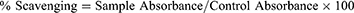Back to Journals » Journal of Inflammation Research » Volume 15
The Neuroprotective Propensity of Organic Extracts of Acacia stenophylla Bark and Their Effectiveness Against Scopolamine-/Diazepam-Induced Amnesia in Mice
Authors Shah D , Iqbal A, Alshehri FS , Ullah A , Ali G , Muhammad T, Ullah R , Sewell RDE , Althobaiti YS
Received 26 May 2022
Accepted for publication 10 August 2022
Published 22 August 2022 Volume 2022:15 Pages 4785—4802
DOI https://doi.org/10.2147/JIR.S376242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Dawood Shah,1 Arshad Iqbal,1 Fahad S Alshehri,2 Aman Ullah,3 Gowhar Ali,4 Tahir Muhammad,5,6 Rahim Ullah,4,7 Robert D E Sewell,8 Yusuf S Althobaiti9,10
1Department of Botany, Islamia College Peshawar, Peshawar, Pakistan; 2Department of Pharmacology and Toxicology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia; 3College of Pharmaceutical Sciences, Shifa Tameer-e-Millat University, Islamabad, Pakistan; 4Department of Pharmacy, University of Peshawar, Peshawar, Pakistan; 5Molecular Neuropsychiatry and Development (MiND) Lab, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada; 6Institute of Medical Science, University of Toronto, Toronto, ON, Canada; 7Department of Pharmacy, Faculty of Life Sciences, Sarhad University of Science and Information Technology, Peshawar, Khyber Pakhtunkhwa, Pakistan; 8Cardiff School of Pharmacy and Pharmaceutical Sciences, Cardiff University, Cardiff, CF10 3NB, UK; 9Department of Pharmacology and Toxicology, College of Pharmacy, Taif University, Taif, Saudi Arabia; 10Addiction and Neuroscience Research Unit, Taif University, Taif, Saudi Arabia
Correspondence: Gowhar Ali, Department of Pharmacy, University of Peshawar, Peshawar, 25120, Pakistan, Tel +92-919216750, Fax +92-91921813, Email [email protected]; [email protected] Yusuf S Althobaiti, Department of Pharmacology and Toxicology, College of Pharmacy, Taif University, P.O. Box 11099, Taif, 21944, Saudi Arabia, Email [email protected]
Background: Alzheimer’s disease (AD) is a neurodegenerative disorder that is more prevalent in the elderly. There is extensive literature on using Acacia species against central nervous system disorders, although Acacia stenophylla has not been investigated for any neuroprotective potential. The purpose of the study was to elucidate the ameliorative effect of ethyl acetate (ASEE) and butanol (ASB) extracts from the bark of A. stenophylla on memory deficits and cognitive dysfunction in scopolamine- or diazepam-induced amnesia in mice.
Methods: The phytochemical constituents of the extracts of A. stenophylla were determined by GC-MS and the in vitro anticholinesterase plus antioxidant activities were also evaluated. Anti-amnesic effects were determined employing the open field test, elevated plus maze (EPM), Morris water maze (MWM), and Y-maze paradigms.
Results: The in vitro cholinesterase assays disclosed a concentration-dependent inhibition of both AChE and BuChE with IC50 values of 28.48 and 44.86 μg/mL for the ASEE extract and 32.04 and 50.26 μg/mL for the ASB extract against AChE and BuChE respectively. DPPH and H2O2 antioxidant assays revealed respective IC50 values for the ASEE extract of 28.04 and 59.84 μg/mL, plus 32.77 and 64.65 μg/mL for ASB extract. The findings revealed that both extracts possessed substantial antioxidant properties. Furthermore, these fractions restored scopolamine- and diazepam-induced memory deficits in a dose-dependent manner, as observed by a significant decrease in the transfer latency in EPM, reduction in escape latency, added time spent in the target quadrant in the MWM, and elevated spontaneous alternation behavior (SAB) in the Y-maze test.
Conclusion: The ameliorative effect of A. stenophylla on scopolamine- and diazepam-induced amnesia can be attributed to antioxidant and anticholinesterase activity. Consequently, the use of A. stenophylla might be exploited in the alleviation of oxidative stress and the management of AD.
Keywords: Acacia stenophylla, amnesia, oxidative stress, cognitive deficits, acetylcholinesterase
Introduction
Dementia is a general term that refers to a group of symptoms associated with an ongoing decline in brain functioning, including cognition. Alzheimer’s disease (AD) is the most common cause of dementia and it is a progressive condition involving a specific type of dementia that is characterized by a gradual loss of memory and learning. The disease was first described in a patient by Alois Alzheimer in 1906. It is widespread, particularly in individuals older than 65 years, and constitutes about 50 to 60% of dementia patients.1,2 In 2018, it was estimated that there were 50 million dementia sufferers globally and this was predicted to rise to 82 million over the ensuing ten-year period, reaching a total of up to 152 million by 2050. The economic impact of dementia is such that it costs an estimated one trillion US dollars, increasing to two trillion dollars by 2030, and in the case of AD, it is not only responsible for memory loss but may also invariably lead to mortality.3 AD is associated with various biological factors such as the excessive deposition of β-amyloid, neurofibrillary tangles, oxidative stress, a decline in central neurotransmission, and an overall deterioration in cognitive function.4
The loss of memory and cognition is partly attributed to cholinergic function deficit, caused by degeneration of cholinergic neurons, a consequent deficiency of acetylcholine neurotransmitters and their receptors located in the forebrain and hippocampus. A limited treatment option for this type of cognitive dysfunction involves inhibition of acetylcholinesterase (AChE) to increase the available supply of acetylcholine neurotransmitters at the cholinergic synapses in the hippocampal and frontal cortical regions.5 In this respect, regulation of acetylcholine in the synaptic cleft is necessary for normal memory function, however, over-accumulation of acetylcholine causes neuronal hyper-stimulation while hypo-secretion of acetylcholine results in memory loss.
