Back to Journals » Infection and Drug Resistance » Volume 16
The Oral Lesion in the COVID-19 Patient: Is It True Oral Manifestation or Not?
Authors Sarasati A , Agustina D, Surboyo MDC
Received 28 March 2023
Accepted for publication 21 June 2023
Published 4 July 2023 Volume 2023:16 Pages 4357—4385
DOI https://doi.org/10.2147/IDR.S411615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Andari Sarasati,1 Dewi Agustina,2 Meircurius Dwi Condro Surboyo3
1Faculty of Dentistry, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia; 2Department of Oral Medicine, Faculty of Dentistry, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia; 3Department of Oral Medicine, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, 60132, Indonesia
Correspondence: Dewi Agustina, Department of Oral Medicine, Faculty of Dentistry, Universitas Gadjah Mada, Jalan Denta No. 1, Sekip Utara, Yogyakarta, 55281, Indonesia, Tel/Fax +6274-515307, Email [email protected]
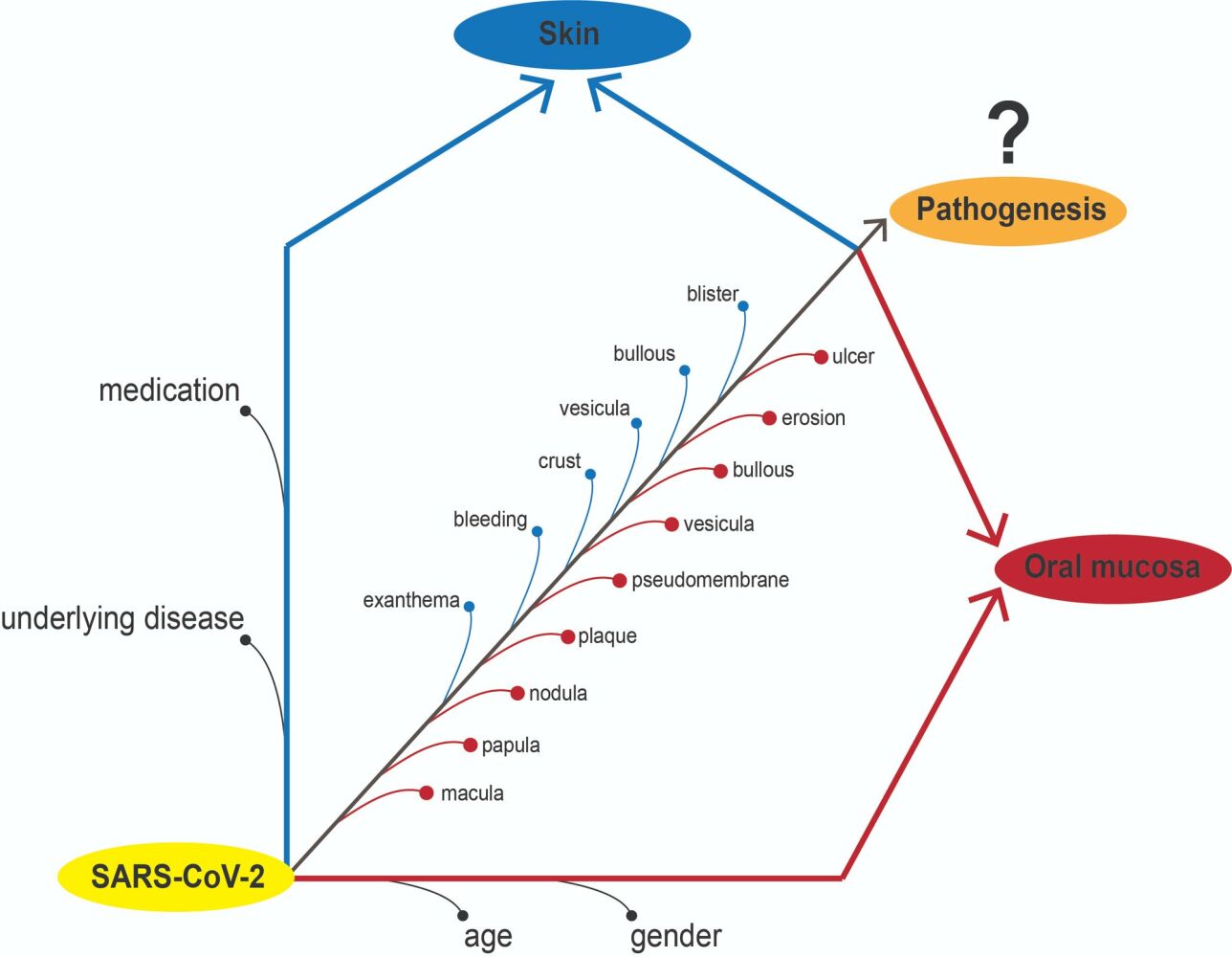
Objective: Many previously reported publications mentioned that oral lesion in COVID-19 patients was varied. The term oral manifestations refer to pathognomonic features that are found consistently with a specific cause and effect. In this context, the oral manifestation of COVID-19 was inconclusive. This systematic review aimed to analyse previously reported publications related to oral lesions in COVID-19 patients to define as oral manifestations or not. The PRISMA guidelines were implemented in this review.
Methods: All umbrella reviews, systematic reviews, systematic reviews and meta-analyses, comprehensive reviews, and original and non-original studies were included. Twenty-one of systematic review, 32 original studies and 68 non-original studies reported the oral lesion in COVID-19 patients.
Results: Most of the publications mentioned that ulcers, macular, pseudomembranes and crusts were frequent oral lesions. The reported oral lesions in COVID-19 patients did not show any pathognomonic features and might be unrelated directly to COVID-19 infections, however, more likely due to gender, age, underlying diseases, and medication.
Conclusion: The oral lesions found in previous studies do not have pathognomonic features and are inconsistent. Therefore, the reported oral lesion, in present time, cannot be defined as an oral manifestation.
Keywords: oral lesion, oral manifestation, underlying disease, medication, COVID-19
Graphical Abstract:
Introduction
Some viruses have a specific manifestation in the oral mucosa or pathognomonic features that can lead a dentist, oral pathologist, or oral medicine specialist to lead to clinical diagnosis. The herpes simplex virus is the cause of primary herpes infection in children. The pathognomonic feature is oral ulceration in the entire mucosa and gingiva,1 and it is called primary gingiva stomatitis.2 The secondary infection presents a specific ulceration in the vermilion of the lips called herpes labialis.3 In other virus infections, like a varicella-zoster infection, the pathognomonic feature was segmental oral ulceration in oral mucosa4 and facial area.5 The measles infection also has pathognomonic features in the oral mucosa called Koplik’s spot and cannot be found in other virus infections.6
Coronavirus infectious disease (COVID-19) is a disease that has been haunting the world for nearly three years. The disease is caused by a viral named SARS-CoV-2.7 The main symptoms are fever, cough, dyspnea, malaise and fatigue, while more serious conditions like respiratory failure and pneumonia could lead to mortality.8,9 SARS-CoV-2 infection, like other viral infections described in a recent report, considered has pathognomonic features in the oral mucosa called COVID tongue.10–13 Further, this condition is known as benign migratory glossitis11–13 and is unable to be considered an oral manifestation. Many kinds of literature have described the oral manifestation or pathognomonic features of COVID-19. But until today, none have concluded the pathognomonic features of COVID-19 because various oral lesion was found in the patient, both hospitalized14 and non-hospitalized, like a casualty.15 The most common oral symptom was dysgeusia16 and xerostomia,17 while the oral lesion was an oral ulcer.16 Further, other oral lesions, vesiculobullous, blisters, and pseudomembranes (Candida albicans infections)14 were reported and more frequent in hospitalized patients.15 The oral lesion looks not specific; in the pediatric patient, the maculopapular (erythematous lesions), ulcers, desquamations (dry and cracked lips), and depapilation lesion (strawberry tongue) were found.18 The doubt of oral manifestation of pathognomonic features arises when accompanied by skin lesions. Most patients have skin lesions similar to herpes simplex virus infection or autoimmune diseases.19 This finding also created doubtfully regarding oral lesions whether is a causality of SARS-CoV-2 (pathognomonic features) or just the casualty.
The oral ulcers, as the common oral lesion found in COVID-19 patients, are mentioned as causality (oral manifestations or pathognomonic features) because of the presence of angiotensin-converting enzyme 2 (ACE-2) in the oral epithelial tissue. It is suspected to be the first receptor for developing oral lesions in SARS-CoV-2-infected patients.20 However, until today, the pathogenesis and interaction between the ACE-2 and SARS-CoV-2 in the oral mucosa has not been able to explain.21,22 The development of various oral lesions in COVID-19 patients looks like a casualty, because it is influenced by various factors such as underlying disease,23,24 immunological and psycho-social factors,25 medication,26–28 and age and gender.29 Nevertheless, various literature has referred to the lesions found as causality, oral manifestation, or pathognomonic features of COVID-19.15–17,19,22,24,26–28 For this reason, this systematic review was composed of various reports regarding oral lesions in COVID-19 and whether the reported lesions can be referred to as oral manifestations or pathognomonic features or not.
