Back to Journals » Drug Design, Development and Therapy » Volume 17
The Molecular Basis of the Anti-Inflammatory Property of Astragaloside IV for the Treatment of Diabetes and Its Complications
Authors Li L, Zhang Y, Luo Y, Meng X, Pan G, Zhang H, Li Y, Zhang B
Received 28 November 2022
Accepted for publication 3 February 2023
Published 10 March 2023 Volume 2023:17 Pages 771—790
DOI https://doi.org/10.2147/DDDT.S399423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Lin Li,1– 3,* Yuwei Zhang,1,3,* Yudan Luo,1 Xianghui Meng,1 Guixiang Pan,4 Han Zhang,1– 3 Yuhong Li,1– 3 Boli Zhang1,3
1Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, People’s Republic of China; 2Ministry of Education Key Laboratory of Pharmacology of Traditional Chinese Medical Formulae, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, People’s Republic of China; 3State Key Laboratory of Component-Based Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, People’s Republic of China; 4Second Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, 300250, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuhong Li; Boli Zhang, Tianjin University of Traditional Chinese Medicine, 10 Poyang Lake Road, Jing Hai District, Tianjin, 301617, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Astragali Radix is a significant traditional Chinese medication, and has a long history of clinical application in the treatment of diabetes mellitus (DM) and its complications. AS-IV is an active saponin isolated from it. Modern pharmacological study shows that AS-IV has anti-inflammatory, anti-oxidant and immunomodulatory activities. The popular inflammatory etiology of diabetes suggests that DM is a natural immune and low-grade inflammatory disease. Pharmacological intervention of the inflammatory response may provide promising and alternative approaches for the prevention and treatment of DM and its complications. Therefore, this article focuses on the potential of AS-IV in the treatment of DM from the perspective of an anti-inflammatory molecular basis. AS-IV plays a role by regulating a variety of anti-inflammatory pathways in multiple organs, tissues and target cells throughout the body. The blockade of the NF-κB inflammatory signaling pathway may be the central link of AS-IV’s anti-inflammatory effect, resulting in a reduction in the tissue structure and function damage stimulated by inflammatory factors. In addition, AS-IV can delay the onset of DM and its complications by inhibiting inflammation-related oxidative stress, fibrosis and apoptosis signals. In conclusion, AS-IV has therapeutic prospects from the perspective of reducing the inflammation of DM and its complications. An in-depth study on the anti-inflammatory mechanism of AS-IV is of great significance for the effective use of Chinese herbal medicine and the promotion of its status and influence on the world.
Keywords: astragaloside IV, anti-inflammatory property, molecular basis, diabetes, complications
Introduction
Diabetes mellitus (DM) is a metabolic disease, clinically characterized by high levels of blood glucose, and symptoms such as polydipsia, polyphagia, polyuria, and weight loss.1 Together with cardiovascular and cerebrovascular diseases and malignant tumors, it constitutes three threats to human life. According to the 2021 Global Diabetes map released officially by the International Diabetes Federation, 537 million adults aged 20–79 years currently have diabetes, which represents 10.5% of the global population in this age group. China has the largest number of diabetics, with the number of cases increasing from 90 million to 140 million over the past 10 years and the number of diabetics in China is predicted to reach 174.4 million by 2045, which will pose a serious threat to people’s health and quality of life. Unfortunately, DM is prone to various acute and chronic complications; the latter covers almost the whole body, including eyes, feet, kidneys, nerves, vasculature, and even the heart and brain, thereby becoming the main cause of disability and death.2
Inflammation is central in the pathogenesis of diabetes and metabolic syndrome, particularly in the development of complications involving innate immunity. In T1D and T2D, dysregulation of glucose and lipid metabolism drives chronic, unresolved tissue inflammation, manifested by increased levels of inflammatory biomarkers and immune cell infiltration.3,4 The primary goal of immune-therapy for type 1 diabetes is to prevent or delay the loss of functional β-cell masses involved in systemic immune dysregulation and autoreactive T cell, cell- or cytokine- directed interventions.5 For T2DM, chronic inflammation caused by obesity is the origin due to destructive behavior in insulin secretion, insulin metabolism, and energy homeostasis. Adipose tissue is the most direct effector of obesity. In adipose tissue, the balance of M1/M2 macrophages tends to be an M1 proinflammatory phenotype,6 which secretes tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and other inflammatory factors to promote low-grade inflammation. On the other hand, systemic circulating factors LPS and free fatty acids accumulate in adipose tissue and combine with toll-like receptor 4 (TLR4), leading to the activation of the inflammatory cascade nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and c-Jun N-terminal kinases 1 (JNK1).7–9 In addition to adipose tissue, carbohydrate metabolism organs, such as skeletal muscle and liver, respond in the same way. In general, activated monocytes and macrophages amplify the chronic inflammatory state by producing proinflammatory cytokines. This inflammatory state is thought to reduce insulin responsiveness in insulin-sensitive tissues, which increases the risk of T2DM and its complications.
Preclinical research supporting the role of inflammation as an underlying etiology of diabetes has increased almost exponentially over the past decade. Clinical trials demonstrating the potential of targeting inflammation for the treatment of DM are still ongoing. Notably, mounting evidence has proven that pharmacotherapies for DM may have direct or indirect anti-inflammatory effects; conversely, some anti-inflammatory approaches may stabilize fluctuating metabolic states. Both preclinical and clinical studies have illustrated that many antihyperglycemic agents, including thiazolidinedione (TZDs),10 metformin,11 dipeptidyl peptidase-4 inhibitors,12 and sodium-glucose cotransporter-2 inhibitors,13 inhibit the inflammatory response associated with their primary mechanisms of action. Several clinical studies have indicated that the IL-1β inhibitor canakinumab and anti-IL-1 treatment prevent and manage DM.14–16 Therefore, it is increasingly accepted that pharmacological intervention of the inflammatory response may provide promising and alternative approaches for the prevention and treatment of DM.
With the advancement of the Chinese medicine business on a global scale, traditional Chinese medicine, especially its active ingredients are becoming widespread and acceptable. Astragali Radix is the dried root of A. membranaceus (Fisch.) Bge. var. mongholicus (Bge.). As a commonly used Chinese medicine for invigorating qi, it has a history of more than two thousand years of clinical application, and it can be traced back to the ancient medical book “Fifty-two Disease Prescriptions”. Modern pharmacological studies have proven that it has anti-inflammatory, antioxidant, antiviral, antidiabetic and immunomodulatory effects.17 It has been used not only as a clinical traditional Chinese prescription, but also as a tea diet and a condiment for cooking. Astragaloside IV (AS-IV) is an active saponin isolated from Astragali Radix, and widely used in TCM preparations for treating DM, such as Astragalus injection18 and Shen-Qi-Jiang-Tang granule,19 and acts as the main active ingredient.20 As the star ingredient of Astragali Radix, AS-IV’s anti-inflammatory effect cannot be ignored. Studies have shown that AS-IV inhibits inflammation by blocking the TLR4/NF-κB, mitogen-activated protein kinase (MAPK), and Janus tyrosine kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways.21–23 Inhibiting the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasomes and regulating the levels of TNF-α, IL-6 and interleukin-1β (IL-1β) inflammatory factors are also ways that AS-IV participates in the complex regulation of the inflammatory response.24,25 In addition, clinical studies have found that AS-IV attenuates the expression of TLR4 in diabetic patients through the NF-κB inflammatory signaling pathway.26 Otherwise, sufficient experimental studies have also reported that the mechanism of AS-IV in the treatment of DM and its complications is related to its anti-inflammatory effects. However, to date, no systematic review has been conducted to assess the anti-inflammatory protection and underlying mechanisms of how AS-IV combats diabetes and its complications. As inflammatory signaling is complex and multifaceted, it is not clear which of the available anti-inflammatory strategies, alone or in combination, will have the best therapeutic potential. In this sense, it is necessary to systematically understand the anti-inflammatory effects of AS-IV in the treatment of DM and complications, which will help to guide the application of AS-IV to treat DM in the clinic.