Oxygen-free radicals have been linked with several diseases including neurodegenerative cognitive disorders like Alzheimer’s and Parkinson’s diseases.6,7 The source of these free radicals is derived from inflammatory activity in neutrophils, macrophages, or the byproduct of the mitochondrial electron transport chain reaction, for example. Reactive oxygen species (ROS) are continuously produced as a byproduct in the form of hydrogen peroxide (H2O2), hydroxyl (OH), and superoxide anion (O2−) during normal metabolic reactions which are then constantly managed by body tissues.8 ROS negatively affect the cell membrane by causing the peroxidation of unsaturated fatty acids that diminish not only membrane fluidity but also its overall structure and function. Such pathological alterations have been observed in AD, multiple sclerosis, liver cirrhosis, and diabetes mellitus.9
A strategy against cognitive dysfunction of AD has been an approach that involves inhibition of AChE and butyrylcholinesterase (BuChE) activity in the central cholinergic system.10−12 Although synthetic anticholinesterase drugs such as tacrine, galantamine, rivastigmine, and donepezil are used primarily for the symptomatic relief of AD, they also have harmful side effects. Therefore, research has focused on the search for effective and safe therapies of natural origin derived from plants. Folkloric uses of various traditional plant species have been used in practice to treat a variety of central nervous system disorders, including anxiety, amnesia, seizures, and Parkinson’s disease. Medicinal plants are an inexpensive and readily available source of biologically active compounds used worldwide for the treatment of various human health issues. In this context, a diversity of plants has been explored in the quest for novel drugs. However, the majority of these plants and their derivatives have yet to be incorporated into conventional therapy.13,14
Enzymatic and non-enzymatic antioxidants have been found to inhibit the harmful effects of ROS in biological systems.15,16 Plants are a rich source of bioactive molecules that play a useful role as antioxidants due to their ability to donate H-atoms to free radicals. Furthermore, these phytochemicals or secondary metabolites play a central role not only in normal body function but also in the prevention of infectious diseases together with anti-inflammatory, antihypertensive, antidiabetic, and anticancer potentials.17–21
There is extensive use of Acacia species against central nervous system disorders as antioxidant or anti-inflammatory agents in a traditional setting. However, limited experimental investigations have been carried out in support of their medicinal use. In this respect, it has been shown in vivo, that the catechin in a methanolic leaf extract obtained from A. Catechu and A. auriculiformis both reversed scopolamine-induced amnesia.22,23 Moreover, a leaf extract from A. modesta,24 an ethanolic A. nilotica root extract25 and an aqueous extract of A. radianna26 all displayed anti-AChE activities. Similarly, two organic extracts of A. sieberiana root exhibited about 60% anti-AChE activity in a microplate assay.27 Bearing in mind the previous literature on the Acacia genus, the current study was conducted to investigate any possibility of an anti-amnesic potential of Acacia stenophylla as a prospective natural agent for the treatment of AD.
Materials and Methods
Plant Material
The bark of Acacia stenophylla was collected in August 2017 from the Pakistan Forest Institute, University of Peshawar. The plant was identified by Prof. Dr. Siraj Ud Din at the Department of Botany, University of Peshawar. The bark was collected according to the guidelines recommended by Trease et al, 2002.28 Following identification and collection, the specimen was preserved, a voucher specimen (AS-Bot-15082017) was deposited in the herbarium of the Department of Botany, Islamia College Peshawar, Pakistan. It was dried in the shade at the botanical garden of Islamia College Peshawar. It took approximately three weeks to dry completely, and then the dried plant bark was ground down to yield circa 7.5 kg powder. All methods were carried out following the relevant guidelines and regulations.
Extraction and Fractionation
The extraction was carried out in a steel drum following the method of Muhammad et al,29 The plant bark powder was completely submerged in 32 liters of commercial-grade methanol for approximately one month with regular stirring every 3 days. The methanolic extract was first filtered through a velvet cloth and then through a Whatman filter paper. Then the filtrate was concentrated using a rotary evaporator set at 50 ℃ and 60 rpm. Subsequently, further concentration was carried out using a water bath maintained at temperature of 50 ℃. The crude methanolic extract was further fractionated into an escalating order of polarity ie N-hexane, chloroform, ethyl acetate, N-butanol, and aqueous fractions.
Gas Chromatography-Mass Spectroscopy Analysis
The GC-MS technique used to determine the bioactive compounds in crude ethyl acetate (ASEE) and N-butanol (ASB) fractions from the bark of A. stenophylla. A Thermo Scientific GC Focus series (code 12550080, serial NO. 10804035) connected to a Thermo Scientific DSQ II spectrometer used for samples analysis. The test sample (1.0 µL) was injected into the GC-MS system coupled to a TR-5-MS capillary column with a length of 30m, i.d. 0.25mm, and a film diameter of 0.50 µm. The injector was kept at a temperature of 250 ℃ and the oven temperature was set to operate at an initial temperature of 50 ℃, the hold time being 60 seconds with an increased rate of 10 ℃ per 60 seconds up to 150 ℃ until the temperature approached 280 ℃ with a rate of 6 ℃ per 60 seconds and a stay time of one minute. Helium gas was used as a mobile phase with a flow rate of 1.0 mL/min, carrying compounds one by one according to the program. The MS settings were such that the temperature of the ion source was 210 ℃, the temperature of interface 250 ℃, scan range: 40–1000 m/z, holdup time of solvent 180 seconds, MS launch time 5 min, MS stop time 35 min, and electron ionization was 70 EV.
In vitro Studies
In vitro Cholinesterase Inhibition Assay
Acetylcholinesterase and butyrylcholinesterase assays were performed following Ellman’s assay method. Acetylthiocholine iodide (ATchI) and butyrylthiocholine iodide (BTchI) were used as substrates, and degradation of these substrates led to the formation of 5-thio-2-nitrobenzoate, which reacted with 5, 5’-dithiobis (2-nitro-benzoate (DTNB) forming a yellow color complex, which was assayed at 412 nm wavelength through a microplate reader.30
The rate of absorbance (V = ΔAB/Δt) showed the enzyme activity (%) and inhibition by test/control samples and was calculated as follows,
Amax shows the maximum enzyme activity in the absence of the inhibitory agent.
Diphenyl-1-Picrylhydrazyl (DPPH) Assay
The in vitro DPPH assay was conducted according to Khan et al protocol31 to investigate the free radical scavenging potential of ASEE and ASB fractions from the bark of A. stenophylla. A 0.1mL solution of standard ascorbic acid and test samples at ascending concentrations of 31.25–1000 mg/mL was mixed with the methanolic solution (0.004%) of DPPH in a 96-microplate reader. After 30 minutes of incubation in the dark at room temperature, the colorization of DPPH was determined at 517 nm.32 Each experiment was carried out in triplicate and the following formula was employed to determine the percentage scavenging potential of the drugs.
Hydrogen Peroxide (H2O2) Free Radical Scavenging Model
The H2O2 free radical scavenging assay was used following the method reported, to examine the antioxidant potential of crude ASEE and ASB fractions derived from A. stenophylla bark.33 A 2.0 mM solution of H2O2 was prepared in 50mM phosphate buffer (pH =7.4). Test drug samples (0.1 mL) from each dilution were used and adjusted to a final volume of 0.4 mL by adding 50 mM phosphate buffer, then 6 mL of H2O2 was added with thorough mixing. The final absorbance was measured at 230 nm relative to a blank after 10 min. The following equation was used to get the percentage scavenging activity with H2O2.
In vivo Activity
Animals
Swiss albino mice of either sex (20–30g bodyweight) were acquired from the Animal House of the Department of Pharmacy, University of Peshawar. The mice were housed in cages (n = 8–10 per cage) at an ambient temperature of 25 ± 3 ℃ and humidity of 56.5 ± 10% with 12-hour light and 12-hour dark phases. They had access to standard laboratory food and water ad libitum. A total of 408 mice were used in all behavioral experiments which were randomly classified into different groups ie 6 mice in each group. In the in vivo behavioral experiments animals were divided into seventeen groups; group 1 was treated with vehicle, groups 2–3 were treated with scopolamine (SCO)/diazepam (DZP), groups 4–5 were treated with SCO/DZP plus donepezil, groups 6–11 were treated with SCO/DZP plus ASEE (50–150 mg/kg), and groups 12–17 were treated with SCO/DZP plus ASB (50–150 mg/kg). The experiments were carried out in a well-aerated soundproof laboratory between 8 am and 5.00 pm. The mice fasted for two hours before each experiment and they were injected intraperitoneally. The experimental procedures on animals were performed under the approval of the Ethical Committee of the Department of Pharmacy, University of Peshawar (registration number: 12/EC-17/Pharm). The experimental procedures on animals were performed according to the relevant guidelines and regulations of the United Kingdom Animals (Scientific procedures) Act 1986.