Materials and Methods
Search Strategy
In this report, PRISMA guidelines for systematic reviews were implemented. The PubMed (https://pubmed.ncbi.nlm.nih.gov), Science Direct (https://www.sciencedirect.com), and Scopus documents (https://www.scopus.com/search/form.uri?display=basic#basic) were searched up to December 22, 2022. All databases were searched using the following terms:(“COVID-19” [All Fields] OR “Sars-Cov-2” [All Fields]) AND “oral manifestation” [All Fields] OR “oral lesion” [All Fields]).
The researchers implemented language restrictions when assessing the records, and only the full-text articles in English were finally qualified for further evaluation. Additionally, a manual search of the bibliographies and the publications identified from a database search for potentially eligible references was performed. In order to identify missing information or data, we attempted to contact the authors of the relevant studies.
Study Assessment and Analysis
All types of articles, including umbrella reviews, systematic reviews, systematic review and meta-analysis and comprehensive review, were included to collect all the evidence. Initially, the records were assessed by two independent authors according to the relevance of the title and/or abstract (A.S and M.D.C.S). At this stage, the full reports were validated independently by another author (D.A), especially in doubtful cases. Studies considered potentially eligible by at least one of the authors in the initial search were then verified in their entirety by all authors.
The umbrella, systematic, systematic, meta-analysis, and comprehensive reviews have analyzed the description and collected the conclusion. The comprehensive review must follow the PRISMA guideline while collecting the data. While the original (pilot, cohort, observational, prospective, retrospective and cross-sectional) and non-original (case reports, case series, letters to the editor, correspondences and clinical images) studies analysed the patient demographic and related like gender, age, underlying disease, history of medication, oral lesion and skin lesion were listed as a primary outcome. Any disagreements between authors (A.S and M.D.C.S) were resolved after consultation with the third author (D.A).
Results
The 21 reviews (umbrella review, systematic review, meta-analysis and comprehensive review) discussed the oral lesion of COVID-19 patients. While the 32 original studies (pilot, cohort, observational, prospective, retrospective and cross-sectional) and 68 non-original studies (case reports, case series, letters to the editor, correspondences and clinical images) reported the oral lesion found in COVID-19 patients with the demographic data like ages, gender, underlying disease, medication and skin lesions (Figure 1).
 |
Figure 1 Schematic literature search. |
The Systematic Review and Systematic Review and Meta-Analysis Report on Oral Lesions in COVID-19 Patients
Systematic reviews and meta-analysis literature that analysed COVID-19 and its oral manifestation and their conclusions are summarized in Table 1. Among 21 studies published until 2022, three were systematic reviews and meta-analyses, 16 were systematic reviews, one was a systematic review of systematic reviews (umbrella review), and one was a form of a comprehensive review.
 |
Table 1 A Systematic and Meta-Analysis Result of Correlation Between COVID-19 and Oral Manifestation |
Around two reviews concluded on clinical findings in the oral cavity that the most prevalent symptom was dry mouth,21 with oral lesions occurring in various sites of the oral mucosa.26 Four studies concluded on data irregularity33 and also unspecific34 and unclear24 lesions with no clear association with COVID-19.22 Around five studies analysed the potential direct causality of COVID-19 infection to oral lesions and concluded that the lesions are related to the disease,14,19 despite not being scientifically proven.27,36 Five studies concluded that oral lesions in COVID-19 likely resulted from various external factors (casualty), such as co-infection,35 medical devices and treatments,15 comorbidities,32 immunosuppression and medications28,32 that could mimic other inflammatory diseases.18 Around three studies concluded in the urge of further research, including clinical evidence-based research30,31 and observational studies17 to confirm the association between COVID-19 and oral lesions.
The Original Studies Report on Oral Lesions in COVID-19 Patients
Thirty-two original studies include one pilot study, one cohort study, five observational studies, four retrospective studies and fourteen cross-sectional studies (Table 2). The pilot study and cohort study reported all patients with all oral lesions.37,38 The observational study reported that the prevalence of oral lesions was 70.34–100% among COVID-19 patients.39–44 The prospective study reported oral lesions from all patients.45 The retrospective reported 1.70–100% among COVID-19 patients,46–50 and the cross-sectional reported 0.67–100% (Figure 2).51–68
 |
Table 2 The Original Study of a Reported Oral Lesion in a COVID-19 Patient |
 |
Figure 2 The large-scale study reported the number of cases of COVID-19 and those with oral lesions. |
The Oral Lesions that Were Found in the Original Studies
Most oral lesions reported in the original study were ulcers.37,39,40,42,43,45,48,52,53,60,63,66–68 Some of the cases were found as atrophy,42,60 erosion,42,48,60 pseudomembrane,39,46,59,60,67 vesico-bullous,39,43 blister,39,67 nodule,60 plaque,59,60 depapilation,39,67,68 macula,40,42,45,46,60,67 petechiae,43 ecchymosis,43 fissure,39 hematoma,48 swelling and bleeding.50 The patient was distributed equally between men and women aged 1–88 (Table 3).
 |
Table 3 The Original Study of an Oral Lesion in a COVID-19 Patient without Underlying Disease and Mediation Related |
Underlying Disease and No Medication-Related
Underlying disease, such as diabetes mellitus and hypertension, is reported as a common condition found,42,44,45,56,57 followed by hyperthyroidism,42 coronary artery disease,44,56 bronchial asthma56 and myocardial infarct.57 While the oral lesions commonly found were ulcers, macula, atrophy,42,44,45,49,56,57 erosion and vesicle,42,56 crust,42 ecchymosis,56 nodule56 and pseudomembrane.42 Three studies did not mentioned details about the underlying disease.54,59,64 This condition is found in 18–70 years old patients (Table 4).
 |
Table 4 The Original Study of an Oral Lesion in a COVID-19 Patient with an Underlying Disease |
The cohort study showed that the oral lesion found in underlying diseases like hypertension, diabetes mellitus, obesity, pulmonary diseases, hypothyroidism, AIDS, and dyslipidemia was ulcers, pseudomembranous, depapilation, erosions and crusts.38
The Medication-Related
The medication-related to oral lesions reported was anti-viral, anti-malaria (hydroxychloroquine), antibiotic and corticosteroid. The skin lesion was found as exanthema, and the oral lesion was anathema (macula and petechiae)51 (Table 5).
 |
Table 5 The Original Study of an Oral and Skin Lesion in a COVID-19 Patient with Medication-Related |
One report mentioned that a skin dan oral lesion was found in the patient without any underlying disease or medication-related. The oral lesions were ulcers, depapilation, crust, pseudomembrane and macula, while the skin lesion was exanthema65 (Table 6).
 |
Table 6 The Original Study of an Oral and Skin Lesion in a COVID-19 Patient without Underlying Disease and Medication-Related |
Underlying Disease and Medication-Related
Related to underlying diseases, diabetes mellitus is reported as a common condition found,41,58,61,62 and hypertension,41,55,58,61,62 cardiovascular disease,41,58,62 asthma,55,58,61 obesity41,58 and renal disease.41,62 The medication-related to the oral lesion was antibiotic,41,62 anticoagulant,41,62 antimalarial,58,62 antiviral,55,58,62 and corticosteroids.41,55,62 Other underlying diseases and medications are listed in Table 7.
 |
Table 7 The Original Study of an Oral Lesion in a COVID-19 Patient with Underlying Disease and Medication-Related |
The oral lesion was found as an ulcer,41,55,58,61,62 atrophy, pseudomembrane,55,61,62 erosion,41,55,61 macula,55,62 petechiae61,62 vesico-bullous,61,62 crust, depapilation, ecchymosis, and41 papule.55
The Non-Original Studies Report on Oral Lesions in COVID-19 Patients
The non-original research was 39 case reports (70 cases),10,69–106 6 case series (64 cases),107–112 18 letters to the editor (23 cases),113–130 2 correspondences (2 cases)131,132 and 3 clinical images (3 cases)133–135 (Table 8).
 |
Table 8 The Individual Case of Oral Lesion in COVID-19 Patient |
No Underlying Disease and Medication
The oral lesion in COVID-19 patients without any underlying disease and medication was reported as not different based on age and gender. Demographic analysis shows that the studies involved 30 females and 29 males ranging from 16 to 78 years old. For the female, the youngest patient was reported as 16 years old,109 and the oldest was 78.127 Generally, ulcerations occur most frequently in the oral cavity of COVID-19 patients, whether in single or multiple ulcers,93,107,109,112,124,127,135 vesiculobullous,131 edematous,114 necrosis,114 bleeding,94,114 depapilation,127 macula,127 erosion,127 pseudomembrane127 and non-white specific lesion.124 The most common site was the tongue, lips or labial, gingiva, palatal, buccal and commissure of the lips (Table 9).
 |
Table 9 The Oral Lesion in COVID-19 Patients without Systematic Condition and Medication |
For the male, the youngest patient was reported as 19 years old,71 and the oldest was 69 years old.109 One study did not mention the details of patients’ ages.106 The oral lesion was reported as an oral ulcer as the typical lesion,71,89,93,95,101,104,107,109,130 swelling,120 petechia,94,134 macula,69 desquamation, crust and papula104 and also pustula.102 The most common site was the tongue, labial mucosa, gingiva, palatal, oropharynx, and floor of the mouth (Table 9).