Pharmacokinetics and Toxicity of AS-IV
AS-IV is rapidly absorbed and widely distributed in various tissues of the body after intravenous injection. Its concentration is highest in the liver and kidney, followed by the lung, heart, spleen, stomach, duodenum, skin, and the least in the brain.27 Previous studies have found that 30% of AS-IV is recycled in bile and 50% in urine and feces, suggesting that nearly 50% of AS-IV may be metabolized in vivo.28 However, AS-IV was barely metabolized in the liver, indicating that there was no first-pass elimination.29 At a dose of 0.75 mg/kg intravenously, the elimination half-life of AS-IV in rats ranged from 34.0 to 131.6 min, with a systemic clearance of 3 mL/kg/min; and at a dose of 0.5 mg/kg intravenously, the elimination half-life in beagles was 50.2 to 68.8 min, and the systemic clearance was 4±1 mL/kg/min, indicating a lower systemic clearance of AS-IV and no significant species differences in the pharmacokinetics of AS-IV in rats and beagles, with the same linear pharmacokinetic profile.30
AS-IV is a special saponin with poor absorption. After oral administration of 10 mg/kg, the absolute bioavailability of AS-IV in rats and beagles was 3.66% and 7.4%.30,31 A study in Caco-2 cells reported that the low absorption of AS-IV mainly results from its poor intestinal permeability, high molecular weight, low lipophilicity and paracellular transport.32 To address this, LS-102, a novel water-soluble derivative of AS-IV was developed and showed a pharmacokinetic profile different from AS-IV with higher bioavailability and similar toxic tolerance.33 Therefore, the use of advanced preparation to develop new dosage forms of AS-IV is feasible and effective, and become a potential strategy for its new drug development.
Even though the toxic effects of AS-IV are ongoing, the acute and long-term toxicity of different dosages of Astragali radix, such as capsule,34 lyophilized powder,35,36 combined nutrient solution37 and injection38 have been reported. And most of them have no obvious acute and long-term toxicity, suggesting that Astragali radix is a relatively safe medicine. For example, the acute toxicity results indicated that LD50 was 90.39 g/kg when Astragalus injection was given intravenously, and LD50 was 108.11 g/kg when Astragalus injection was injected intraperitoneally. Chronic toxicity studies were conducted in the rats at daily dosage from 6 to 33 g/kg. After 30-day-treatment, the normal food intake, weight gain, indices of uric, hematology, serum biochemistry, organ coefficient, and histopathological observation indicated no chronic toxicity. Existing researches have shown that AS-IV has no obvious toxicity or adverse reactions. Continuous oral administration of AS-IV (10 mg/kg/d) did not affect liver and renal function in rats for 14 weeks.39,40 To further ensure the safety of AS-IV, Jiangbo Zhu et al evaluated its acute toxicity, maternal toxicity, embryonic toxicity and fetal toxicity. Maternal and fetal toxicity were observed after intravenous administration of AS-IV (0.5–1.0 mg/kg) in SD rats and New Zealand rabbits during pregnancy. Further studies indicated that maternal exposure to AS-IV (1.0 mg/kg, 4w) directly leads to the delay in pups fur formation, eye opening, and cliff parry reflex (Neurodevelopmental marker), while it has no effect on memory and learning of newborn rats.41 Thus, based on the existing researches, AS-IV should still be used with caution in pregnant women to treat diabetes and its complications. Toxicity evaluation is the premise of clinical transformation. Pharmacological effects of AS-IV have been extensively reported, but toxicity studies are few. Although the reproductive toxicity has been reported, there is still a need of systematical toxicity evaluation, including acute toxicity, subacute toxicity, chronic toxicity, genotoxicity, and immunotoxicity.
Anti-Inflammatory Effects of AS-IV for the Treatment of Diabetes Mellitus
AS-IV demonstrates antidiabetic effects on regulating glucose and lipid metabolism, targeting skeletal muscle, liver, and adipose tissue. Adipose tissue inflammation is a key contributor to ectopic lipid accumulation in the development of insulin resistance and T2DM. Inflammation causes increased circulating free fatty acids (FFAs) by promoting lipolysis and halting lipogenesis in adipocytes. When targeting adipose tissue, AS-IV improved insulin resistance through its antilipolytic action. In TNF-α-stimulated 3T3-L1 adipocytes, AS-IV inhibited ERK phosphorylation to enhance the perilipin level, which coated the surface of intracellular lipid droplets to halt the entrance of lipase to the triacylglycerol stored within the lipid droplets.42 In addition to anti-lipolysis action, AS-IV demonstrated activity that promotes lipid production by upregulating key enzymes in lipogenesis, including lipoprotein lipase (LPL), fatty acid synthase (FAS) and glycerol-3-phosphate acyltransferase (GPAT) in adipocytes.42
Adipose tissue stores excess energy in the neutral lipid form of triglyceride (TG). However, once the storage function is destroyed, circulating FFAs derived from lipolysis favors TG synthesis in non-adipose tissues. This ectopic lipid, which accumulates in the liver, skeletal muscle, pancreas and other tissues, induces inflammation and ultimately leads to insulin resistance.43–45 Recent studies have revealed the contributory action of AS-IV on whole-body insulin sensitivity by relieving ectopic lipids in liver and skeletal muscle, partly relying on anti-inflammatory effects. Zhou46 reported that AS-IV was an effective and specific inhibitor of protein tyrosine phosphatase 1B (PTP1B), which not only initiates the insulin signaling pathway by dephosphorylating key tyrosine residues of the insulin receptor (IR) and insulin receptor substrate-1 (IRS-1), but also inhibits lipogenesis in liver tissue by downregulating SREBP1, in an insulin resistance model and a metabolic-associated fatty liver disease (MAFLD) model. In particular, they further indicated that AS-IV and PTP1B could be well-combined through hydrogen bonding by molecular docking analysis. Otherwise, it is clear that inflammatory cytokines such as TNF-α and IL-6 promote the development of insulin resistance by triggering glycogenolysis.47,48 In HFD and STZ treated C57BL/6J mice, with the inhibitory effects of AS-IV on glycogen phosphorylase (GP) and glucose-6-phosphatase (G6Pase), the two key enzymes in glycogenolysis, hepatic glucose production was reduced.49 Similarly, AS-IV demonstrated anti-inflammatory effects on IR in skeletal muscle. As an inhibitor of TLR4, AS-IV effectively halted the inflammatory response in palmitate-stimulated C2C12 myotubes. Zhu50 reported that AS-IV impeded the IKK/IκBα/NF-κB cascade, blocked IL-6 and TNF-α expression and subsequently activated the IRS/Akt pathway against insulin resistance by palmitate in C2C12 myotubes. However, there are no data to illustrate the mechanism of AS-IV’s action on TLR4 inhibition.