Acute Toxicity Study
The Organization for Economic Co-Operation and Development (OECD) guidelines were followed to determine the acute toxicity of the drug. Forty mice of both sexes were randomly assigned to five groups (n=8 mice/group). Each group has administrated a dose on an increasing scale with 300 mg/kg increments of (300, 600, 900, 1200, and 1500 mg/kg i.p. body weight). According to OECD recommendations and the inaccessibility of the information on lethality, the starting dose level was determined at 300 mg/kg for welfare reasons and then the animals were observed for the first four hours and then up to 72h.
Grouping of Animals
Mice were assigned to 17 groups (n=6) randomly. Group 1 (control group) was injected with normal saline; groups 2–3 (amnesic groups) were administered scopolamine (1.0 mg/kg) or diazepam (1.0 mg/kg) respectively; groups 4–5 (positive control groups) were given scopolamine plus donepezil (3.0 mg/kg) or diazepam plus donepezil (3.0 mg/kg); groups 6–17 (tested groups) were administered ASEE or ASB crude plant extract at doses of 50, 100, and 150 mg/kg combined with scopolamine or diazepam respectively (Figure 1).
 |
Figure 1 Experimental schedule of behavioral activity of scopolamine and diazepam administered mice. |
Open Field Test
The Open Field Test (OFT) paradigm was used to assess any change in the exploratory behavior of each animal. The number of line crossings and the time spent in the periphery and center of the arena were recorded during a 5-minute test period following a previously described method.34,35
Elevated Plus Maze
The elevated plus maze (EPM) consisted of a plus-shaped apparatus consists of two open (16 × 5 cm) and two covered (16 × 5×12 cm) arms arranged at right angles to each other with a central platform (5 × 5 cm) at a height of 25 cm above the ground. Transfer latency (TL) was noted as the time to relocate the animal from the open arm to the covered arm. To habituate mice to the maze, each animal was trained one day before the commencement of the test. Thus, each animal was gently placed in one of the open arms, allowed to enter the covered arms, and allowed to stay there for 10 seconds before being returned to the home cage. Animals that did not enter the covered arm within 90 seconds were manually placed in the covered arm with care and the transfer latency was recorded as 90 seconds. The above procedure was repeated after 24 hours to examine memory retention.30
Morris Water Maze
The Morris Water maze test (MWM) was performed with a slightly modified protocol.36,37 The MWM equipment comprised a circular water pool (80 cm in diameter with a 40 cm high wall) that was partitioned into four quadrants ie, Q1, Q2, Q3, and Q4 with external visual signs on the laboratory walls for navigation. The pool was filled with water to a depth of 30 cm and milk whitener was added to make the water opaque and it was maintained at a temperature of 26.0 ± 1.0 ℃. A platform, 6 cm in diameter was positioned in the center of Q3 and it was maintained in position throughout the test activity. MWM was used to estimate the effect of ASEE and ASB extracts of Acacia stenophylla bark on changes in spatial memory. On the first day of the protocol, each mouse was allowed 60 seconds in the pool to swim and explore and then transferred to the home cage. The animals were then administered test extracts daily for five consecutive days. Thirty minutes after dosing with bark extract, scopolamine or diazepam were injected in all but the control group. Thirty minutes later, each animal underwent two consecutive platform location trials of 120s with an interval of 30 minutes on four consecutive days. The mouse platform location time or escape latency (EL) was noted. Animals were then permitted to reside on the platform for the 20s and then transferred to the home cage. Animal failure to locate the platform during the 120s trial meant that it was guided manually to the platform and then transferred to the home cage. On the fifth day, each mouse underwent a 120s probe trial in the absence of the platform and after administration of bark extract and/or scopolamine/diazepam. This determined the time each animal spent in the correct quadrant (Q3) as an indication of memory retention.
Y-Maze Test
The Y-maze is an exploratory paradigm that was used to determine the effect of the ASEE and ASB fractions on the exploratory and locomotor behavior of mice. The maze consisted of a three-arm Y-shaped apparatus made from opaque polyvinyl plastic mounted on a white board.35,38 The three arms were set at a 120-degree angle, each arm being 40cm long, 15cm high and 10cm wide. All arms of the maze were permanently closed. On the first protocol day, each animal was allowed a trial period of 60 seconds in the maze for habituation and then returned to the home cage. On the test day, 60 minutes before each trial, animals were injected with crude ASEE, ASB, or donepezil, and 30 minutes later, scopolamine or diazepam was administered to induce amnesia. Each animal was then gently placed in the center of the three arms and allowed to explore freely for 6 min. The new arm alternations for each animal were recorded manually. An arm entrance was counted when all four animal limbs crossed the threshold line of the arm. The entry of each mouse into three arms in a consecutive manner was recorded as correct alternation behavior. The percentage of alternation behavior was computed using the equation given below.
Statistical Analysis
The data of the in vivo OFT, time spent in target quadrants of MWM and Y-maze were analyzed by one-way ANOVA followed by Bonferroni’s post hoc test, while EPM, escape latency times in the MWM, in vitro anticholinesterase and antioxidant data were analyzed by two-way ANOVA followed by the Bonferroni’s post hoc test using Graph-Pad Prism Version-9 software (GraphPad Software Inc San Diego, CA, US). Significance: #p < 0.05, ##p< 0.01, ###p< 0.001, and #### p< 0.0001 showed significance between vehicle and SCO/DZP groups, and *p < 0.05, **p< 0.01, ***p< 0.001, ****p< 0.0001 showed significance between SCO/DZP and treated groups. Data were expressed as mean ±SEM.
Results
Gas Chromatography-Mass Spectroscopy
GC-MS analysis of the ethyl acetate fractions of Acacia stenophylla bark revealed the following eleven types of phytoconstituents which are classified in decreasing order of their percentage peak area, ie dodecyl acrylate (2.08%), 3-chloropropionic acid, heptadecyl ester (2.08%), isooctyl (2-methyl-4-chlorophenoxy) acetate (0.54%), spirost-8-en-11-one, 3-hydroxy (0.54%), phenol, 2,4-bis (1,1-dimethylethyl) (0.35%), hexadecanoic acid, methyl ester (0.28%), cyclooctasiloxane, hexadecamethyl (0.25%), hexasiloxane, tetradecamethyl (0.25%), acetic acid, 2-methylpropyl ester (0.22%), cyclononasiloxane, octadecamethyl (0.20%), cyclodecasiloxane, eicosamethyl (0.20%), cyclononasiloxane, octadecamethyl (0.20%), tetracosamethyl-cyclododecasiloxane (0.20%), benzene, 1,3-bis (1,1-dimethylethyl) (1.7%), cyclopentanone, 2-methyl (0.09%), cyclohexanone (0.09%), cyclotetrasiloxane, octamethyl (0.08%) (Figure 2A). Similarly the compounds found in the butanol fraction are ranked in descending order of the percentage peak area ie acetic acid, butyl ester (2.58%), silane, diethoxydimethyl (0.23%), dodecyl acrylate (0.17%), 3-chloropropionic acid, heptadecyl ester (0.17%), decane (0.11%), hexadecanoic acid, methyl ester (0.07%), cyclononasiloxane, octadecamethyl (0.04%), tetracosamethyl-cyclododecasiloxane (0.04%), heptasiloxane, hexadecamethyl (0.04%), cyclononasiloxane, octadecamethyl (0.03%), 3-isopropoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy) tetrasiloxane (0.03%), 3-butoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris (trimethylsiloxy) tetrasiloxane (0.03%), cyclononasiloxane, octadecamethyl (0.03%), heptasiloxane, hexadecamethyldiazene (0.03%), [1-(2,2-dimethylhydrazino)-2-methylpropyl]ethyl (0.02%), butane, 1,1’-[(1-methylethylidene) bis (oxy)]bis (0.02%), Gly-Gly-Gly (0.02%), cyclooctasiloxane, hexadecamethyl (0.02%), hexasiloxane, tetradecamethyl (0.02%) (Figure 2B).