Oral lesions and skin lesions were also observed in the patient with COVID-19. The oral lesion was ulcer,76,98,117,118,122,127 erosion and hemorrhagic crust,98 blister,98 depapilation,72 desquamation117 and erythema76,117—the skin lesion including the erythematous macula, urticaria, exanthema and perioral ulcer (Table 10).
 |
Table 10 The Oral Lesion with Skin Lesion in COVID-19 Patients without Underlying Disease and Medication |
The Medication-Related
The oral lesion of COVID-19 was also related to medication. Antibiotics and analgesics were reported to be the most used drugs for COVID-19 patients. Antibiotics include amoxicillin-clavulanic,101 azithromycin,73,77,81,83,101,116,126 moxifloxacin,91 levofloxacin,77 penicillin,96 ceftriaxone,95 cefixime,110 piperacillin-tazobactam,100 doxycycline100 and cefadroxil105 (Table 11).
 |
Table 11 The Oral Lesion in COVID-19 Patients Related to Medication |
The anti-inflammatory and antipyretic drugs include dipyrone,83,113 acetaminophen,90,96,101,119,132 ibuprofen,81 acetylsalicylic acid,77 paracetamol73,95,101 Steroids also prescribe dexamethasone,77,111,113 prednisone77 and methylprednisolone.100 Other types of drugs include proton pump inhibitors,91 anti-malaria,73,95,116 anti-virus,73,100,116 antihistamine,90 mucolytic,77,101,111 anticholinergic,77 anti-coagulant,95,96,100,111 antiparasitic,126 anti-gout,111 anti-gerd,111 anti-depressant110,111 and multivitamin73,81,91,95,101,105,111 (Table 11).
The use of medication during COVID-19 treatment also has a side effect on the oral mucosa as oral lesions, observed in male and female patients in diverse age groups. In the female, the oral manifestation was crust,83,101 macula,113 depapilation,100,101,113 bullous,90 ulcer77,101 and pseudomembrane.77 In the male, there was depapilation,100 bleeding,95 ulcers,94,100,111,126 macula90 and pseudomembrane111 (Table 11).
Oral lesion-related medication is sometimes also found with a skin lesion. The most common lesion was ulcer,73,81,96,105,110,116,119 crust,96,105,116,132 macula,136 vesicle,132 and vesiculobullous,96 with skin lesions in the form of petechiae,81 macula,96,110,119 papula,96,119,132 exanthem73,110 and targetoid lesions.116 The oral lesion mostly affected on lip,96,105,116,119,132 while others were on other mucosae73 (Table 12).
 |
Table 12 The Oral and Skin Lesion in COVID-19 Patients Related Medication |
Underlying Disease
Underlying Disease and No Medication-Related
Oral lesions found in the COVID-19 patient with the underlying disease were also reported. Most of the lesion was ulcer,42,74,85,89,99,110,123 vesicle,42,80 erythema,42,80 pseudomembrane,42 erosion,42,80 crust42,111 and atrophy.42,94 Other lesions were depapilation,74 oedema,42 macule,42 petechiae94 and plaque103 (Table 13).
 |
Table 13 The Oral Lesion Found in COVID-19 Patient with Underlying Disease |
The underlying disease found in men and women was different. Most hypertension,42,80,89 diabetes mellitus,42,85,89,111 hyperthyroidism,42,74 osteoarthritis,80 hypothyroidism,111 rheumatoid arthritis,94 severe dystonia,123 epilepsy,123 arterial hypertension,110 chronic hepatopathy,110 hypercholesterolemia,110 gastroesophageal reflux disease,110 HIV103 and asthma99 (Table 13).
One study only reported oral and skin lesions found in COVID-19 patients with underlying disease. The males of 60 and 63 years old with chronic cholecystitis, renal cyst, and inguinal hernia found an erosive and radiating stria in the buccal and tongue. In contrast, the skin lesion was found as a pruritic macule in the arm’s skin, arm and flexure surface79 (Table 14).
 |
Table 14 The Oral Lesion Accompanies Skin Lesions Found in COVID-19 Patient with Underlying Disease |
Underlying Disease and Medication-Related
The case presented 13 females with a range of 42–84 years old and 14 males with a range of 46–86 years old. The underlying disease frequently reported was diabetes mellitus,84,87,91,92,94,95,107,115,129 hypertension,70,82,84,87,90,92,94,95,100,107,108,115 stroke,100 obesity,47,84,92,107 CVD,82,121,129 hypothyroidism,92,108,129 COPD,92,100,107 carcinoma,92,107 renal disease92,107 and cardiac disease.10 Other conditions such as rheumatoid arthritis,91 allergies,94 chronic sinusitis,70 coronary and peripheral artery disease,92 vascular disease,91 hypercholesterinemia,121 hyperlipidemia,92 pancreatitis,107 Parkinson's disease,107 peripheral neuropathy,91 rectal tumour,108 HIV,78 depression,91 follicular lymphoma,125 kidney transplant82 and autosomal dominant polycystic kidney disease82 were also reported (Table 15).
 |
Table 15 The Oral Lesion is Found in COVID-19 Patient with Underlying Disease and Medication Related |
Most of the prescribed drug was antibiotics,47,70,82,87,90,91,107,108,115,121,125,129 anti-coagulant,47,82,91,107 anti-viral,78,92,100,115 NSAIDs,70,90 steroid,47,90,92,100,107,125 anti-diabetic,94,95 anti-hypertension,94,95 or cardiac drug,10 anti-malaria,82,125 anti-allergic,94 and bronchodilator.70 Another study only mentions intensive care medicine84 and covalent plasma administration.92 Azithromycin, Ceftriaxone, dexamethasone and remdesivir are antibiotics, steroids and anti-virals that are frequently prescribed (Table 15).
The oral lesion was ulcer,47,78,82,87,92,100,107,108,115,121,125 papula-plaque,82,90,125,129 pseudomembrane,92,94,129 crust,84,100,107,108 erosion,94,125 hemorrhagic,95,107 depapilation,10 ecchymosis,94 macula,90 vesicle70 and white patches.91 The location of the lesion dominated in the tongue,10,82,87,91,92,94,107,115,129 lip,47,70,84,94,100,107,108 palate90,91,94,95,129 and labial91,92,100,125 (Table 15).
The oral lesion in COVID-19 patients with underlying disease and medication are also found with skin lesions. Six studies reported that the condition consists of three females and two males, ages 41 to 82. The oral lesion found was ulcer,86,88,108,110,128 blister and bullae,89 crusted,108 macula128 and white patches.110 The skin lesion found as bullae,86 rash89 and exanthema,110 petechia-like and vesiculobullous.128 Other cases reported perioral ulcers88 and fungal infection108 (Table 16).
 |
Table 16 The Oral and Skin Lesion Found in COVID-19 Patient with Underlying Disease and Medication Related |
The underlying disease found was hypertension,88,89,108,128 obesity,89,110 Hodgkin’s lymphoma stage II,86 hyperlipidemia,88 dyslipidaemia,108 hypothyroidism,108 diabetes mellitus,128 arterial hypertension,110 myocardial infarction110 and septic shock.110 The medication prescribes like chemotherapy medication,86,88 antibiotic,108 antivirus,86,89 antimalarial,89 corticosteroid,128 anti-vomiting128 and anti-hypertension110 (Table 16).
Discussion
COVID-19 patients are reported to suffer from various oral lesions throughout or preceding the disease onset.28 Various questions and hypotheses emerged along with the increasing report of the incidence, especially regarding whether it is a manifestation of the viral infection (causality) or a result of large numbers of unidentified risk factors (casualty). Attempts to analyze the lesions and their correlation to COVID-19 are resulting to thereabouts inconclusive results. Various systematic reviews termed that the lesions are COVID-19 oral manifestations,15–17,19,22,24,25,27,28,123 that is also determined by individual and environmental factors,16 secondary infection and psychosocial factors,25,27 immunosuppressive conditions15 caused by medications15,28 and diseases,23,24,26,29 or might be as primary direct causality since ACE2 is expressed in the oral cavity.15,17,19,22,25–27 We report contradictory findings based on analyses of the patterns of COVID-19 patients with clinical oral lesions and by further breaking down the reported various factors that might be involved. The lesions reported by 70 works of literature were analyzed to see whether they are symptoms or conditions resulting from COVID-19 infection as an oral manifestation or pathognomonic features.