Some hypoglycemic drugs have intrinsic anti-inflammatory activities associated with their primary mechanisms of action. The anti-inflammatory actions of hypoglycemic drugs, including TZDs and metformin are related to adenosine monophosphate activated protein kinase (AMPK) activation and NF-κB inhibition.13 Regarding AS-IV, preclinical studies suggest that AS-IV has the potential for the treatment of DM in some aspects of anti-inflammation (Table 1). First, AS-IV may have the potential to be developed as a health care product for obese patients, since AS-IV initially inhibits lipolysis in adipocytes. By decreasing circulating FFAs derived from fat, early administration of AS-IV in overweight or obesity populations, even in the absence of DM, may prevent IR. Additionally, preclinical studies suggest that AS-IV helps to mobilize liver glucose metabolism, prevent ectopic lipid accumulation, and halt liver and skeletal muscle inflammation and insulin resistance. Moreover, FFA has been reported to trigger chronic and low-grade inflammation in human islets.51 Exposure of islets to high glucose levels promotes the expression of proinflammatory cytokines. Since AS-IV has shown significant anti-inflammatory effects, whether it can prevent pancreatic inflammation in diabetic patients needs to be actively explored in the future.
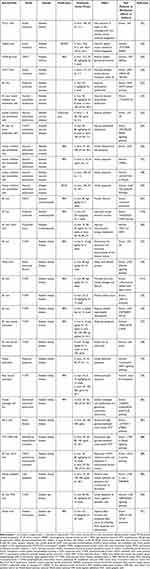 |
Table 1 Summary of Anti-Inflammatory Activities of AS-IV on the Treatment of DM and Its Complications |
Anti-Inflammatory Effects of AS-IV for the Treatment of Diabetic Complications
Diabetic Vascular Disease
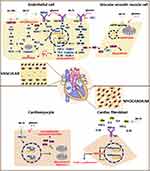
Under diabetes, blood vessels are directly exposed to high levels of glucose and FFA, which triggers oxidative stress and inflammation. A large number of preclinical studies have confirmed that AS-IV plays an anti-inflammatory role in the prevention and treatment of diabetic vascular diseases. Endothelial dysfunction is a pathophysiological step in the early stage of DM macrovascular complications. Excessive inflammatory cytokines are vital contributors to endothelial dysfunction caused by high glucose. In a rodent model of T2DM, AS-IV has been reported to improve endothelial dysfunction,52 especially endothelium-dependent relaxation in the aorta.53 Although vascular smooth muscle cells and endothelial cells are both vulnerable to damage by hyperglycemia, most studies of AS-IV have focused on endothelial cells as the target cells. In TNF-α and LPS-stimulated endothelial cells, AS-IV effectively halts the enhanced expression of the adhesion molecules E-selectin and VCAM-1 by inhibiting the nuclear transportation of NF-κB.54 By targeting TLR4, in both the aorta of diabetic rats and HUVECs treated with high glucose, AS-IV reduced inflammatory cytokine content, including IL-6 and TNF-α, and the expression of VCAM-1 and ICAM-1,53 by inactivating the NF-κB signaling pathway. In addition to TLR4, recent research has indicated that AS-IV targets the P2X7 receptor (P2X7R) to inhibit inflammation via the P2X7R dependent p38 MAPK signaling pathway. P2X7R is an ATP-gated cation channel. Phosphorylated p38 by P2X7R promotes the recruitment of leukocytes to the site of inflammation, accelerates inflammation, and causes endothelial dysfunction by destroying the eNOS/NO signaling pathway as a result. A study demonstrated an inhibitory effect of AS-IV on the P2X7R/p38 MAPK signaling pathway.55 Moreover, inflammatory cytokines phosphorylate ASK1 and subsequently activate the JNK signaling pathway, which promotes proinflammatory gene expression and mitochondrial dependent apoptosis. An in vitro study indicated that AS-IV ameliorated HG-induced inflammation and apoptosis in HUVECs by inactivating ASK and JNK phosphorylation, due to the reductive effect of AS-IV on TNF-α and IL-1β.56 In addition, Xu57 reported that AS-IV reversed TNF-α-induced HUVEC injury by promoting PPARγ expression.
Notably, inflammation induces apoptosis, while apoptosis further accelerates inflammation and induces DM vascular lesions. Several studies have reported that AS-IV relieves oxidative stress and oxidized low-density lipoprotein (ox-LDL) induced apoptosis of endothelial cells. NADPH oxidase 4 (NOX4)-dependent reactive oxygen species aggregation is the main cause of vascular endothelial inflammation and cell apoptosis. Ma et al reported that AS-IV ameliorated H2O2-induced oxidative stress by inhibiting NOX4 expression, and subsequently suppressed the TGF-β1/Smad2 pathway and halted the apoptosis process.58 However, there are no data to demonstrate changes in inflammatory factors in their report. In T2D, endothelial cell apoptosis triggered by ox-LDL induces vascular complications. In HUVECs, ox-LDL downregulated miR-140-3p expression, which could promote cell proliferation and inhibit apoptosis. A current study provides evidence that AS-IV suppresses ox-LDL-induced apoptosis in HUVECs by upregulating of miR-140-3p expression and inactivating the downstream Krüppel‑like factor 4 (KLF4)/PI3K/Akt signaling pathway.59 However, there is a controversy that the result is not consistent with those in other studies due to the inhibited role of AS-IV on the PI3K/Akt signaling pathway, which is worthy of further study.
The low-grade inflammatory reaction of the blood vessel wall induced by hyperglycemia is the basis for the occurrence and development of diabetic vascular complications. Under inflammatory conditions, inflammatory mediators continuously stimulate vascular endothelial cells, and the stability of the connection between endothelial cells is destroyed, along with cell gaps, as a result of increased vascular permeability and leakage of macromolecular substances.60 When these leaked macromolecular substances deposit into the blood vessel wall, tissue edema and acceleration of atherosclerosis occur. Hyperglycemia is the trigger of endothelial dysfunction, partly attributed to enhanced vascular permeability, which is determined by translocation and activation of protein kinase C (PKC), redistribution of filamentous actin (F-actin) and formation of intercellular gaps. Rearrangement of F-actin contributes to the maintenance of structural integrity. It has been reported that AS-IV improves barrier dysfunction induced by high glucose in HUVECs, by inhibiting PKC translocation from the cytosol to the membrane and improving the redistribution of the F-actin cytoskeleton.61 However, it is not clear whether AS-IV regulates rearrangement by exerting its anti-inflammatory effects, which remains to be further studied. Whether AS-IV inhibits the activation of PKC by inhibiting inflammation and thereby protects vascular permeability also remains to be further illustrated.
In addition to endothelial cells, one of the main causes of vascular dysfunction in DM is the reconstruction of the vascular wall caused by the abnormal proliferation and migration of vascular smooth muscle cells (VSMCs), thereby promoting the formation of vascular plaque.62,63 Studies have shown that inflammatory cytokines are key inducers of smooth muscle cell proliferation. In particular, TNF-α shows mitotic characteristics on smooth muscle cells by directly activating the NF-κB and p42/44 signaling pathways.61 An in vitro experiment revealed that AS-IV retarded proliferation and promoted apoptosis in VSMCs under high glucose conditions.64 The mechanism involved in intervention with the cell cycle, regulating phenotypic modulation, and protecting mitochondria, thereby halting the process of vascular remodeling. However, it is unclear whether the inhibitory effect of AS-IV on VSMC proliferation is related to its anti-inflammatory action.