Anticholinesterase Activities of ASEE and ASB Extracts
The crude ASEE and ASB extracts exhibited concentration-dependent inhibition of cholinesterase enzymes. The ASEE extract disclosed 15.6 and 76% inhibition at a concentration of 15.62 and 1000 µg/mL concentration with an IC50 value of 28.48 µg/mL against AChE while producing 14 and 79% inhibition at 15.62 and 1000 µg/mL with an IC50 value of 44.86 µg/mL against BuChE. Whereas the ASB extract exhibited 14.5 and 75.3% inhibition at 15.62 and 1000 µg/mL concentration with an IC50 value of 32.04 µg/mL against AChE. The ASB extract also revealed 16 and 73.6% inhibition at 15.62 and 1000 µg/mL with an IC50 value of 50.26 µg/mL against BuChE (Figure 3). These results provide compelling evidence of anticholinesterase activity for the ASEE and ASB extracts.
ASEE and ASB Diminish Free Radical Scavenging and ROS in vitro
The antioxidant activity of ASEE and ASB extracts was shown in the DPPH assay and H2O2 radical scavenging paradigm. The data presented below (Table 1) disclosed concentration-dependent free radical scavenging activities for both ASEE and ASB fractions compared to standard ascorbic acid. In the DPPH assay, the percentage scavenging effect of ASEE was observed to be 20.14–80.30% over the concentration range 31.25–1000 µg/mL with an IC50 value of 28.04 µg/mL. Similarly, the percentage free radical scavenging strength of ASB was 20.0–78.14% at concentrations of 31.25–1000 µg/mL with an IC50 value of 32.77 µg/mL. A dose-related antioxidant potential of ASEE and ASB fractions was also detected in the H2O2 free radical scavenging assay. Hence, the percentage antioxidant potential of ASEE was 20.49–80.30% at concentrations of 31.25–1000µg/mL, the IC50 value being 59.84 µg/mL, while the ASB fraction produced 09.67–85.31% antioxidant activity at concentrations of 31.25–1000µg/mL. The IC50 value for the ASB extract was 64.65µg/mL. These findings indicate that both ASEE and ASB fractions may well have the potential to target oxidative stress.
 |
Table 1 Evaluation of the Antioxidant Activity of ASEE and ASB Fractions Using DPPH Assay and H2O2 Free Radical Scavenging Paradigm |
Acute Toxicity of ASEE and ASB in Mice
Behavioral responses of mice at different doses of ASEE and ASB were determined. No aggressiveness, spontaneous locomotor activity, catalepsy, or any overt bizarre behaviors were observed at doses up to 1200 mg/kg. However, some bizarre responses were observed at 1500 mg/kg, so the maximum tolerated dose (MTD) was recorded as 1200 mg/kg.
The Activity of the ASEE and ASB Fractions on Scopolamine-Induced Behaviors in the Open Field Test (OFT)
The OFT was used to evaluate the general locomotor activity and exploratory behavior of the animals and the parameters evaluated included the time spent in the center of the field, the incidence of rearing, and any manifestation of grooming activity. The findings suggested at the outset that both ASEE and ASB had a dose-dependent tendency to increase the duration of time spent in the center of the apparatus, but this deduction did not prove to be statistically significant (Figure 4A). However, the occurrence of rearing in the scopolamine-treated group was significantly increased compared to the vehicle-administered control group, and donepezil (anticholinesterase), antagonized this scopolamine effect manifested by a reduction increase in the overall number of rears. Both extracts of A. stenophylla induced a gradual reduction in the number of rears at higher doses (100 and 150 mg/kg; Figure 4B). Similarly, the effect of the bark extracts on the number of grooming episodes was elevated in the scopolamine-treated group compared to the controls while donepezil reduced this occurrence of grooming. In addition, ASEE and ASB extract both reversed scopolamine-augmented grooming in a dose-dependent manner (Figure 4C). Consequently, these results indicate that A. stenophylla bark extracts counteracted general locomotor activity and exploratory behaviors related to scopolamine-induced amnesia.
ASEE and ASB Improved Scopolamine and Diazepam-Induced Cognitive Deficits
To assess the aptitude of ASEE and ASB against scopolamine-induced learning and memory consolidation, we performed the EPM behavioral test. Hence, scopolamine exerted an impairment of learning and memory consolidation by increasing transfer latencies (TLs) on day 1 (acquisition) and day 2 (retention trial) in comparison with controls. This increase in transfer latency reflected a disturbance in animal mnestic activity and donepezil (3.0 mg/kg) was statistically effective (p < 0.001) in reversing this scopolamine amnesia on both days. ASEE and ASB (50 mg/kg) were both ineffective in reducing TL on day 1, although ASEE (50 mg/kg) reduced TL on day 2. However, at a higher dose (100mg/kg) the two bark extracts reduced animal TLs on days 1 and 2. What is more, the effects of ASEE and ASB at the highest dose (150 mg/kg) on TL were comparable to that of donepezil (Figure 5A).
ASEE and ASB Activity on Spatial Memory Deficits in the Morris Water Maze (MWM) Behavioral Test
In the MWM behavioral test, a distinct protraction of platform escape latency (EL) ie spatial memory, was observed in mice following scopolamine treatment, while donepezil counteracted this effect of scopolamine by reducing EL on days 3 and 4 of the protocol. ASEE and ASB exerted their effects dose-dependently, although no noticeable reversal of memory deficit was recorded among the treated groups on days 1 and 2, except for ASEE (150 mg/kg). However, on day 4, both ASEE and ASB at doses of 50mg/kg evoked a notable decline in EL, while at 100 mg/kg, organic bark extracts instigated a reduction in EL on days 3 and 4. Administration of the highest dose of ASEE and ASB (150 mg/kg) produced the most marked mitigation of platform EL on days 3 and 4 (Figure 6A). To investigate memory retention, a probe trial was carried out on day 5 by removing the hidden platform, and animals were allowed to swim freely in the water pool. The test revealed that the scopolamine-treated group spent a shorter time in the target quadrant compared to the corresponding controls. This effect was reversed not only by donepezil but also by extracts of ASEE and ASB bark (Figure 6B).
