. Environ Toxicol Chem. 1996;15(8):1329–1334. doi:10.1002/etc.5620150811
239. Müller WEG, Wang XH, Sinha B, Wiens M, Schröder HC, Jochum KP. NanoSIMS: insights into the organization of the proteinaceous scaffold within hexactinellid sponge spicules. ChemBioChem. 2010;11(8):1077–1082. doi:10.1002/cbic.201000078
240. Hench LL, Splinter RJ, Allen WC, Greenlee TK. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res Symp. 1971;5:117–141.
241. Zhu H, Zheng K, Boccaccini AR. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021;129:1–17. doi:10.1016/j.actbio.2021.05.007
242. Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17(11):967–978. doi:10.1007/s10856-006-0432-z
243. Rahaman MN, Day DE, Bal BS, et al. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355–2373. doi:10.1016/j.actbio.2011.03.016
244. Moffa M, Camposeo A, Fasano V, et al. Biomineral amorphous lasers through light-scattering surfaces assembled by electrospun fiber templates. Laser Photonics Rev. 2018;12(1):1700224. doi:10.1002/lpor.201700224
245. Szwed-Georgiou A, Płociński P, Kupikowska-Stobba B, et al. Bioactive materials for bone regeneration: biomolecules and delivery systems. ACS Biomater Sci Eng. 2023;9(9):5222–5254. doi:10.1021/acsbiomaterials.3c00609
246. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy?. Arch Dermatol Res. 2007;298(9):413–420. doi:10.1007/s00403-006-0713-x
247. Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. Br J Radiol. 2003;76(Spec No 1):S11–S22. doi:10.1259/bjr/12913493
248. Yan GX, Kléber AG. Changes in extracellular and intracellular pH in ischemic rabbit papillary muscle. Circ Res. 1992;71(2):460–470. doi:10.1161/01.RES.71.2.460
249. Gan Q, Zhu J, Yuan Y, Liu H, Zhu Y, Liu C. A proton-responsive ensemble using mesocellular foam supports capped with N,O-carboxymethyl chitosan for controlled release of bioactive proteins. J Mater Chem B. 2015;3(11):2281–2285. doi:10.1039/C5TB00219B
250. Tao B, Deng Y, Song L, et al. BMP2-loaded titania nanotubes coating with pH-responsive multilayers for bacterial infections inhibition and osteogenic activity improvement. Colloids Surf B Biointerfaces. 2019;177:242–252. doi:10.1016/j.colsurfb.2019.02.014
251. Banerjee I, Mishra D, Das T, Maiti TK. Wound pH-responsive sustained release of therapeutics from a poly(NIPAAm-co-AAc) hydrogel. J Biomater Sci Polym Ed. 2012;23(1–4):111–132. doi:10.1163/092050610X545049
252. Schäcke H, Müller IM, Müller WEG. Tyrosine kinase from the marine sponge Geodia cydonium: the oldest member belonging to the receptor tyrosine kinase class II family. In: Müller WEG, editor. Use of Aquatic Invertebrates as Tools for Monitoring of Environmental Hazards. Stuttgart, New York: Gustav Fischer Verlag; 1994:201–211.
253. Schopf JW. Microfossils of the early Archean apex chert: new evidence of the antiquity of life. Science. 1993;260(5108):640–646. doi:10.1126/science.260.5108.640
254. Müller WEG, Brümmer F, Batel R, Müller IM, Schröder HC. Molecular biodiversity. Case study: porifera (sponges). Naturwissenschaften. 2003;90(3):103–120. doi:10.1007/s00114-003-0407-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.