In general, sufficient preclinical studies have confirmed the role of AS-IV in the prevention and treatment of diabetic vascular disease. AS-IV not only directly inhibits inflammation-related pathways, but also plays a role indirectly by suppressing apoptosis (Figure 1, Table 1). The literature has revealed the anti-inflammatory effect of AS-IV on diabetic vascular disease from the aspects of pharmacological effects, pathways and target receptors in preclinical studies, providing a basis for clinical research. Notably, preclinical studies suggest that AS-IV is an inhibitor of TLR4 and NOX4. The latest findings indicated that it is an inhibitor of P2X7R and a miR140 agonist. However, there are many challenges including but not limited to clarifying the exact target of AS-IV in the treatment of diabetic vascular disease, due to protein interaction technology, including coimmunoprecipitation, protein chip, and fluorescence resonance energy transfer.
Diabetic Cardiomyopathy
Diabetic cardiomyopathy is one of the cardiovascular complications of diabetes. The main pathological features are myocardial hypertrophy, interstitial fibrosis, diffuse microvascular injury and diastolic dysfunction. Oxidative stress and inflammation induced by hyperglycemia can cause vascular endothelial cell damage or dysfunction, and abnormal microvascular constriction, causing coronary microvascular disorders, and further leading to capillary basement membrane thickening, myocardial fibrosis, and eventually diabetes cardiomyopathy. In addition, the activation of the inflammatory factors TNF-α and IL-6 and the NLRP3 inflammasome65 favors the development of myocardial tissue fibrosis and fibroblast transformation, and ultimately leads to heart failure.66 In this sense, inflammation is closely related to the development of diabetic cardiomyopathy.
AS-IV improves diabetic myocardial fibrosis at the level of cardiac structure, restores myocardial systolic and diastolic functions at the level of cardiac function, and regulates energy metabolism and lipid metabolism at the level of cardiac metabolism. The mechanism of AS-IV’s action against diabetic cardiomyopathy has been reported to be related to inhibition of oxidative stress and inflammation, thereby restoring NO-mediated myocardial diastolic function.
Myocardial lipid metabolism disorder characterized by excessive intake of FFA and storage of TG in cardiomyocytes is an important cause of myocardial fibrosis by activating inflammation. In T2DM and HFD rats, AS-IV effectively inhibited increased systemic TC, TG and myocardial FFA, as well as inflammatory factors, including TNF-α, IL-6, and IL-1β.67 As a result, AS-IV improved myocardial systolic function by preventing fibrosis. Furthermore, Lin reported that AS-IV improved cardiac diastolic function by activating the endothelial eNOS/NO/cGMP pathway. Diabetes leads to mitochondrial dysfunction and insufficient ATP production, which contributes to energy metabolism disorders in the heart. Energy metabolism disorders induce excessive production of ROS, which activate mediators of inflammation. PGC-1α and nuclear respiratory factor 1 (NRF1) are energy metabolism associated pathway proteins. PGC-1α promotes the expression of oxidative respiratory chain subunits and the replication and transcription of mtDNA through interaction with NRF1.68 In high glucose-induced H9c2 cells and STZ-injured mice, AS-IV increased the expression of PGC-1α and NRF1, resulting in increased ATP generation.69
The etiology of diabetic cardiomyopathy is complex. Anti-inflammatory strategies are used to treat diabetic cardiomyopathy through multiple pathways in the long run. AS-IV facilitates protective effects on cardiomyocytes mainly by inhibiting lipids, alleviating energy metabolism, and improving oxidative stress and inflammation (Figure 1, Table 1). However, the possible targets of the actions and the specific protein with which AS-IV interacts are not clear. In addition to cardiomyocytes, how AS-IV modulates fibroblasts and Purkinje cells and even epicardial fat and pericardial fat is unknown.
Diabetic Kidney Disease
Diabetic kidney disease (DKD) is one of the most serious complications of DM and has a large tendency to progress to end-stage renal disease (ESRD). The pathophysiology of DKD is complicated and involves oxidative stress, inflammation, transforming growth factor-β (TGF-β)-mediated fibrosis, and a variety of adhesion molecules. However, inflammation can be considered a center linked to several molecular pathways. Since tubular and glomerular cells are particularly sensitive to inflammation, inflammation caused by toxic metabolites during hyperglycemia is the trigger of early pathological changes in the glomerulus and tubules. Mounting clinical studies have shown that inhibiting the inflammatory response can effectively delay the continuous progression of diabetic nephropathy.70–72
Many preclinical studies have suggested that AS-IV can effectively inhibit the inflammatory response to treat or prevent diabetic nephropathy. In vivo studies of streptozotocin (STZ)-induced diabetic rats,73 KKAy74 and db/db75 mice indicated that AS-IV reduced albuminuria and attenuated glomerular sclerosis and tubulointerstitial fibrosis, especially decreased the serum levels of TNF-α, MCP-1 and ICAM-1 due to NF-κB inhibition.73 A study by Zhang74 elucidated that in STZ-induced diabetic rats, AS-IV minimized early kidney damage and fibrosis, which was related to the inhibition of inflammation-related NLR signaling events. Meanwhile, they also demonstrated that AS-IV suppressed ERK1/2 activation, and reduced the production of IL-1β and TNF-α in DKD caused by iatrogenic hyperinsulinemia.75
Mechanistically, the target cells of AS-IV for the treatment of DKD mainly focus on glomerular podocytes, mesangial cells, and renal tubular epithelial cells (Figure 2). Glomerular basement membrane (GBM) thickening, mesangial hyperplasia and extracellular matrix (ECM) accumulation are the early pathological processes of glomerulosclerosis, which are mainly related to podocyte loss, mesangial cell proliferation, and contraction dysfunction. In addition, early pathological changes in renal tubules, characterized by thickening tubular BM, interstitial edema, and inflammatory infiltrate, are mainly related to renal tubular epithelial cells. Many studies support that AS-IV has an early preventive effect on both glomerulosclerosis and renal tubulointerstitial fibrosis via multiscale mechanisms related to anti-inflammation.
Podocytes are an important part of the glomerular filtration membrane. The loss of podocytes in the form of apoptosis is the trigger of glomerulosclerosis, which is mainly associated with glomerular hyperfiltration resulting in increased renal albumin permeability. Inflammation is a key factor in inducing podocyte apoptosis. Tumor-necrosis factor receptor associated factor 5 (TRAF5) triggers the NF-κB inflammatory pathway leading to podocyte apoptosis, which is negatively regulated by miR-378. AS-IV exhibited its anti-inflammatory action by targeting the increase in miR-378 expression in STZ-induced diabetic rats and high glucose-stimulated podocytes.76 Otherwise, endoplasmic reticulum (ER) stress is involved in the pathogenesis of podocyte apoptosis. Studies in STZ-induced diabetic rats and high glucose-stimulated conditionally immortalized mouse podocytes show that AS-IV could protect the kidney from ER stress-induced apoptosis under diabetic conditions, by regulating the PERK-ATF4-CHOP signaling pathway, balancing Bax/Bcl-2 expression and inhibiting caspase-3 activation.77 Wang used PBA as an ER function restorer and tunicamycin as an ER stress inducer to further confirm these results. In addition to high glucose stimulation, AS-IV also inhibited loss of podocytes due to ER stress induced by palmitic acid, when targeting transient receptor potential channel 6 (TRPC6).78 As TRPC6 is a Ca2+ permeable cation channel, and its reduction directly affects the decrease of intracellular Ca2+ concentration, the inhibitory effect of AS-IV on TRPC6 determines its regulation of intracellular calcium signaling. AS-IV not only inhibits podocyte apoptosis induced by calcium-dependent ER stress, but also reverses increased calcium-dependent transcription factor nuclear factor of activated T cells (NFAT2) expression, thereby halting the expression of pro-apoptotic proteins.79 Moreover, a preliminary study by Gui40 found that the inhibition of podocyte apoptosis by AS-IV was related to its antioxidative stress effect.