The anti-amnesic activity of ASEE and ASB extract on diazepam-induced amnesia was disclosed in the MWM test and the effects were shown to be dose-dependent in shortening the EL behavior. Although ASEE and ASB did not possess anti-amnesic properties at 50 mg/kg, ASEE (100–150 mg/kg) did show significant activity on days 1, 3, and 4 of the procedure. Similarly, on days 3 and 4, ASB (100–150 mg/kg) markedly reduced platform ELs (Figure 7A). On day 5, probe trial was performed as before by removing the water pool platform. Subsequently, diazepam-treated mice were found to spend less time in the target quadrant compared to controls and this was countered not only by donepezil but also by ASEE and ASB extracts (Figure 7B). Accordingly, the findings with the MWM are inclined to corroborate the EPM behavioral outcomes and indicate that ASEE and ASB extracts demonstrably improve spatial learning and memory acquisition and consolidation.
ASEE and ASB Improvement of Scopolamine and Diazepam-Induced Spatial Memory and Exploratory Behavior
The Y-maze behavioral test was employed to evaluate spatial memory and exploratory behavior in mice. The scopolamine-treated animal group expressed a shortened percentage of spontaneous alternation behavior (SAB) and this SAB decrement was reversed by donepezil. Neither ASEE nor ASB at the dose level of 50 mg/kg improved the decline in SAB generated by scopolamine. However, ASEE and ASB (100–150mg/kg) significantly cause an increase in the %-SAB, while in case of the ASB (150mg/kg), the improvement was comparable to that of donepezil (Figure 8A). ASEE and ASB also produced analogous effects against diazepam evoked cognitive deficit in the Y-maze. So, diazepam reduced the percentage of SAB considerably while ASEE and ASB (150 mg/kg) both retrieved the diazepam-induced memory impairment by raising SAB (Figure 8B). Therefore, it was deduced that the result of the Y-maze behavioral tests revealed that the organic A. stenophylla bark extracts had substantially improved mouse exploratory behavior and spatial memory following scopolamine- and diazepam-induced cognitive impairments.
Discussion
Medicinal plants are a rich source of secondary metabolites that are used for various therapeutic purposes. Phytochemicals such as flavonoids, alkaloids, tannins, and phenolic compounds are well-known bioactive agents found in medicinal plants and have been found to possess substantial therapeutic effects against a variety of health-related complications. To uncover the main curative agent, it is necessary to identify the biologically active constituents of medicinal plants.39,40 Several acetylcholinesterase inhibitors such as donepezil, galantamine, piracetam, and rivastigmine have been used for AD management. However, because they offer only symptomatic relief and/or due to their side effects, no agreeable treatment is currently available for AD. Herein, we have extracted ASEE and ASB fractions from the bark of A. stenophylla and studied their spatial memory neuroprotective and cognitive propensities. We also performed GC-MS analysis followed by studies on anticholinesterase activity, antioxidant effects, and their actions on cognition and memory-related behavior.
Plants are a rich source of biologically active constituents that have been used for the treatment of various neurological disorders and AD is the commonest form of dementia.41–43 After the extraction of AEE and ASB fractions, GC-MS analysis was used to examine the concentration of biologically active compounds isolated from the bark of A. stenophylla. GC-MS analysis revealed various bioactive secondary metabolites found in the extracts. These active constituents, regardless of their low or high concentration, are known to alleviate the development of various diseases such as neurodegenerative, cardiovascular, and cancer-related conditions.44,45 The availability of various biologically active constituents in the bark of A. stenophylla confirmed that there may be possible anti-amnesic, antioxidant and inflammatory constituents.
Nootropics are mental performance enhancers that may either be synthetic (eg piracetam) or naturally occurring (eg Ginkgo biloba) and both types of agents are capable of increasing mental functions such as attention, concentration, memory, and motivation.46 Some natural nootropics are effective in boosting brain function through dilatation of small arteries and veins in the brain increasing blood circulation and facilitating the supply of essential nutrients and oxygen to the tissues. Other nootropic mechanisms involve antioxidant activity and enhancement of ACh levels thereby increasing cholinergic receptor binding in the hippocampus and frontal cortex.47,48
Diazepam-induced amnesia is mediated primarily through benzodiazepine receptors, while scopolamine-induced memory impairment is largely attributed to a cholinergic deficit in some brain areas.49,50 Consequently, we exploited both of these mechanisms to induce memory deficits to broaden model validities and their pertinence to clinical disease. ACh plays an important role in memory and learning processes and brain concentrations of Ach and its activity decline during memory impairment.35 In this regard, inhibition of AChE and BuChE facilitate cholinergic transmission by decreasing the hydrolysis of acetylcholine. This has been reported to be a strategy for the symptomatic management of AD as a synthetic target being part of a multi-functional approach.51 Our anticholinesterase studies identified ASEE and ASB extracts as inhibitors of both AChE and BuChE to potentially increase cholinergic transmission providing enhanced cognition and improved learning and memory consolidation. The unequivocal monophasic dose-related anti-amnesic and cognitive enhancement propensity of ASEE and ASB fractions might be attributed to the presence of secondary metabolites such as phenol, 2.4-bis (1,1-dimethyl ethyl), hexadecanoic acid, methyl ester, and cyclohexanone. A possible neuroprotective effect might be exerted by the hexadecanoic acid found in the ASEE fraction that produces anticholinesterase activity to increase cholinergic neurotransmission.52 The ameliorative action of ASEE on cognitive impairment could also be attributed to the presence of active phenolic constituents such as phenol, 2.4-bis (1,1-dimethyl ethyl), which have been shown to inhibit the activity of AChE and BuChE.53,54 Similarly, the bioactive constituent, cyclohexanone, found in the crude ASEE and ASB fractions may also act as an AChE and BuChE inhibitor.55 Our study verified that ASEE and ASB extracts produced a concentration-related inhibition of AChE and BuChE (Figure 4) likely to give rise to the elevated cholinergic function and conducive to enhanced learning and memory. Different plants of acacia species are effective for memory impairment such as Acacia auriculiformis (200–400 mg/kg) caused memory improvement.56 Acacia obtusifolia and Acacia catechu inhibit acetylcholinesterase activity (IC50 values: 81.6 and 204.38 μg/mL).57,58 Acacia farnesiana and Acacia sieberiana root cause AChE inhibition (58–60%) and Acacia Senegal and Acacia seyalcause a minor decline in the AChEactivity in mice.27,59,60 Acacia stenophyllabarkextracts are more effective than these plants in memory improvement. Butanolic extract of Acacia cyanophylla inhibits acetylcholinesterase activity (IC50 value: 16.03 μg/mL), this is more effective than Acacia stenophylla bark extracts.61