Preceding systematic reviews were summarized to understand the current understanding of oral lesions in COVID-19 patients (Table 1). All systematic reviews give varying conclusion but generally show uncertainty and skepticism towards the concept of oral lesions as COVID-19 manifestation. A meta-analysis also discovered that data on oral lesion prevalence was highly heterogenous, while data on xerostomia show lower heterogeneity,17 indicating that the most common symptom found on COVID-19 was dry mouth, as also concluded by another review.21 A systematic review concludes that oral lesions suffered by COVID-19 patients were very diverse and indistinctive,24,34 and mostly unrelated to the SARS-CoV-2 virus.17,22,30 The indistinctive lesions would explain the heterogeneity of oral lesion prevalence in COVID-19 patients. Various concepts regarding the correlation between COVID-19 and oral lesions were also stated, such that the virus infected ACE2 receptors in the oral mucosal tissue, thus leading to lesion onset through inflammatory mechanism,19,30 and that the lesions might progress along with the disease progression.29 However, despite the statement of direct causality, all these studies mostly concluded the doubt that COVID-19 infection might result in oral manifestations, as no substantial evidence could be found regarding it.17 These studies suggest that other factors such as comorbidities,32 medication and immunosuppression28,32 are potentially the leading cause of these lesions as various interrelated factors’ casualty. This notion led to inconclusive conclusions in these reviews that oral lesions in COVID-19 patients might not be an oral manifestation of the viral infection. Detailed investigation and analysis of these oral lesions and their clinical signs along with COVID-19 patterns is needed to be done on each case published from various case reports, case series, cross-sectional, letters to editors and observational studies to uncover more of the relation between clinical oral lesion with COVID-19 conditions.
A plethora of oral diseases had been found to occur in the oral cavity of patients infected with SARS-CoV-2. We found that oral lesions in COVID-19 are very diverse but mostly in the form of ulcers37–43,47,52–57,59–64,71,73,74,76–78,81,82,85–89,92–94,96,98,107–112,115,116,118,119,121–128,131,135 and erosion.38,41,42,47,55,56,60,61,80,94,96,98,125,127,132 Some studies also reported an occurrence of recurrent oral lesions, aphthous-like ulcers,53 hemorrhagic ulcers38,41,42,83,84,96,107,108,111,116,117,132 and macula (erythema).42,55,56,76,80,81,91 While the findings often took place as an ulcer, other oral findings are also found in the form of plaques,55,59,60,82,90,125,129 pseudomembrane,38,39,42,46,55,59–62,77,81,91,92,94,111,127,129 depapilation,38,39,41,72,74,113,127 or bleeding lesion.37,94,95,114 Most of these lesions were found in the tongue,71,72,79,81,82,87,90–92,94,98,107,109–113,115,116,118,120,122,127–129,132,134 palate,80,85,89–91,94,107,112,115,128,129 lip70,77,81,83,84,88,89,94,96,98,107,108,110,111,117,119,128,132 and buccal mucosa.71,80,98,112,126,128,129,132 In this context, the diversity of types of oral lesions and locations in COVID-19 patients raises a question regarding its causality and casualty. This shows that COVID-19 patients did not show particular clinical patterns and tendencies that could be assumed as pathognomonic lesions of COVID-19.
Unsolved hypotheses of the mechanism of the lesion formation could possibly be solved by analyzing underlying diseases and medications that underwent by COVID-19 patients with oral lesions. Most of these patients have various medical conditions that alter and worsen their immune status to respond to viral infection. We found that most of the patients included in the study suffered from cardiovascular diseases,38,41,42,55–58,61,62,70,80,82,84,87–92,94,107,108,110,115,121,128 pulmonary diseases38,92 and diabetes,38,41,42,55–58,61,62,84,85,87,89,91,92,94,107,111,115,128,129 that could worsen their immune status whether independent or dependent to COVID-19 infection and resulted in the lesion due to wane host defense.137 Observations on these patients also show the diversity of the reported underlying diseases that seem to likely be inconsistent and unrelated to the oral lesions. Patients with underlying diseases were separately analysed (Tables 4, 13 and 14) to see whether it could be a key player in the lesion progression, but it was later found that no evident differences in clinical patterns were found in the oral cavity in patients without underlying diseases. This leads to the notion that underlying diseases might not aid the lesion manifesting in COVID-19 patients.
Most patients with underlying diseases received high doses of various medications, like antibiotics,41,62 immunosuppressants,41,55,62 NSAIDs,58 and anti-virals.55,58,62 Meanwhile, in patients without underlying diseases who received medications, we found that most of them used antibiotics,77,83,91,95,126 NSAIDs,83,113 vitamins,91,95,111 corticosteroids,77,94,111,113 analgesics,85,95 and antivirals.95 We found that these drugs resulted in mostly ulcers in patients without underlying diseases (Table 11 and Table 15) or with underlying diseases (Table 7), despite the large diversity of the following lesion forms. We also found the same inconclusive lesions in patients without underlying diseases and without medications (Table 4 and Table 9). These drugs may not directly cause the specific manifestation of lesions in COVID-19 patients. Previous systematic reviews stated that steroids could cause immunosuppression that could lead to oral lesion formation.15,24,26 Lesions occur in those patients could also occur in non-COVID-19 patients or in patients without steroid prescriptions. This can be inferred that medication is not a plausible factor that could aid COVID-19 manifesting in the oral cavity.
COVID-19 patients often receive multiple medications, which could promote the risk of drug reactions.138 Hydroxychloroquine has been used to treat COVID-19 and was reported to be one of the most prevalent erythema multiforme-triggering drugs and various other side effects in patients.138–142 The lesion was caused by promoted CD8+ lymphocyte infiltration to the epithelial tissue, thus leading to the necrosis of the cells and subepithelial cleft forming as a hypersensitivity reaction to the drugs consumed.140 This led to the hypothesis that the crusts suffered by the patients potentially are actually not an oral manifestation of SARS-CoV-2, but probably erythema multiforme, which is the distinct pathognomonic features that also include hemorrhagic crusts along with targetoid lesions on the skin.138,142 Our findings highlighted that most of the skin lesion-related medications were exanthema and skin or genital ulceration (Tables 5, 12 and 16). In contrast, some cases also reported similar skin lesions in the patient without any medication (Tables 6, 10 and 14).
Despite the effort to break down possible influencing factors of oral COVID-19 lesions through underlying diseases and patients’ medications, we found no relevance to specific manifestations in the oral tissue. Oral lesions in patients with and without underlying diseases and medications vary with no particular patterns or pathognomonic pattern. The lesions might be resulted from various unidentified interrelated factors with unknown mechanisms, resulting in varying forms of lesions. These lesions are unlikely to be called oral manifestations of COVID-19 since data show clinical signs that are not in accordance with the viral infection itself.
Contrary to previous studies, we also found no correlation between COVID-19 severity and oral lesions.29 Patients with severe COVID-19 symptoms were often to be hospitalized. Oral ulcers in these patients may also occur because of mechanical ventilation and intubation. However, there is no distinguishing oral lesion found in patients with severe COVID-19 symptoms compared to non-hospitalized patients, as both presented with mostly ulcers.41 This corroborates our statement that oral lesions in COVID-19 patients are not dependent on the viral infection to manifest whether in a mild or advanced stage of the disease. However, despite some studies reported regarding the matter, the number of studies that include the intubation treatment in their reports is limited to make a proper analysis and conclusion to the exact cause of the lesion and how significant the mechanical trauma to the ulcers that reported.
Compared to other diseases with established and distinct oral clinical patterns, such as herpes zoster, primary gingiva stomatitis, and measles, COVID-19 did not show clear and consistent causality to the oral tissue and casualty to form distinct pathognomonic features. Based on the analyses done of the lesions and their clinical patterns, it is difficult and unlikely to conclude that these oral lesions occur as the result of COVID-19 infection, and SARS-CoV-2 does not seem to have any specific manifestations in the oral cavity. We found 121 works of literature (21 reviews, 32 original studies and 68 non-original studies) that could lead to these findings on the inconclusiveness of oral lesions in COVID-19 patients. However, we found irregularity and unclarity in these reports regarding lesion descriptions and terms, such as their type, shape, size, and location. This makes the data heterogeneous and difficult to analyse in a more accurate way. This could be due to the fact that most of the authors who reported the oral lesion in those studies probably were not dentists, let alone oral medicine specialists, thus possibly lead to inaccurate lesion descriptions. The findings of oral lesions in COVID-19 patients has prompted numerous authors to hypothesize that these lesions are attributable to COVID-19 infection itself. Through meticulous analysis on the clinical signs, we found that it is crucial to emphasize that the presence of these lesions does not necessarily establish a causal relationship with COVID-19 infection itself, especially when the clear cause and effect are not found yet. Further comprehensive investigations are imperative to discern potential confounding factors and establish a clearer understanding of the etiology behind these oral lesions in COVID-19 patients. Dentists and oral medicine specialists need an active role in uncovering more about these oral lesions in COVID-19 patients in the future research, especially in its pathogenesis.