The function of podocytes depends on adherence to the glomerular basement membrane (GBM) through its foot process. When podocytes infiltrate an inflammatory environment, the overexpression of inflammatory chemokines such as MCP-1/CCL2 participates in the reorganization of the podocyte actin skeleton, leading to the disappearance of the foot process and subsequent exfoliation from the GBM.80–82 It has been proven that the continuous high glucose (HG) environment causes poor adhesion between podocytes and GBM, which promotes the occurrence and development of DKD. α3β1 integrin and integrin-linked kinase (ILK) are important factors that mediate podocyte adhesion. Chen83 indicated that in HG stimulated immortalized mouse podocyte cells, AS-IV exhibited α3β1 integrin upregulation and ILK suppression effects on preventing podocyte exfoliation.
Glomerular mesangial hyperplasia caused by proliferation of mesangial cells is an early pathological manifestation of glomerulonephritis, which can lead to glomerular sclerosis as the lesion progresses and eventually to end-stage renal disease. Sun84 elaborated that AS-IV attenuated HG-induced MC proliferation in DKD, which was partly attributed to regulating the NF-κB pathway. Both oxidative stress and the PI3K/Akt signaling pathway participate in the kidney injury caused by the activation of NF-κB. ROS can regulate the activity of AKT and calcium channels. AS-IV could block the NADPH oxidase/ROS/Akt/NF-κB pathway and enhance TRPC6 expression, thereby promoting the influx of calcium to increase mesangial cell contractile function.
Tubular-interstitial fibrosis is an important pathological feature of DKD. Long-term hyperglycemia induces interstitial damage and inflammatory cell infiltration, and releases a variety of proinflammatory and profibrotic cytokines, which are the central driving factors of progressive renal interstitial fibrosis.85 Notably, tubular epithelial-mesenchymal transition (EMT) is a major cause of renal tubular-interstitial fibrosis. TGF-β1 is a major inflammatory factor and a key mediator of EMT. It favors the transcription and expression of mesenchymal markers and inhibits epithelial markers by activating multiple downstream signal transduction pathways. In addition, in high glucose stimulation, macrophages release IL-1β, TNF-α and other inflammatory factors, accompanied by TGF-β1/Smads activation.86 When targeting renal tubules, AS-IV inhibits DM-induced renal tubular EMT via TGF-β1/mTORC1/p70S6K, TGF-β1/Smads, and Wnt/β-catenin signaling pathway. When renal tubular epithelial cells injury is activated, the phenotype is transformed into fibroblasts, and the corresponding characteristic proteins are changed accordingly, evidenced by the decreased expression of epithelial markers (E-cadherin and occludin) and the increased expression of mesenchymal markers (α-SMA and N-cadherin), which are controlled by transcription factors including snail and twist. EMT further initiates ECM deposition by enhancing ECM proteins (FN and Col IV). Chen87 revealed that AS-IV attenuated HG-stimulated EMT in renal tubular epithelial HK-2 cells. AS-IV treatment observably abrogated mTORC1/p70S6K pathway activation to halt EMT transformation and ECM deposition, and ultimately inhibited fibrosis. The activated TGF-β1/Smads signaling pathway promotes the expression of α-SMA protein, which speeds up the development of renal fibrosis. Wang74,88 proved that the protective effects of AS-IV on renal interstitial fibrosis were attributed to halting the TGF-β1/Smads signaling pathway in a model of diabetic KKAy mice and high glucose-induced renal proximal tubular epithelial cells (PTCs). Moreover, docking and molecular dynamics stimulation studies confirmed that AS-IV regulated the Wnt/β-catenin pathway by binding with the GSK-3β-APC-Axin protein complex. By this means, AS-IV facilitated reduction in EMT-related proteins and alleviation in inflammatory factors release.89 In addition, the apoptosis of renal tubular epithelial cells induced by inflammation is also an irreversible part of diabetic renal tubular damage. Wang89 reported that AS-IV can reverse renal tubular epithelial cell apoptosis and proliferation of fibroblasts by downregulating the expression of TGF-β1 and blocking the p38 MAPK pathway.
A large number of animal and cell experiments have confirmed that AS-IV can regulate the structure and function of podocytes, mesangial cells and renal tubular epithelial cells, and save the death destiny of cells caused by DM, thereby inhibiting glomerular and renal tubular lesions in the early stage of DKD (Figure 2, Table 1) due to its anti-inflammatory effect. It is suggested that diabetic patients use AS-IV in advance for the prevention of DKD, while controlling blood glucose in the early stage. However, for diabetic patients with DKD, whether AS-IV can delay the continuous progression of DKD is worthy of further experimental study. Moreover, mounting evidence suggests that ROS, inflammation and fibrosis promote each other as part of a vicious cycle that leads to the development and progression of DKD. In diabetic patients, when stimulated by high blood glucose, oxidative stress occurs in renal tubules and glomerular cells, leading to an inflammatory response, which in turn triggers EMT, resulting in excessive deposition of the extracellular matrices (ECM) in the glomerulus and tubule interstitium, leading to interstitial fibrosis and glomerular sclerosis. Therefore, the exact mechanism of the intervention effect of AS-IV on podocytes, mesangial cells and renal tubular epithelial cells through its anti-inflammatory effect remains to be further studied. How AS-IV blocks the vicious cycle of ROS, inflammation and fibrosis in the kidneys of DKD patients also needs to be actively explored. Otherwise, the effects of AS-IV on glomerular endothelial cells in a high glucose environment are also of concern.
Diabetic Eye Disease
Inflammation is a significant event that is involved in the pathogenesis of diabetic eye disease, including diabetic retinopathy (DR) and diabetic macular edema. As a part of blood-retinal barriers, retinal pigment epithelial cells are vulnerable in the early development of DR. A recent study demonstrated that AS-IV inhibited apoptosis of retinal pigment epithelial cells by upregulating the expression of miR-128 in DM rats, probably via the PI3K/Akt signaling pathway.90 However, the exact molecular mechanism is not clear. Neurodegeneration is an early pathogenesis of DR. Retinal ganglion cells (RGCs) are essential for maintaining visual function. A study in db/db mice by Ding91 demonstrated that AS-IV, acting as an AR inhibitor, improved RGC function and protected the optic nerve by inhibiting RGC apoptosis and inflammation due to the inhibitory action of AS-IV on ERK1/2 and NF-κB activation, thereby downregulating IL-1, IL-6, TNF-β, and VEGF. At present, the mechanism of AS-IV in the prevention of DR is relatively preliminary. In addition to RGC and retinal pigment epithelial cells, whether AS-IV has effects on retinal astrocytes, Müller cells and pericyte cells is also of concern. Whether AS-IV has an early preventive effect on diabetic eye disease needs further verification.