Reactive oxygen species (ROS) and related free radicals such as lipid peroxides are produced during various metabolic reactions in the body, while antioxidants scavenge these free radicals by interacting and neutralizing them.62,63 Oxidative stress is characterized by an imbalance between the rate of free radical production and the antioxidant defense of the body.2 Oxidative brain damage and neuroinflammation are the prime culprits involved in the pathogenesis of AD along with several other factors such as environmental issues and genetic anomalies.64,65 Neurons consume more oxygen and produce energy at a high rate therefore they are more vulnerable to oxidative damage from ROS. The mechanism of amyloid-beta (Aβ) accumulation is unknown, but ROS generation during self-aggregation of Aβ is a potential mechanism by which Aβ may induce neuronal damage.66 It has been reported that oxidative stress induces apoptosis. Hence, the generation of ROS mediates the events leading to apoptosis which causes cell death. Apoptosis in the presence of ROS generation involves caspase activation and this initiates an augmented apoptotic pathway by activating other caspases including caspase-3 leading to AD and rapid cell death.67 In this regard, antioxidants reduce apoptosis and caspase-3 which is beneficial for AD treatment. ASEE and ASB extract both exhibited significant antioxidant potential as shown in the DPPH and H2O2 assays. The results disclosed a concentration-dependent free radical scavenging proclivity for the ASEE and ASB fractions in the DPPH assay. Maximum free radical scavenging activities were observed at a high concentration (1000µg/mL) for ASEE and ASB with corresponding IC50 values in the 28–33 µg/mL range. In the H2O2 free radical scavenging assay, both ASEE and ASB fractions produced good antioxidant responses with IC50 values between 59–65 µg/mL. It was also noted that the free radical scavenging potential of both ASEE and ASB did not exceed that of the ascorbic acid standard but the antioxidant action was significant (Table 1). The antioxidant effectiveness of ASEE and ASB extracts may be attributed to a capability for electron or hydrogen donation, like other species of Acacia previously reported.7,68,69
An acute toxicity study was carried out to establish a safety profile associated with the A. stenophylla bark extracts and a maximum tolerated dose (MTD) of 1200 mg/kg was determined for both extracts. The open-field test was employed to assess the general locomotor activity and exploratory behaviors affected by the extracts. The outcome showed that on its own, scopolamine markedly increased the incidence of rearing and grooming. However, the frequencies of rearing and grooming were dose-dependently reduced by ASEE and ASB, suggesting a reversal of scopolamine-induced amnesia-related general locomotor activity and exploratory behaviors. Subsequently, to ascertain the anti-amnesic potential of ASEE and ASB extracts, behavioral models were employed to examine any learning, memory, or related cognitive aptitudes. Scopolamine or diazepam caused substantial increases in animal TLs on training and retention days in the elevated plus-maze, while ASEE and ASB extracts reversed these cognitive deficits induced by scopolamine and diazepam. Similarly, the MWM was used to evaluate learning and memory consolidation and to substantiate the EPM findings. The donepezil standard, as well as the ASEE and ASB extracts, all manifested a recovery from scopolamine and diazepam memory impairment by reducing platform EL during training sessions and the time spent in the target quadrant after platform removal. Finally, to assess exploratory and spatial memory-related functions, the Y-maze behavioral test was used. Scopolamine or diazepam was found to reduce spontaneous alternation behavior, reflecting a deficit in exploratory and spatial memory that was countered by ASEE and ASB. Various etiological pathways have been described to detect AD. These pathways include cholinesterase level, cerebral blood flow, beta-amyloid (Aβ) protein level, neurotransmitter fluctuations, neuroinflammation, and oxidative stress. The drugs that decrease the level of cholinesterase enzymes, Aβ plaques formation, neuroinflammation, and oxidative stress have the potential to reduce memory impairment.70,71 Acacia stenophylla bark extracts inhibited the cholinesterase and oxidative stress, and also improved the behavioral changes in amnesic mice. The Acacia stenophylla bark contains cyclic ketone derivatives, it has been reported that these derivatives reduce cholinesterases activities, Aβ plaques formation, neuroinflammation, and oxidative stress in the mice model of Alzheimer’s disease.30,72 The possible mechanism of action of Acacia stenophylla bark extracts to ameliorate amnesia is their anti-cholinesterase and anti-oxidant activities and the anti-inflammatory, anti-oxidant, and anti-cholinesterase properties of their active constituents derivatives. The schematic representation of ASEE and ASB extracts is shown in Figure 9. The Acacia stenophylla reduces memory impairment without causing motor coordination deficits. In the next phase of this study, we will evaluate the effect of Acaciastenophyllaon APP level, BACE-2, α-, β-, and γ-secretase, tau protein, neuron degeneration, and overall gliosis.
 |
Figure 9 Schematic representation of ASEE and ASB extracts showing potential antioxidant and anticholinesterase effects and mitigates scopolamine and diazepam-induced cognitive dysfunction. |
Data Sharing Statement
All the data has been either included in the manuscript.
Ethics Statement
Institutional Review Board Statement: The experimental procedures on animals were performed under the approval of Ethics Committee of the Department of Pharmacy, University of Peshawar (registration number: 12/EC-17/Pharm). The experimental procedures on animals were performed according to with the relevant guidelines and regulations of the United Kingdom Animals (Scientific procedures) Act 1986.
Acknowledgments
The authors thank the Department of Pharmacy, University of Peshawar, and the Department of Botany, Islamic college University Peshawar, for providing the facilities necessary to conduct this research. Yusuf S. Althobaiti was supported by Taif University Researchers Supporting Project number (TURSP-2020/78), Taif University, Taif, Saudi Arabia. Fahad S Alshehri would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by grant code (22UQU4310453DSR04).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All the authors declare no conflicts of interest in relation to this work.
References
1. Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137–147. doi:10.1136/jnnp.66.2.137
2. Singhal AK, Naithani V, Naithani V. Pharmacology, neurological diseases. Medicinal plants with a potential to treat Alzheimer and associated symptoms. Int J Nutr Pharmacol Neurol Dis. 2012;2(2):84. doi:10.4103/2231-0738.95927
3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446.
4. Korolev IOJMSRJ. Alzheimer’s disease: a clinical and basic science review. Med Stud Res J. 2014;4(1):24–33.
5. Lee JS, Kim HG, Lee HW, et al. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci Rep. 2015;5:9651. doi:10.1038/srep09651
6. Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci. 2007;81(3):177–187. doi:10.1016/j.lfs.2007.05.005
7. Saha MR, Dey P, Begum S, et al. Effect of acacia catechu (L.f.) willd. on oxidative stress with possible implications in alleviating selected cognitive disorders. PLoS One. 2016;11(3):e0150574. doi:10.1371/journal.pone.0150574
8. Scartezzini P, Speroni E. Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol. 2000;71(1–2):23–43. doi:10.1016/S0378-8741(00)00213-0
9. Meszaros AV, Weidinger A, Dorighello G, et al. The impact of inflammatory cytokines on liver damage caused by elevated generation of mitochondrial reactive oxygen species. Free Radical Bio Med. 2016;100:S57. doi:10.1016/j.freeradbiomed.2016.10.149
10. Bhadra S, Dalai MK, Chanda J, Mukherjee PK. Evaluation of bioactive compounds as acetylcholinesterase inhibitors from medicinal plants. Evid-Based Validat Herb Med. 2015;273–306. doi:10.1016/B978-0-12-800874-4.00013-1
11. Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315–335. doi:10.2174/1570159X11311030006
12. Mukherjee PKJDIJ. Evaluation of Indian traditional medicine. Drug Info J. 2001;35(2):623–632. doi:10.1177/009286150103500235
13. Esimone C, Ebebe I, Chah K, Onyeka CJJOTMP. Comparative antibacterial effects of Psidium guajava aqueous extract. J Trop Med Plants. 2003;4(2):185–190.