On the other hand, it is still debatable whether all reported oral lesions are solely a result of the SARS-CoV-2 infection. One piece of evidence demonstrated that, out of 14 patients, the oral lesions in 13 expressed the spike protein of SARS-CoV-2 and exhibited higher ACE2 expression.43 This indicates the presence of SARS-CoV-2 components in the oral mucosa, but the subsequent processes that occurred have not been determined. Although the study conducted by Soares et al identified the presence of SARS-CoV-2 components within oral lesions, further analyses and investigations involving a larger population of patients and other types of oral lesions are required to comprehensively determine the potential significance and impact of these viral components on oral lesion development. Hence, the direct causality of SARS-CoV-2 in the oral mucosa remains uncertain. Other studies have also corroborated that oral lesions in COVID-19 patients may be secondary lesions associated with trauma events, immune impairment, or adverse reactions to therapeutic interventions.143 In the subsequent study, if it can elucidate how the interplay between ACE2, the SARS-CoV-2 spike protein, and the initiation of oral lesions occurs, then the identified lesions can be confidently classified as oral manifestations.
This review has strengths as we conducted comprehensive systematic analyses from various kinds of original and non-original literature. Various types of literature, such as pilot study, cohort, observational, prospective, retrospective, cross-sectional, case reports, case series, and even letters to editor, that report COVID-19 patients with specific oral lesion conditions were included and analyzed. Through these approaches, detailed analysis and observation on oral lesions on COVID-19 could be done, and a conclusive conclusion could be reached that the lesions that suffered by COVID-19 patients are not oral manifestations of the disease. However, we were unable to determine the bias in this study, as we adhere to descriptive approaches to explain the conformity between oral lesions and COVID-19. Therefore, extensive, and comprehensive research is needed to know the cause of these lesions in COVID-19 patients and discover their pathognomonic features.
Conclusion
Oral lesions in reported studies do not have pathognomonic features and are vary, so in present time they cannot be defined as an oral manifestation. The suspicious factors such as underlying diseases and medications might be classified as predisposing factors. This would arise several possibilities for pathogenesis stacked across one another, making it possible to indirect causality of oral lesion development.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huang CW, Hsieh CH, Lin MR, Huang YC. Clinical features of gingivostomatitis due to primary infection of herpes simplex virus in children. BMC Infect Dis. 2020;20(1):782. doi:10.1186/s12879-020-05509-2
2. Crimi S, Fiorillo L, Bianchi A, et al. Herpes virus, oral clinical signs and QoL: systematic review of recent data. Viruses. 2019;11(5):463. doi:10.3390/v11050463
3. Leung AKC, Barankin B. Herpes labialis: an update. Recent Pat Inflamm Allergy Drug Discov. 2017;11(2). doi:10.2174/1872213X11666171003151717
4. Lamichhane S, Humagain M, Subba M, Neupane M, Dawadi A. Necrotizing stomatitis in varicella zoster infection. Kathmandu Univ Med J. 2020;18(70):210–213.
5. Clarkson E, Mashkoor F, Abdulateef S. Oral Viral Infections. Dent Clin North Am. 2017;61(2):351–363. doi:10.1016/j.cden.2016.12.005
6. Tanaka M, Harada T. Koplik spots in measles. Postgrad Med J. 2019;95(1126):454. doi:10.1136/postgradmedj-2019-136739
7. Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20. doi:10.1038/s41580-021-00418-x
8. da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7–8):377–382. doi:10.1007/s00508-020-01760-4
9. Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19. J Chine Med Ass. 2020;83(3):217–220. doi:10.1097/JCMA.0000000000000270
10. Sharma S, Bhardwaj A. COVID tongue. J Indian Soc Periodontol. 2022;26(5):498–500. doi:10.4103/jisp.jisp_437_21
11. Scotto G, Fazio V, Spirito F, Lo Muzio E, Lo Muzio L. COVID Tongue: suggestive hypothesis or clinical reality? Oral Dis. 2022;28:2618–2619. doi:10.1111/odi.14134
12. Hathway RW. COVID tongue. Br Dent J. 2021;230(3):114. doi:10.1038/s41415-021-2666-z
13. Surboyo MDC, Santosh ABR, Kuntardjo Y, Indriyani I, Ayna VKP, Ernawati DS. COVID tongue: reports, debate, and scope for research. Dental J Advan Stud. 2022;2022:1.
14. Cuevas-Gonzalez MV, Espinosa-Cristóbal LF, Donohue-Cornejo A, et al. COVID-19 and its manifestations in the oral cavity. Medicine. 2021;100(51):e28327. doi:10.1097/MD.0000000000028327
15. Orilisi G, Mascitti M, Togni L, et al. Oral manifestations of COVID-19 in hospitalized patients: a systematic review. Int J Environ Res Public Health. 2021;18(23):12511. doi:10.3390/ijerph182312511
16. Nijakowski K, Wyzga S, Singh N, Podgórski F, Surdacka A. Oral manifestations in SARS-CoV-2 positive patients: a systematic review. J Clin Med. 2022;11(8):2202. doi:10.3390/jcm11082202
17. Aragoneses J, Suárez A, Algar J, Rodríguez C, López-Valverde N, Aragoneses JM. Oral manifestations of COVID-19: updated systematic review with meta-analysis. Front Med. 2021;8. doi:10.3389/fmed.2021.726753
18. Nascimento RB, Araujo NS, Silva JC, Xavier FCA. Oral manifestations of multisystemic inflammatory syndrome in children (MIS‐C) and Kawasaki disease associated to COVID‐19: a systematic review. Spec Care Dentist. 2022;42(3):266–280. doi:10.1111/scd.12669
19. Erbaş GS, Botsali A, Erden N, et al. COVID‐19‐related oral mucosa lesions among confirmed SARS‐CoV‐2 patients: a systematic review. Int J Dermatol. 2022;61(1):20–32. doi:10.1111/ijd.15889
20. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi:10.1038/s41368-020-0074-x
21. Qi X, Northridge ME, Hu M, Wu B. Oral health conditions and COVID-19: a systematic review and meta-analysis of the current evidence. Aging Health Res. 2022;2(1):100064. doi:10.1016/j.ahr.2022.100064
22. Di Spirito F, Iandolo A, Amato A, et al. Prevalence, features and degree of association of oral lesions in COVID-19: a systematic review of systematic reviews. Int J Environ Res Public Health. 2022;19(12):7486. doi:10.3390/ijerph19127486
23. Temgoua MN, Endomba FT, Nkeck JR, Kenfack GU, Tochie JN, Essouma M. Coronavirus disease 2019 (COVID-19) as a multi-systemic disease and its impact in low- and middle-income countries (LMICs). SN Compr Clin Med. 2020;2(9):1377–1387. doi:10.1007/s42399-020-00417-7
24. Doceda MV, Gavriiloglou M, Petit C, Huck O. Oral health implications of SARS-CoV-2/COVID-19: a systematic review. Oral Health Prev Dent. 2022;20(1):207–218. doi:10.3290/j.ohpd.b2960801
25. Uzêda-E-Silva VD, de Sá IB, Martins J, Pedreira N, Vieira VPS, de Silva BHM. Oral lesions associated with COVID-19: a systematic review. Stomatologija. 2021;23(1):3–8.
26. Bhujel N, Zaheer K, Singh RP. Oral mucosal lesions in patients with COVID-19: a systematic review. Br J Oral Maxillofac Surg. 2021;59(9):1024–1030. doi:10.1016/j.bjoms.2021.06.011
27. Sharma P, Malik S, Wadhwan V, Gotur Palakshappa S, Singh R. Prevalence of oral manifestations in COVID‐19: a systematic review. Rev Med Virol. 2022;32(6). doi:10.1002/rmv.2345
28. Fakhruddin KS, Samaranayake LP, Buranawat B, Ngo H. Oro-facial mucocutaneous manifestations of Coronavirus Disease-2019 (COVID-19): a systematic review. PLoS One. 2022;17(6):e0265531. doi:10.1371/journal.pone.0265531
29. da Santana LAM, Vieira W, Gonçalo RIC, Lima Dos Santos MA, Takeshita WM, Miguita L. Oral mucosa lesions in confirmed and non-vaccinated cases for COVID-19: a systematic review. J Stomatol Oral Maxillofac Surg. 2022;123(5):e241–50. doi:10.1016/j.jormas.2022.05.005
30. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a 6-month update. J Dent Res. 2021;2021:002203452110296.