Diabetic Peripheral Neuropathy
Among the complications of diabetes, peripheral neuropathy is by far the most prevalent and appears relatively early. Diabetes disturbs the expression of ion channels in axons, which contributes to axon injury.92 Sodium/potassium (Na/K) ATPase plays a vital role in exporting intra-axonal Na+. Reduced Na/K ATPase activity impairs normal nerve physiology. It has been reported that AS-IV as an aldose reductase (AR) inhibitor could ameliorate peripheral nerve damage by lowing motor nerve conduction velocity and increasing nerve blood flow, due to the increasing effect on Na+K+-ATPase activity in both erythrocytes and nerves.93 Moreover, AS-IV promoted GSH activity and increased AGEs, thereby retarding peripheral nerve impairment in STZ-treated rats. Studies have shown that activation of the inflammatory cascade pathway is one of the mechanisms of diabetic peripheral neuropathy.94 Inflammatory factors such as TNF-α and IL-6 can induce microangiopathy and neuronal apoptosis. Notably, there is a certain connection between inflammation and aldose reductase.95 Aldose reductase inhibitors can block NF-κB-dependent inflammatory signals, which suggests that the therapeutic effect of AS-IV as an aldose reductase inhibitor on diabetic peripheral neuropathy may be attributed to the regulation of inflammation.
Diabetic Wound Healing
An in vivo study of diabetes reported that AS-IV has potential efficacy on impaired wound healing. Delayed wound healing is a neglected complication of diabetes. It is caused by multiple factors involved in dysregulated leukocyte chemotaxis, extracellular matrix deposition, and decreased neovascularization. Normal wound healing usually goes through 4 stages: hemostasis, inflammation, proliferation, and remodeling.96 However, in diabetic wounds, leukocytes accumulate slowly during the inflammatory phase and their chemotactic effect is reduced, which makes the wounds chronically inflammatory for a long time.97,98 Accordingly, it is difficult for macrophages to switch from a proinflammatory phenotype to an anti-inflammatory phenotype, so the increased expression of metalloproteinases and decreased expression of proangiogenic factors causes the degradation of the extracellular matrix and the reduction in angiogenesis,99 which ultimately leads to difficulty in wound healing. Scientists elucidated that AS-IV accelerated diabetic wound healing by enhancing the expression of extracellular matrix-related genes and angiogenic factors in terms of promoting collagen deposition and improving new blood vessel formation.100 In addition, the beneficial effect of AS-IV was associated with the development of polarized alternatively activated macrophages. After encapsulation by MMP-2-responsive nanocarriers, insoluble astragaloside appears to be more effective in promoting wound healing in diabetes.101
Perspectives
Existing literatures have shown that AS-IV is effective in treating DM and its complications, including cardiovascular, neurological, kidney disease, etc. Since they are a series of diseases that progress continuously, it is necessary to take them as a whole. Therefore, from the perspective of the time dimension of disease progression, the follow-up preclinical studies urgently need to clarify which period of DM should be administered and what dose should be given to delay the progression of DM, as well as the complications occurring at different periods, so as to provide hints for clinical medication.
Diabetes is a chronic inflammatory disease. AS-IV has anti-inflammatory effects in the treatment of diabetes and its complications, and the mechanism of action involves many aspects. MAPKs, NF-κB, Akt, and TGF-β are the main star molecules. However, it is not clear exactly what the target proteins are, nor how AS-IV interacts with them. Therefore, follow-up studies need to uncover these by using transgenic and gene knockout techniques, molecular docking, protein interaction and structural pharmacology research methods, especially identify the target proteins of AS-IV in different target organs. After all, identifying specific sites of actin is helpful for further structural modification of AS-IV and target-drug development. Moreover, at the cellular level, the types of functional cells affected by AS-IV need to be further explored, not limited to retinal ganglion cells in diabetic eye disease, for example, and even neurons, glia (astrocytes and Müller cells), and pericytes changed dramatically at the function and structure levels. Inflammation is characterized by the activation of immune and nonimmune cells that protect the host from pathogens. Of future note, the effect of AS-IV on systemic and local immune cells deserves exploration. Since AS-IV may modulate multiple cells in an organ, the modulatory effect on cell-to -cell inflammatory signaling in an organ is also worth investigating.
Pharmacokinetics and toxicology information of AS-IV is a vital premise of clinical transformation. Pharmacokinetics evidence indicated the low oral bioavailability of AS-IV, which reduces its transformation value and application value. The physicochemical properties are the root cause. AS-IV is characterized by large molecular weight, low solubility both in water and lipid, and poor drug permeability. At present, although AS-IV has not been successfully developed into a medicine, researchers have tried to increase the oral bioavailability of AS-IV by modern methods, such as a novel water-soluble derivative33 and nanomicelle,102 named LS-102 and AST-NMs. It is believed that with the improvement of preparation technology and the optimization of dosing regimen, the clinical transformation of AS-IV is expected to be further advanced. As for safety, AS-IV is an active saponin isolated from Astragali Radix, which has been widely used in numerous TCM preparations, such as Qi-Shen-Yi-Qi pill,103 Shen-Qi-Jiang-Tang granule19 and Zhen-Qi-Fu-Zheng granule.104 Thousands of years of clinical usage indicates a good safety. At present, although there is no systematic toxicology study, anecdotal studies show that AS-IV has no obvious toxicity or adverse reactions. Thinking in another way, we believe that the blank of toxicity research is an urgent research topic.
Conclusion
Inflammation is increasingly recognized as a well-established mediator of diabetes and its complications. Mounting preclinical evidence has proven that AS-IV has a prominent anti-inflammatory effect in the prevention and treatment of diabetes and its complications. It plays a role by regulating a variety of anti-inflammatory pathways in multiple organs, tissues and target cells throughout the body (Figure 3). AS-IV can regulate glucose metabolism and reduce lipid accumulation, thereby inhibiting insulin resistance caused by inflammation of adipose tissue. The blockade the of NF-κB inflammatory signaling pathway may be the central link of AS-IV’s anti-inflammatory effect, reducing the tissue structure and functional damage stimulated by inflammatory factors. In addition, AS-IV can improve diabetes and its complications by inhibiting inflammation-related oxidative stress, fibrosis and apoptosis signals.