14. Ullah B.Pharmacognosy of Skimmia Laureola (Dc.) Siebold. & Zucc. Ex Walp. And Zanthoxylum Armatum Dc. Family Rutaceae [Doctoral Dessertation]. Uni of Peshawar; 2012.
15. Nimse SB, Pal DJRA. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adva. 2015;5(35):27986–28006. doi:10.1039/C4RA13315C
16. Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. 2017;38(7):592–607. doi:10.1016/j.tips.2017.04.005
17. Sajid M, Khan MR, Ismail H, et al. Antidiabetic and antioxidant potential of Alnus nitida leaves in alloxan induced diabetic rats. J Ethnopharmacol. 2020;251:112544. doi:10.1016/j.jep.2020.112544
18. Farooq U, Khan T, Shah SA, et al. Isolation, characterization and neuroprotective activity of folecitin: an in vivo study. Life. 2021;11(8):825. doi:10.3390/life11080825
19. Fakri Mustafa Y, Riyadh Khalil R, Tareq Mohammed E, Bashir M, Khudhayer Oglah M. Effects of structural manipulation on the bioactivity of some coumarin-based products. Arch Razi Inst. 2021;76(5):1297. doi:10.22092/ari.2021.356100.1776
20. Khalil RR, Mohammed ET, Mustafa YF. Various promising biological effects of cranberry extract: A; 2021.
21. Khalil RR, Mohammed ET, Mustafa YF. Evaluation of in vitro antioxidant and antidiabetic properties of cydonia oblonga seeds’ extracts. J Med Chem Sci. 2022;5(6):1048–1058.
22. Biradar S, Tarak K, Kulkarni V, Habbu P, Smita D. Toxicology. Antiamnesic and antioxidant effect of Acacia catechu-catechin in normal, aged and scopolamine challenged cognitive deficit mice. J Pharmacol Toxicol. 2012;7(5):231–241. doi:10.3923/jpt.2012.231.241
23. Shetty M, Kameshwar Y, Shenoy S, Bhandarkar AP, Vaishnav R, Shenoy G. Behavioral and biochemical evaluation of antiamnesic activity of Acacia auriculiformis leaf extract in rats. World J Pharm Res. 2015;4(4):1903–1915.
24. Khan N, Ahmad M, Khan R, Khan S, Muhammad NJWASJ. Investigation of Acacia modesta leaves for in-vitro antioxidant activity, enzyme inhibition and cytotoxicity. World Appl Sci J. 2014;30(3):286–293.
25. Sulaiman C, Sadashiva C, George S, Goplakrishnan V, Balachandran IJACL. Chromatographic studies and in vitro screening for acetyl cholinesterase inhibition and antioxidant activity of three Acacia species from South India. Ana Chem Lett. 2013;3(2):111–118. doi:10.1080/22297928.2013.806405
26. Benamar H, Rached W, Derdour A, Marouf AJJOBS. Screening of Algerian medicinal plants for acetylcholinesterase inhibitory activity. J Biol Sci. 2010;10(1):1–9. doi:10.3923/jbs.2010.1.9
27. Eldeen I, Elgorashi E, Van Staden J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J Ethnopharmacol. 2005;102(3):457–464. doi:10.1016/j.jep.2005.08.049
28. Trease GE, Evancs WC. Pharmacognosy.
29. Muhammad N, Saeed M, Khan HJBC. medicine a. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complement Altern Med. 2012;12(1):1–8. doi:10.1186/1472-6882-12-59
30. Ullah R, Ali G, Subhan F, et al. Attenuation of spatial memory in 5xFAD mice by targeting cholinesterases, oxidative stress and inflammatory signaling using 2-(hydroxyl-(2-nitrophenyl) methyl) cyclopentanone. Int Immunopharmacol. 2021;100:108083. doi:10.1016/j.intimp.2021.108083
31. Khan J, Ali G, Khan R, Ullah R, Ullah SJNS. Attenuation of vincristine-induced neuropathy by synthetic cyclohexenone-functionalized derivative in mice model. Neurol Sci. 2019;40(9):1799–1811. doi:10.1007/s10072-019-03884-6
32. Ahmad SI, Ali G, Muhammad T, Ullah R, Umar MN, Hashmi AN. Synthetic β-hydroxy ketone derivative inhibits cholinesterases, rescues oxidative stress and ameliorates cognitive deficits in 5XFAD mice model of AD. Mol Biol Rep. 2020;47:9553–9566. doi:10.1007/s11033-020-05997-0
33. Ayaz M, Junaid M, Ullah F, et al. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front Pharmacol. 2017;8:697. doi:10.3389/fphar.2017.00697
34. Ullah R, Ali G, Rasheed A, et al. The 7-Hydroxyflavone attenuates chemotherapy-induced neuropathic pain by targeting inflammatory pathway. Int Immunopharmacol. 2022;107:108674. doi:10.1016/j.intimp.2022.108674
35. Ullah R, Ali G, Ahmad N, et al. Attenuation of spatial memory in 5xFAD mice by halting cholinesterases, oxidative stress and neuroinflammation using a cyclopentanone derivative. Pharmaceuticals. 2020;13(10):318. doi:10.3390/ph13100318
36. Morris RJJONM. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi:10.1016/0165-0270(84)90007-4
37. Ullah R, Ali G, Baseer A, et al. Tannic acid inhibits lipopolysaccharide-induced cognitive impairment in adult mice by targeting multiple pathological features. Int Immunopharmacol. 2022;110:108970. doi:10.1016/j.intimp.2022.108970
38. Kim DH, Hung TM, Bae KH, et al. Gomisin A improves scopolamine-induced memory impairment in mice. Eur J Pharmacol. 2006;542(1–3):129–135. doi:10.1016/j.ejphar.2006.06.015
39. Vuorelaa P, Leinonenb M, Saikkuc P, et al. Natural products in the process of finding new drug candidates. Curr Med Chem. 2004;11(11):1375–1389. doi:10.2174/0929867043365116
40. Ullah R, Badshah W, Ali G, et al. Cassia artemisiodes attenuates nociceptive and diabetes-induced neuropathic pain modalities apropos antioxidant and anti-inflammatory mechanisms. Biomed Pharmacother. 2022;149:112834. doi:10.1016/j.biopha.2022.112834
41. Joy MKI, Akhter N, Reza R, et al. Ex-vivo acetylcholinesterase and butyrylcholinesterase inhibitory activities assay of G. asiatica and G. tiliaefolia (Tiliaceae) leaves. Annu Res Rev Biol. 2019;1–10. doi:10.9734/arrb/2019/v34i230149
42. Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39(1):41–51. doi:10.1038/ng1935
43. Farooq U, Sahibzada MUK, Khan T, et al. Folecitin isolated from hypericum oblongifolium exerts neuroprotection against lipopolysaccharide-induced neuronal synapse and memory dysfunction via p-AKT/Nrf-2/HO-1 signalling pathway. Evid-Based Complement Altern Med. 2022;2022:1–10. doi:10.1155/2022/9419918
44. Ansari N, Khodagholi F. Natural products as promising drug candidates for the treatment of Alzheimer’s disease: molecular mechanism aspect. Curr Neuropharmacol. 2013;11(4):414–429. doi:10.2174/1570159X11311040005