31. Dar-Odeh N, Bobamuratova DT, Alnazzawi A, et al. Jaw-related complications in COVID-19 patients; a systematic review. CRANIO®;2022. 1–8. doi:10.1080/08869634.2022.2031438
32. Samaranayake LP, Fakhruddin KS, Ngo HC, Bandara HM, Leung YY. Orofacial mycoses in coronavirus disease-2019 (COVID-19): a systematic review. Int Dent J. 2022;72(5):607–620. doi:10.1016/j.identj.2022.02.010
33. Di Spirito F, Caggiano M, Di Palo MP, et al. Oral lesions in pediatric subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Appl Sci. 2022;12(18):8995. doi:10.3390/app12188995
34. Surboyo MDC, Ernawati DS, Budi HS. Oral mucosal lesions and oral symptoms of the SarS-coV-2 infection. Minerva Dent Oral Sci. 2021;70(4):161–168. doi:10.23736/S2724-6329.21.04493-9
35. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. 2020;2020:002203452095728.
36. Reis VP, Bezerra AR, Maia ABP, Marques LC, Conde DC. An integrative review of oral manifestations in patients with COVID‐19: signs directly related to SARS‐CoV‐2 infection or secondary findings? Int J Dermatol. 2022;61(3):278–290. doi:10.1111/ijd.15881
37. Kady DM, Gomaa EA, Abdella WS, Ashraf Hussien R, ElAziz RH A, Khater AGA. Oral manifestations of COVID-19 patients: an online survey of the Egyptian population. Clin Exp Dent Res. 2021;2020:1–9.
38. Schwab G, Palmieri M, Zerbinati RM, et al. Lack of direct association between oral mucosal lesions and SARS-CoV- 2 in a cohort of patients hospitalised with COVID-19. J Oral Microbiol. 2022;14(1). doi:10.1080/20002297.2022.2047491
39. Favia G, Tempesta A, Barile G, et al. Covid-19 symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the university hospital policlinic of bari with a purpose of a new classification. J Clin Med. 2021;10(4):757. doi:10.3390/jcm10040757
40. Fidan V, Koyuncu H, Akin O. Oral lesions in Covid 19 positive patients. Am J Otolaryngol. 2021;42(3):102905. doi:10.1016/j.amjoto.2021.102905
41. Batista AAF, Ramos KPP, Do Amaral MA, et al. Oral lesions in patients with COVID-19 hospitalized in an intensive care unit: a case-series study. Braz Oral Res. 2022;36:e108. doi:10.1590/1807-3107bor-2022.vol36.0108
42. Subramaniam T, Nikalje M, Jadhav S. Oral manifestations among COVID-19: an observational study of 713 patients. Dent Res J (Isfahan). 2021;18(1):67. doi:10.4103/1735-3327.324026
43. Soares CD, Souza LL, de Carvalho MGF, et al. Oral manifestations of coronavirus disease 2019 (COVID-19). Am J Surg Pathol. 2022;46(4):528–536. doi:10.1097/PAS.0000000000001825
44. Bianco E, Maddalone M, Ferdeghini C, Mirabelli L, Hari S. Oral Manifestations in Hospitalized COVID Patients. World J Dent. 2022;13(5):434–440. doi:10.5005/jp-journals-10015-2082
45. Naser AI, Al-Sarraj MN, Deleme ZH. Oral and maxillofacial lesions in COVID 19 infection from Mosul Hospital in Iraq: epidemiological study and approach to classification and treatment. J Oral Res. 2021;10(6):1–14. doi:10.17126/joralres.2021.069
46. Bardellini E, Bondioni MP, Amadori F, et al. Non-specific oral and cutaneous manifestations of Coronavirus Disease 2019 in children. Med Oral Patol Oral Cir Bucal. 2020;2020:1.
47. de Paula Eduardo F, Gobbi MF, Bergamin LG, Migliorati CA, Bezinelli LM. Oral care and photobiomodulation protocol for the prevention of traumatic injuries and lip necrosis in critically ill patients with COVID-19: an observational study. Lasers Dent Sci. 2021;5(4):239–245. doi:10.1007/s41547-021-00144-9
48. Eduardo F, Bezinelli P, Gobbi MF, Bergamin LG, de Carvalho DLC, Corrêa L. Oral lesions and saliva alterations of COVID‐19 patients in an intensive care unit: a retrospective study. Spec Care Dentist. 2022;42(5):494–502. doi:10.1111/scd.12705
49. Santos NMV, Brito DH, Santos TG, et al. Oral manifestations in hospitalized children with COVID-19. Braz Oral Res. 2022;36:e139. doi:10.1590/1807-3107bor-2022.vol36.0139
50. Saraf S, Nalawade T, Mallikarjuna R, Al Kashmiri A. The retrospective pilot study of the prevalence of olfactory dysfunction or loss of smell, loss of taste and oral manifestations among COVID-19 positive health workers in Muscat, Oman. Indian J Otolaryngol Head Neck Surg. 2022;2022:1.
51. Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol. 2020;156(10):1134. doi:10.1001/jamadermatol.2020.2550
52. Abubakr N, Salem ZA, Kamel AHM. Oral manifestations in mild-to-moderate cases of covid-19 viral infection in the adult population. Dent Med Probl. 2021;58(1):7–15. doi:10.17219/dmp/130814
53. Katz J, Yue S. Increased odds ratio for COVID-19 in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2021;50(1):114–117. doi:10.1111/jop.13114
54. Alade O, Folayan MO, Adeniyi A, et al. Differences in oral lesions associated with tobacco smoking, E-Cigarette Use and COVID-19 infection among adolescents and young people in Nigeria. Int J Environ Res Public Health. 2022;19(17):10509. doi:10.3390/ijerph191710509
55. Villarroel-Dorrego M, Chacón L, Rosas R, Barrios V, Pernía Y, Vélez H. Hallazgos bucales en pacientes COVID-19. Actas Dermosifiliogr. 2022;113(2):183–186. doi:10.1016/j.ad.2021.08.007
56. Chawla J, N Y, Bakshi SS, et al. Oral manifestations associated with COVID-19 disease: an observational cross sectional study. J Oral Biol Craniofac Res. 2022;12(2):279–283. doi:10.1016/j.jobcr.2022.03.008
57. Muthyam A, Reddy M, Kulkarni S, Srilatha A, Sahithi K, Satyanarayana D. Oral manifestations in COVID-19 patients: an observational study. J Family Med Prim Care. 2022;11(3):1000. doi:10.4103/jfmpc.jfmpc_1264_21
58. Tuter G, Yerebakan M, Celik B, Kara G. Oral manifestations in SARS-CoV-2 infection. Med Oral Patol Oral Cir Bucal. 2022;27(4):e330–9. doi:10.4317/medoral.25259
59. El Tantawi M, Sabbagh HJ, Alkhateeb NA, et al. Oral manifestations in young adults infected with COVID-19 and impact of smoking: a multi-country cross-sectional study. PeerJ. 2022;10:e13555. doi:10.7717/peerj.13555
60. Ganesan A, Kumar S, Kaur A, et al. Oral Manifestations of COVID-19 infection: an analytical cross-sectional study. J Maxillofac Oral Surg. 2022;21:1326–1335. doi:10.1007/s12663-021-01679-x
61. Binmadi NO, Aljohani S, Alsharif MT, Almazrooa SA, Sindi AM. Oral Manifestations of COVID-19: a cross-sectional study of their prevalence and association with disease severity. J Clin Med. 2022;11(15):4461. doi:10.3390/jcm11154461
62. Elamrousy W, Nassar M, Issa D. Prevalence of oral lesions in COVID-19 Egyptian patients. J Int Soc Prev Community Dent. 2021;11:6.
63. Folayan MO, Zuniga RAA, Ezechi OC, et al. Associations between emotional distress, sleep changes, decreased tooth brushing frequency, self-reported oral Ulcers and SARS-Cov-2 infection during the first wave of the COVID-19 pandemic: a global survey. Int J Environ Res Public Health. 2022;19(18):11550. doi:10.3390/ijerph191811550
64. Hans M, Hans VM, Kahlon N, Sagar M, Pandey AK, Das A. Gustatory dysfunction and oral ulceration in COVID-19 patients: a cross sectional study. Dent Res J. 2022;19:43. doi:10.4103/1735-3327.346401
65. Özen T, Kahraman FC, Öcal S, Ovalı HF. Skin, mucosa and nail findings in hospitalized pediatric patients with Coronavirus disease-2019 (COVID-19). An Bras Dermatol. 2022;98:208–215. doi:10.1016/j.abd.2022.03.006
66. Bhuyan R, Bhuyan SK, Mohanty JN, et al. A preliminary survey on the oral manifestation of COVID-19 in the First and Second Waves in Bhubaneswar, City of Odisha, India. Nat J Commun Med. 2022;13(5):294–297. doi:10.55489/njcm.130520221617
67. Natto ZS, Afeef M, Khalil D, et al. Characteristics of oral manifestations in symptomatic non-hospitalized COVID-19 Patients: a cross-sectional study on a sample of the Saudi Population. Int J Gen Med. 2021;14:9547–9553. doi:10.2147/IJGM.S331611
68. Nuno‐Gonzalez A, Martin‐Carrillo P, Magaletsky K, et al. Prevalence of mucocutaneous manifestations in 666 patients with COVID‐19 in a field hospital in Spain: oral and palmoplantar findings. Br J Dermatol. 2021;184(1):184–185. doi:10.1111/bjd.19564
69. Kahraman FC, Çaşkurlu H. Mucosal involvement in a COVID ‐19‐positive patient: a case report. Dermatol Ther. 2020;33:4.