Furthermore, the oral bioavailability of AS-IV is only 2.2%, greatly limiting its clinical application. Therefore, in view of the excellent antidiabetic effect of AS-IV, the development of new biological agents and the provision of convenient drug delivery routes to increase bioavailability have become urgent problems to be solved.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by funds from the National Natural Science Foundation of China [81830112], National Key Research and Development Project of China [2020YFA0708004].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Carracher AM, Marathe PH, Close KL. American association of diabetes educators. J Diabetes. 2017;9(12):1054–1057. doi:10.1111/1753-0407.12603
2. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi:10.1152/physrev.00045.2011
3. Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. 2020;16(2):81–90. doi:10.1038/s41574-019-0286-3
4. Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113(9):1009–1023. doi:10.1093/cvr/cvx108
5. von Scholten BJ, Kreiner FF, Gough SCL, von Herrath M. Current and future therapies for type 1 diabetes. Diabetologia. 2021;64(5):1037–1048. doi:10.1007/s00125-021-05398-3
6. Esser N, Lhomme L, De RA, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56(11):2487–2497. doi:10.1007/s00125-013-3023-9
7. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. doi:10.1172/JCI92035
8. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi:10.1172/JCI28898
9. Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113(4):389–398. doi:10.1093/cvr/cvx012
10. Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437(7059):759–763. doi:10.1038/nature03988
11. Cameron AR, Morrison VL, Levin D, et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res. 2016;119(5):652–665. doi:10.1161/CIRCRESAHA.116.308445
12. Kim SC, Wu S, Fang X, et al. Postconditioning with a CpG containing oligodeoxynucleotide ameliorates myocardial infarction in a murine closed-chest model. Life Sci. 2014;119(1–2):1–8. doi:10.1016/j.lfs.2014.09.029
13. Okamoto A, Yokokawa H, Sanada H, Naito T. Changes in levels of biomarkers associated with adipocyte function and insulin and glucagon kinetics during treatment with dapagliflozin among obese type 2 diabetes mellitus patients. Drugs R D. 2016;16(3):255–261. doi:10.1007/s40268-016-0137-9
14. Ruscitti P, Masedu F, Alvaro S, et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, open-label, randomised controlled trial. PLoS Med. 2019;16(9):e1002901. doi:10.1371/journal.pmed.1002901
15. Ridker PM, MacFadyen JG, Glynn RJ, et al. Inhibition of Interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71(21):2405–2414. doi:10.1016/j.jacc.2018.03.490
16. Everett BM, Donath MY, Pradhan AD, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71(21):2392–2401. doi:10.1016/j.jacc.2018.03.002
17. Liu P, Zhao H, Luo Y. Anti-aging implications of Astragalus Membranaceus (Huangqi): a well-known Chinese tonic. Aging Dis. 2017;8(6):868–886. doi:10.14336/AD.2017.0816
18. Fu TX, Wu GL. Effect of Astragalus injection on inflammatory mediators and the expression of adiponectin in renal tissue of rats with diabetic nephropathy. Chin J Clin Pharmacol. 2019;35(2):150–153. Chinese.
19. Chen MM, Jia JH, Tan YJ, et al. Shen-Qi-Jiang-Tang granule ameliorates diabetic nephropathy via modulating tumor necrosis factor signaling pathway. J Ethnopharmacol. 2023;303:116031. doi:10.1016/j.jep.2022.116031
20. Tan YQ, Chen HW, Li J. Astragaloside IV: an effective drug for the treatment of cardiovascular diseases. Drug Des Devel Ther. 2020;14:3731–3746. doi:10.2147/DDDT.S272355
21. Wu Y, Fan Z, Chen Z, et al. Astragaloside IV protects human cardiomyocytes from hypoxia/reoxygenation injury by regulating miR-101a. Mol Cell Biochem. 2020;470(1–2):41–51. doi:10.1007/s11010-020-03743-5
22. Shi H, Zhou P, Gao G, et al. Astragaloside IV prevents acute myocardial infarction by inhibiting the TLR4/MyD88/NF-κB signaling pathway. J Food Biochem. 2021;45(7):e13757. doi:10.1111/jfbc.13757
23. Yang L, Xing F, Han X, et al. Astragaloside IV regulates differentiation and induces apoptosis of activated CD4+ T cells in the pathogenesis of experimental autoimmune encephalomyelitis. Toxicol Appl Pharmacol. 2019;362:105–115. doi:10.1016/j.taap.2018.10.024
24. Zhang T, Wang H, Lu M, et al. Astragaloside IV prevents myocardial hypertrophy induced by mechanical stress by activating autophagy and reducing inflammation. Am J Transl Res. 2020;12(9):5332–5342.
25. Zhao P, Wang Y, Zeng S, Lu J, Jiang TM, Li YM. Protective effect of astragaloside IV on lipopolysaccharide-induced cardiac dysfunction via downregulation of inflammatory signaling in mice. Immunopharmacol Immunotoxicol. 2015;37(5):428–433. doi:10.3109/08923973.2015.1080266
26. Li M, Yu L, She T, et al. Astragaloside IV attenuates Toll-like receptor 4 expression via NF-κB pathway under high glucose condition in mesenchymal stem cells. Eur J Pharmacol. 2012;696(1–3):203–209. doi:10.1016/j.ejphar.2012.09.033
27. Chang YX, Sun YG, Li J, et al. The experimental study of Astragalus membranaceus on Meridian tropsim: the distribution study of astragaloside IV in rat tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;911:71–75. doi:10.1016/j.jchromb.2012.10.024
28. Du Y, Zhang Q, Chen GG, Wei P, Tu CY. Pharmacokinetics of Astragaloside IV in rats by liquid chromatography coupled with tandem mass spectrometry. Eur J Drug Metab Pharmacokinet. 2005;30(4):269–273. doi:10.1007/BF03190631
29. Li L, Hou X, Xu R, Liu C, Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31(1):17–36. doi:10.1111/fcp.12232
30. Zhang Q, Zhu LL, Chen GG, Du Y. Pharmacokinetics of astragaloside iv in beagle dogs. Eur J Drug Metab Pharmacokinet. 2007;32(2):75–79. doi:10.1007/BF03190995
31. Zhang W, Zhang C, Liu R, et al. Quantitative determination of Astragaloside IV, a natural product with cardioprotective activity, in plasma, urine and other biological samples by HPLC coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;822(1–2):170–177. doi:10.1016/j.jchromb.2005.05.034
32. Huang CR, Wang GJ, Wu XL, et al. Absorption enhancement study of astragaloside IV based on its transport mechanism in caco-2 cells. Eur J Drug Metab Pharmacokinet. 2006;31(1):5–10. doi:10.1007/BF03190635
33. Qing LS, Chen TB, Sun WX, et al. Pharmacokinetics comparison, intestinal absorption and acute toxicity assessment of a novel water-soluble astragaloside IV derivative (astragalosidic acid, LS-102). Eur J Drug Metab Pharmacokinet. 2019;44(2):251–259. doi:10.1007/s13318-018-0515-5
34. Zhang L, Li LZ, Gong HY. Chronic toxicity of Fufanghuangqi Capsula in experimental animals. Acta Acad Med CPAPF. 2005;2:108–110. Chinese.
35. Yang YH, Chen YX, Ouyang HT, Cheng JP. Study on safety tests and acute toxicological test of lyophilized powder of Astragalus for injection. Acta Acad Med Xuzhou. 2007;2:88–91. Chinese.
36. Ouyang HT, Yang JY, Yu SY, et al. Long-term toxicity studies of lyophilized powder of astragalus membranaceus extract in rats and dogs. Chin J Modern Med. 2008;15:2173–2175+2179. Chinese.
37. Zhan GY, Sun JQ, Zhan J, Wang HQ. The toxicology research of astragalus membranaceus composite nutrient liquid. J Guiyang Med Coll. 1994;3:271–273. Chinese.
38. Han R, Zhu LJ, Pan JX, Zhang KP. Acute and chronic toxicity studies of huangqi injection in mice and rats. Chin Wild Plant Res. 2004;4:50–53. Chinese.