45. Sivanath C, Amutha S, Hemalatha G, Mini M, Karthikeyan GJIJCMAS. Identification of various chemical compounds in methanolic and chloroform crude extracts of groundnut seed coat by GC-MS. Int J Curr Microbiol App Sci. 2017;6(6):1952–1956. doi:10.20546/ijcmas.2017.606.228
46. Lanni C, Lenzken SC, Pascale A, et al. Cognition enhancers between treating and doping the mind. Pharmacol Res. 2008;57(3):196–213. doi:10.1016/j.phrs.2008.02.004
47. Suliman NA, Mat Taib CN, Mohd Moklas MA, Adenan MI, Hidayat Baharuldin MT, Basir R. Establishing natural nootropics: recent molecular enhancement influenced by natural nootropic. Evid Based Complement Alternat Med. 2016;2016:4391375. doi:10.1155/2016/4391375
48. Bhattacharya S, Bhattacharya A, Kumar A, Ghosal S. Antioxidant activity ofBacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14(3):174–179. doi:10.1002/(SICI)1099-1573(200005)14:3<174::AID-PTR624>3.0.CO;2-O
49. Parle M, Dhingra D. Ascorbic acid: a promising memory-enhancer in mice. J Pharmacol Sci. 2003;93(2):129–135. doi:10.1254/jphs.93.129
50. Saraf MK, Prabhakar S, Khanduja KL, Anand A. Bacopa monniera attenuates scopolamine-induced impairment of spatial memory in mice. Evid-Based Complement Altern Med. 2011;2011:1–10. doi:10.1093/ecam/neq038
51. Luo W, Li YP, He Y, et al. Synthesis and evaluation of heterobivalent tacrine derivatives as potential multi-functional anti-Alzheimer agents. Eur J Med Chem. 2011;46(6):2609–2616. doi:10.1016/j.ejmech.2011.03.058
52. Pahwa P, Goel RK. Asparagus adscendens root extract enhances cognition and protects against scopolamine induced amnesia: an in-silico and in-vivo studies. Chem Biol Interact. 2016;260:208–218. doi:10.1016/j.cbi.2016.10.007
53. Kim CR, Choi SJ, Kim JK, et al. 2,4-Bis (1,1-dimethylethyl) phenol from Cinnamomum loureirii improves cognitive deficit, cholinergic dysfunction, and oxidative damage in TMT-treated mice. Biol Pharm Bull. 2017;40(6):932–935. doi:10.1248/bpb.b16-00997
54. Ren J, Wang J, Karthikeyan S, Liu H, Cai JJIJOB. Biophysics. Natural anti-phytopathogenic fungi compound phenol, 2, 4-bis (1, 1-dimethylethyl) from Pseudomonas fluorescens TL-1. Indian J Biochem Biophys. 2019;56(2):162–168.
55. Zha GF, Zhang CP, Qin HL, et al. Biological evaluation of synthetic alpha, beta-unsaturated carbonyl based cyclohexanone derivatives as neuroprotective novel inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-beta aggregation. Bioorg Med Chem. 2016;24(10):2352–2359. doi:10.1016/j.bmc.2016.04.015
56. Sharma A, Shetty M, Parida A, Adiga S, Kamath S. Effect of ethanolic extract of Acacia auriculiformis leaves on learning and memory in rats. Pharmacognosy Res. 2014;6(3):246. doi:10.4103/0974-8490.132605
57. Kim DH, Yoon BH, Kim Y-W, et al. The seed extract of cassia obtusifolia ameliorates learning and memory impairments induced by scopolamine or transient cerebral hypoperfusion in mice. J Pharmacol Sci. 2007;105(1):82–93. doi:10.1254/jphs.FP0061565
58. Lakshmi T, Rajendran R. In vitro acetyl cholinesterase inhibitory assay of acacia catechu willd ethanolic seed extract. Pharmacogn J. 2015;7(5). doi:10.5530/pj.2015.5.5
59. Tengku N, Raja S. Inhibition of acetylcholinesterase and NADH oxidase by Acacia farnesiana. Panacea J Pharm Pharm Sci. 2013;1:15–17.
60. Albasher G, Alharthi NS, Alkahtani S, et al. Behavioral and physiological assessments to evaluate the effect of Acacia Senegal and Acacia seyal in albino mice. Pharmacogn Mag. 2020;16(70):410. doi:10.4103/pm.pm_20_20
61. Ghribia L, Ghouilaa H, Omrib A, Besbesb M, Janneta HB. Antioxidant and anti–acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac J Trop Biomed. 2014;4:S417–S423. doi:10.12980/APJTB.4.2014C1038
62. Diplock AT, Charleux JL, Crozier-Willi G, et al. Functional food science and defence against reactive oxidative species. Br J Nutr. 1998;80(Suppl 1):S77–112. doi:10.1079/BJN19980106
63. Onoja SO, Omeh YN, Ezeja MI, Chukwu MN. Evaluation of the in vitro and in vivo antioxidant potentials of aframomum melegueta methanolic seed extract. J Trop Med. 2014;2014:159343. doi:10.1155/2014/159343
64. Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71(2):621S–629S. doi:10.1093/ajcn/71.2.621s
65. Wang ZQ, Porreca F, Cuzzocrea S, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869–878. doi:10.1124/jpet.103.064154
66. Ghoneum MH, El Sayed NS. Protective effect of biobran/MGN-3 against sporadic alzheimer’s disease mouse model: possible role of oxidative stress and apoptotic pathways. Oxid Med Cell Longev. 2021;2021:8845064. doi:10.1155/2021/8845064
67. Musaogullari A, Mandato A, Chai Y-C. Role of glutathione depletion and reactive oxygen species generation on caspase-3 activation: a study with the kinase inhibitor staurosporine. Front Physiol. 2020;11. doi:10.3389/fphys.2020.00998
68. Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001;49(7):3420–3424. doi:10.1021/jf0100907
69. Zia-Ul-Haq M, Ahmad S, Bukhari SA, Amarowicz R, Ercisli S, Jaafar HZ. Compositional studies and biological activities of some mash bean (Vigna mungo (L.) Hepper) cultivars commonly consumed in Pakistan. Biol Res. 2014;47:23. doi:10.1186/0717-6287-47-23
70. Shal B, Khan A, Khan AU, et al. Coagulansin-A improves spatial memory in 5xFAD Tg mice by targeting Nrf-2/NF-κB and Bcl-2 pathway. Int Immunopharmacol. 2022;109:108860. doi:10.1016/j.intimp.2022.108860
71. Shal B, Khan A, Khan AU, et al. Alleviation of memory deficit by bergenin via the regulation of reelin and Nrf-2/NF-κB pathway in transgenic mouse model. Int J Mol Sci. 2021;22(12):6603. doi:10.3390/ijms22126603
72. Ullah R, Ali G, Khan A, Ahmad S, Al-Harrasi A. Cyclopentanone derivative attenuates memory impairment by inhibiting amyloid plaques formation in the 5xFAD mice. Int J Mol Sci. 2021;22(17):9559. doi:10.3390/ijms22179559
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.