70. Eghbali Zarch R, Hosseinzadeh P. COVID ‐19 from the perspective of dentists: a case report and brief review of more than 170 cases. Dermatol Ther. 2021;34(1):1–6. doi:10.1111/dth.14717
71. Dominguez‐Santas M, Diaz‐Guimaraens B, Fernandez‐Nieto D, Jimenez‐Cauhe J, Ortega‐Quijano D, Suarez‐Valle A. Minor aphthae associated with SARS‐CoV‐2 infection. Int J Dermatol. 2020;59(8):1022–1023. doi:10.1111/ijd.15004
72. Chaughtai S, Chaughtai Z, Asif A. Conservative treatment with mouthwashes followed by tongue photo biomodulation therapy in Covid-19: a case report. J Med Case Rep. 2022;16(1):367. doi:10.1186/s13256-022-03519-z
73. Putra BE, Adiarto S, Dewayanti SR, Juzar DA. Viral exanthem with “Spins and needles sensation” on extremities of a COVID-19 patient: a self-reported case from an Indonesian medical frontliner. Int J Infect Dis. 2020;96:355–358. doi:10.1016/j.ijid.2020.05.020
74. Al-Khanati NM, Riad A, Sahloul ME, Klugar M. Aphthous-like stomatitis of COVID-19 patients. Braz J Oral Sci. 2020;19:1–4. doi:10.20396/bjos.v19i0.8661354
75. Chérif MY, de Filette JMK, André S, Kamgang P, Richert B, Clevenbergh P. Coronavirus disease 2019–related Kawasaki-like disease in an adult: a case report. JAAD Case Rep. 2020;6(8):780–782. doi:10.1016/j.jdcr.2020.06.023
76. Malih N, Hajinasrollah G, Zare M, Taheri M. Unexpected Presentation of COVID-19 in a 38-year-old male patient: a case report. Case Rep Dermatol. 2020;12(2):124–131. doi:10.1159/000509994
77. Berlingieri G, Alvares CMA, Serrano RV, Palma LF, Campos L. Phototherapies for COVID-19-associated opportunistic oral infections. Photodiagnosis Photodyn Ther. 2022;37:102678. doi:10.1016/j.pdpdt.2021.102678
78. Campeanu AT, Dumea E, Rus M, et al. A rare case of plasmablastic lymphoma in a patient with HIV and SARS-CoV-2 infections. Curr Oncol. 2022;29(3):1537–1543. doi:10.3390/curroncol29030129
79. Saleh W, SHawky E, Halim GA, Ata F. Oral lichen planus after COVID-19, a case report. Ann Med Surg. 2021;72:103051. doi:10.1016/j.amsu.2021.103051
80. Saleh W, Ata F, Elashry MM. Is COVID-19 infection triggering oral herpes zoster? A case report. SAGE Open Med Case Rep. 2021;9:2050313X2110657. doi:10.1177/2050313X211065793
81. Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: case report. Int J Infect Dis. 2020;100:154–157. doi:10.1016/j.ijid.2020.08.071
82. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int J Infect Dis. 2020;97:326–328. doi:10.1016/j.ijid.2020.06.012
83. Kitakawa D, Oliveira FE, Neves de Castro P, Carvalho LF. Short report - Herpes simplex lesion in the lip semimucosa in a COVID-19 patient. Eur Rev Med Pharmacol Sci. 2020;24(17):9151–9153. doi:10.26355/eurrev_202009_22863
84. Ramires MC, Mattia MB, Tateno RY, Palma LF, Campos L. A combination of phototherapy modalities for extensive lip lesions in a patient with SARS-CoV-2 infection. Photodiagnosis Photodyn Ther. 2021;33(January):102196. doi:10.1016/j.pdpdt.2021.102196
85. Pauli MA, Pereira LM, Monteiro ML, de Camargo AR, Rabelo GD. Painful palatal lesion in a patient with COVID-19. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(6):620–625. doi:10.1016/j.oooo.2021.03.010
86. De Medeiros VLS, Monteiro-Neto AU, França DDT, Castelo Branco R, de Miranda Coelho ÉO, Takano DM. Pemphigus Vulgaris After COVID-19: a case of induced autoimmunity. SN Compr Clin Med. 2021;3(8):1768–1772. doi:10.1007/s42399-021-00971-8
87. Nejabi MB, Noor NAS, Raufi N, et al. Tongue ulcer in a patient with COVID-19: a case presentation. BMC Oral Health. 2021;21(1):1–5. doi:10.1186/s12903-021-01635-8
88. Siotos C, Bonett AM, Hansdorfer MA, Siotou K, Kambeyanda RH, Dorafshar AH. Medical device related pressure ulcer of the lip in a patient with COVID-19: case report and review of the literature. J Stomatol Oral Maxillofac Surg. 2020;14(4):337–339.
89. Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021;27(S3):710–712. doi:10.1111/odi.13382
90. Tapia ROC, Labrador AJP, Guimaraes DM, Valdez LHM. Oral mucosal lesions in patients with SARS‐CoV‐2 infection. Report of four cases. Are they a true sign of COVID‐19 disease? Spec Care Dentist. 2020;3(July):12520.
91. Riad A, Gomaa E, Hockova B, Klugar M. Oral candidiasis of COVID-19 patients: case report and review of evidence. J Cosmet Dermatol. 2021;20(6):1580–1584. doi:10.1111/jocd.14066
92. Yeom J, Wolk R, Griffin L, Freedman PD, Reich RF. Atypical herpetic ulcerations in COVID-19 positive patients: a report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;135:268–271. doi:10.1016/j.oooo.2022.07.015
93. Khodavirdipour A, Asadimanesh M, Masoumi SA. Impact of SARS-CoV-2 genetic blueprints on the oral manifestation of COVID-19: a case report. Glob Med Genet. 2021;08(4):183–185. doi:10.1055/s-0041-1735538
94. Rafałowicz B, Wagner L, Rafałowicz J. Long COVID oral cavity symptoms based on selected clinical cases. Eur J Dent. 2022;16(02):458–463. doi:10.1055/s-0041-1739445
95. Manzalawi R, Alhmamey K, Abdelrasoul M. Gingival bleeding associated with COVID‐19 infection. Clin Case Rep. 2021;9(1):294–297. doi:10.1002/ccr3.3519
96. Dalipi ZS, Dragidella F, Dragidella DK. Oral manifestations of exudative erythema multiforme in a patient with COVID-19. Case Rep Dent. 2021;2021:1–8.
97. Garcez AS, Delgado MGT, Sperandio M, Dantas Silva FT, de Assis JSR, Suzuki SS. Photodynamic therapy and photobiomodulation on oral lesion in patient with coronavirus disease 2019: a case report. Photobiomodul Photomed Laser Surg. 2021;39(6):386–389. doi:10.1089/photob.2020.4977
98. Palaia G, Pernice E, Pergolini D, et al. Erythema multiforme as early manifestation of COVID-19: a case report. Pathogens. 2022;11(6):654. doi:10.3390/pathogens11060654
99. Al-Akhali MS, Halboub E, Ibraheem W, Khan HK, Hummadi AM. Recurring oral erythema multiforme-like lesions elicited by COVID-19 infection: a case report. Braz Dent Sci. 2022;25(1):e2960. doi:10.4322/bds.2022.e2960
100. Sircar K, Popli DB, Jha OK, Sircar M, Hasan S. Oral mucosal lesions in moderate-to-severe COVID-19 Disease - An Indian critical care unit experience. J Datta Meghe Inst Med Sci Univ. 2022;17(5):S63–6. doi:10.4103/jdmimsu.jdmimsu_137_22
101. Mahmoud MS, Taha MS, Mansour OI, et al. Oral mucosal lesions during SARS-CoV-2 infection: a case series and literature review. Egypt J Otolaryngol. 2022;38(1):18. doi:10.1186/s43163-022-00203-3
102. Ingale Y, Bommanavar S, Ingale M. Mucormycotic osteomyelitis involving maxilla in SARS-CoV-2 prediabetic patient: a case report 1 2* 3 4. J Krishna Inst Medical Sci Univ. 2022;11(1):105–110.
103. Ding X, Ma X, Xu Y, Xu L. HIV-associated mycobacterium Avium Complex, Oral Candida, and SARS-CoV-2 Co-Infection: a rare case report. Infect Drug Resist. 2022;15:7037–7042. doi:10.2147/IDR.S390333
104. Emelyanova N, Isayeva G, Komir I, Shalimova A, Buriakovska O, Vovchenko M. Changes in the oral cavity of a patient after suffering from coronavirus infection COVID-19: a clinical case. Acta Med Mediterr. 2021;37:827–831.
105. Talahatu LB, Kaban BE, Ayuningtyas NF, et al. Management of patients with aphthous-like ulcers related to aplastic anaemia in the COVID-19 pandemic era through teledentistry: a case report. Dental J. 2022;55(1):49–55. doi:10.20473/j.djmkg.v55.i1.p49-55
106. Indu S. Multiple oral ulcerations – an initial manifestation of COVID 19 infection: a personal experience!! J.Oral Maxillofac Pathol. 2020;24(2):227. doi:10.4103/jomfp.JOMFP_324_20
107. Brandão TB, Gueiros LA, Melo TS, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;00(00):1–7.
108. Teixeira IS, Leal FS, Tateno RY, Palma LF, Campos L. Photobiomodulation therapy and antimicrobial photodynamic therapy for orofacial lesions in patients with COVID-19: a case series. Photodiagnosis Photodyn Ther. 2021;34:102281. doi:10.1016/j.pdpdt.2021.102281
109. Riad A, Kassem I, Hockova B, Badrah M, Klugar M. Tongue ulcers associated with SARS‐CoV‐2 infection: a case series. Oral Dis. 2020;2020:13635.
110. Hocková B, Riad A, Valky J, et al. Oral Complications of ICU Patients with COVID-19: case-series and review of two hundred ten cases. J Clin Med. 2021;10(4):581. doi:10.3390/jcm10040581
111. Sachet P, Rocha BA, Lima FS, et al. Management of orofacial lesions with antimicrobial photodynamic therapy and photobiomodulation protocols in patients with COVID-19: a multicenter case series. Photodiagnosis Photodyn Ther. 2022;38:102743. doi:10.1016/j.pdpdt.2022.102743
112. Riad A, Kassem I, Stanek J, Badrah M, Klugarova J, Klugar M. Aphthous stomatitis in COVID‐19 patients: case‐series and literature review. Dermatol Ther. 2021;34(1):1–4.
113. Tomo S, Miyahara GI, Simonato LE. Oral mucositis in a SARS‐CoV‐2‐infected patient: secondary or truly associated condition? Oral Dis. 2020;2020:13570.
114. Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID‐19. Oral Dis. 2020;2020:13462.
115. Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID‐19). Oral Dis. 2020;27:13465.
116. Demirbaş A, Elmas ÖF, Atasoy M, Türsen Ü, Lotti T. A case of erythema multiforme major in a patient with COVID 19: the role of corticosteroid treatment. Dermatol Ther. 2020;13:21–23.
117. Labé P, Ly A, Sin C, et al. Erythema multiforme and Kawasaki disease associated with COVID‐19 infection in children. J Eur Acad Dermatol Venereol. 2020;34. doi:10.1111/jdv.16666
118. Gueiros LA, Neves MCS, Marques CDL. Chikungunya fever and COVID‐19: oral ulcers are a common feature. Oral Dis. 2022;28(S1):1008–1009. doi:10.1111/odi.13717
119. Hockova B, Riad A, Klugar M, Azar B. Self-case report of oral and skin lesions associated with positivity of COVID-19. J Cosmet Dermatol. 2021;20:1–2.
120. McGoldrick DM, Sarai R, Green J. Tongue and floor of mouth swelling: a potential rare manifestation of COVID-19. Br J Oral Maxillofac Surg. 2021;59(4):500–501. doi:10.1016/j.bjoms.2021.03.001
121. Kämmerer T, Walch J, Flaig M, French LE. COVID‐19‐associated herpetic gingivostomatitis. Clin Exp Dermatol. 2021;46(1):174–176. doi:10.1111/ced.14402
122. Chaux-Bodard AG, Deneuve S, Desoutter A. Oral manifestation of Covid-19 as an inaugural symptom? Oral Surg Oral Med Oral Radiol. 2020;26(2):18. doi:10.1051/mbcb/2020011
123. Cant A, Bhujel N, Harrison M. Oral ulceration as presenting feature of paediatric inflammatory multisystem syndrome associated with COVID-19. Br J Oral Maxillofac Surg. 2020;58(8):1058–1059. doi:10.1016/j.bjoms.2020.06.037
124. Glavina A, Biočina‐Lukenda D, Mravak‐Stipetić M, Markeljević J. Oral symptoms and lesions in SARS‐CoV‐2‐positive patient. Oral Dis. 2020;15:
125. Gabusi A, Gissi DB, Rossi R, Foschini MP, Montebugnoli L. Persistent lesions in oral cavity after SARS-CoV-2 infection. Oral Dis. 2021;2020:1–2.
126. Bezerra TM, Feitosa SG, Carneiro DTO, Costa FWG, Pires FR, Pereira KMA. Oral lesions in COVID‐19 infection: is long‐term follow‐up important in the affected patients? Oral Dis. 2020;28:2570–2571
127. Rodríguez MD, Romera AJ, Villarroel M. Oral manifestations associated with COVID‐19. Oral Dis. 2020;28:960.
128. Soares C, Carvalho RA, Carvalho KA, Carvalho MG, Almeida O. Letter to Editor: oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. 2020;25(4):e563–4. doi:10.4317/medoral.24044
129. Riad A, Gad A, Hockova B, Klugar M. Oral candidiasis in non‐severe COVID‐19 patients: call for antibiotic stewardship. Oral Surg. 2020;15:465.
130. Aranda Romo S, Rizo VHT, Noyola Cherpitel DE, et al. Oral lesions as the only signs of recurrent SARS‐CoV‐2 infection. Oral Dis. 2022;28(S2):2614–2615. doi:10.1111/odi.14098
131. Soares CD, Mosqueda-Taylor A, de Carvalho MGF, de Almeida OP. Oral vesiculobullous lesions as an early sign of COVID-19: immunohistochemical detection of SARS-CoV-2 spike protein. Br J Dermatol. 2021;184(1):e6. doi:10.1111/bjd.19569
132. Aghazadeh N, Homayouni M, Sartori‐Valinotti JC. Oral vesicles and acral erythema: report of a cutaneous manifestation of COVID‐19. Int J Dermatol. 2020;2020:15047.
133. Dilsiz A, Parlak E, Gül SS. Oral and ocular manifestations in a patient with coronavirus disease-2019: clinical presentation and management. Rev Soc Bras Med Trop. 2022;55:e06992021. doi:10.1590/0037-8682-0699-2021
134. Casu C, Orrù G. Tongue papillitis and volatile sulfur compounds (VSC) values in a COVID-19 patient. Pan Afrn Medl J. 2022;41. doi:10.11604/pamj.2022.41.5.28915
135. Sweet S, Moayedi S, Torres M. Oral aphthous ulcers associated with COVID-19. Vis J Emerg Med. 2022;29:101423. doi:10.1016/j.visj.2022.101423
136. Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part I. Acute ulcers. Clin Exp Dermatol. 2009;34(3):289–294. doi:10.1111/j.1365-2230.2009.03220.x
137. Liu D, Yuan X, Gao F, et al. High number and specific comorbidities could impact the immune response in COVID-19 Patients. Front Immunol. 2022;13:899930.
138. Ergun T, Ergenç İ, Seven S, et al. Drug eruption: a mimicker of Coronavirus disease-2019 rash. Turkderm Turk Arch Dermatol Venereol. 2022;56(1):34–38.
139. Mohammad Zadeh N, Mashinchi Asl NS, Forouharnejad K, et al. Mechanism and adverse effects of COVID-19 drugs: a basic review. Int J Physiol Pathophysiol Pharmacol. 2021;13(4):102–109.
140. Bennardo L, Nisticò SP, Dastoli S, et al. Erythema multiforme and covid‐19: what do we know? Medicina. 2021;57(8):2–7.
141. Bouabdella S, Benkaraache M, Almheirat Y, Zizi N, Dikhaye S. Erythema multiforme eruption due to SARS-COV 2: case report. Ann Med Surg. 2021;68(June):102591. doi:10.1016/j.amsu.2021.102591
142. Etaee F, Eftekharian M, Naguib T, Daveluy S. Erythema multiforme inCOVID-19 patients and following COVID-19 vaccination: manifestations, associations and outcomes. J Eur Acad Dermatol Venereol. 2022;36(7):e522–4. doi:10.1111/jdv.18063
143. Zarpellon A, Matuck BF, Dolhnikoff M, et al. Oral lesions and SARS‐CoV‐2: a postmortem study. Oral Dis. 2022;28(S2):2551–2555. doi:10.1111/odi.14047
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