39. Yu SY, Ouyang HT, Yang JY, et al. Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J Ethnopharmacol. 2007;110(2):352–355. doi:10.1016/j.jep.2006.09.024
40. Gui D, Guo Y, Wang F, et al. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One. 2012;7(6):e39824. doi:10.1371/journal.pone.0039824
41. Xuying W, Jiangbo Z, Yuping Z, et al. Effect of astragaloside IV on the general and peripartum reproductive toxicity in Sprague-Dawley rats. Int J Toxicol. 2010;29(5):505–516. doi:10.1177/1091581810376840
42. Jiang B, Yang Y, Jin H, et al. Astragaloside IV attenuates lipolysis and improves insulin resistance induced by TNFalpha in 3T3-L1 adipocytes. Phytother Res. 2008;22(11):1434–1439. doi:10.1002/ptr.2434
43. Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–15435. doi:10.1073/pnas.0904944106
44. Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–1375. doi:10.1053/j.gastro.2008.01.075
45. Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S. Increased whole-body adiposity without a concomitant increase in liver fat is not associated with augmented metabolic dysfunction. Obesity. 2010;18(8):1510–1515. doi:10.1038/oby.2010.90
46. Zhou X, Wang LL, Tang WJ, Tang B. Astragaloside IV inhibits protein tyrosine phosphatase 1B and improves insulin resistance in insulin-resistant HepG2 cells and triglyceride accumulation in oleic acid (OA)-treated HepG2 cells. J Ethnopharmacol. 2021;268:113556. doi:10.1016/j.jep.2020.113556
47. Huang L, Yue P, Wu X, et al. Combined intervention of swimming plus metformin ameliorates the insulin resistance and impaired lipid metabolism in murine gestational diabetes mellitus. PLoS One. 2018;13(4):e0195609. doi:10.1371/journal.pone.0195609
48. Wei X, Gu N, Feng N, Guo X, Ma X. Inhibition of p38 mitogen-activated protein kinase exerts a hypoglycemic effect by improving β cell function via inhibition of β cell apoptosis in db/db mice. J Enzyme Inhib Med Chem. 2018;33(1):1494–1500. doi:10.1080/14756366.2018.1477138
49. Lv L, Wu SY, Wang GF, et al. Effect of astragaloside IV on hepatic glucose-regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Phytother Res. 2010;24(2):219–224. doi:10.1002/ptr.2915
50. Zhu R, Zheng J, Chen L, Gu B, Huang S. Astragaloside IV facilitates glucose transport in C2C12 myotubes through the IRS1/AKT pathway and suppresses the palmitate-induced activation of the IKK/IκBα pathway. Int J Mol Med. 2016;37(6):1697–1705. doi:10.3892/ijmm.2016.2555
51. Böni-Schnetzler M, Meier DT. Islet inflammation in type 2 diabetes. Semin Immunopathol. 2019;41(4):501–513. doi:10.1007/s00281-019-00745-4
52. Zhang N, Wang XH, Mao SL, Zhao F. Astragaloside IV improves metabolic syndrome and endothelium dysfunction in fructose-fed rats. Molecules. 2011;16(5):3896–3907. doi:10.3390/molecules16053896
53. Leng B, Tang F, Lu M, Zhang Z, Wang H, Zhang Y. Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Life Sci. 2018;209:111–121. doi:10.1016/j.lfs.2018.07.053
54. Zhang WJ, Hufnagl P, Binder BR, Wojta J. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb Haemost. 2003;90(5):904–914. doi:10.1160/TH03-03-0136
55. Leng B, Li C, Sun Y, et al. Protective effect of astragaloside IV on high glucose-induced endothelial dysfunction via inhibition of P2X7R dependent P38 MAPK signaling pathway. Oxid Med Cell Longev. 2020;2020:5070415. doi:10.1155/2020/5070415
56. You L, Fang Z, Shen G, et al. Astragaloside IV prevents high glucose-induced cell apoptosis and inflammatory reactions through inhibition of the JNK pathway in human umbilical vein endothelial cells. Mol Med Rep. 2019;19(3):1603–1612. doi:10.3892/mmr.2019.9812
57. Xu ME, Xiao SZ, Sun YH, Ou-Yang Y, Zheng XX. Effects of astragaloside IV on pathogenesis of metabolic syndrome in vitro. Acta Pharmacol Sin. 2006;27(2):229–236. doi:10.1111/j.1745-7254.2006.00243.x
58. Ma Y, Li W, Yin Y, Li W. AST IV inhibits H2O2-induced human umbilical vein endothelial cell apoptosis by suppressing Nox4 expression through the TGF-β1/Smad2 pathway. Int J Mol Med. 2015;35(6):1667–1674. doi:10.3892/ijmm.2015.2188
59. Qian W, Qian Q, Cai X, et al. Astragaloside IV inhibits oxidized low-density lipoprotein-induced endothelial damage via upregulation of miR-140-3p. Int J Mol Med. 2019;44(3):847–856. doi:10.3892/ijmm.2019.4257
60. Reglero-Real N, Colom B, Bodkin JV, Nourshargh S. Endothelial cell junctional adhesion molecules: role and regulation of expression in inflammation. Arterioscler Thromb Vasc Biol. 2016;36(10):2048–2057. doi:10.1161/ATVBAHA.116.307610
61. Li HB, Ge YK, Zhang L, Zheng XX. Astragaloside IV improved barrier dysfunction induced by acute high glucose in human umbilical vein endothelial cells. Life Sci. 2006;79(12):1186–1193. doi:10.1016/j.lfs.2006.03.041
62. Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118(4):692–702. doi:10.1161/CIRCRESAHA.115.306361
63. Hénaut L, Mary A, Chillon JM, Kamel S, Massy ZA. The impact of uremic toxins on vascular smooth muscle cell function. Toxins. 2018;10(6):218. doi:10.3390/toxins10060218
64. Yuan W, Zhang Y, Ge Y, Yan M, Kuang R, Zheng X. Astragaloside IV inhibits proliferation and promotes apoptosis in rat vascular smooth muscle cells under high glucose concentration in vitro. Planta Med. 2008;74(10):1259–1264. doi:10.1055/s-2008-1081290
65. Zhang X, Fu Y, Li H, et al. H3 relaxin inhibits the collagen synthesis via ROS- and P2X7R-mediated NLRP3 inflammasome activation in cardiac fibroblasts under high glucose. J Cell Mol Med. 2018;22(3):1816–1825. doi:10.1111/jcmm.13464
66. Li L, Zhao Q, Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018;68–69:490–506. doi:10.1016/j.matbio.2018.01.013
67. Wang Z, Zhu Y, Zhang Y, et al. Protective effects of AS-IV on diabetic cardiomyopathy by improving myocardial lipid metabolism in rat models of T2DM. Biomed Pharmacother. 2020;127:110081. doi:10.1016/j.biopha.2020.110081
68. Vernier M, Giguère V. Aging, senescence and mitochondria: the PGC-1/ERR axis. J Mol Endocrinol. 2021;66(1):R1–R14. doi:10.1530/JME-20-0196
69. Zhang Z, Wang J, Zhu Y, Zhang H, Wang H. Astragaloside IV alleviates myocardial damage induced by type 2 diabetes via improving energy metabolism. Mol Med Rep. 2019;20(5):4612–4622. doi:10.3892/mmr.2019.10716
70. Perez-Gomez MV, Sanchez-Niño MD, Sanz AB, et al. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin Investig Drugs. 2016;25(9):1045–1058. doi:10.1080/13543784.2016.1196184
71. Saoud R, Jaffa MA, Habib A, et al. Modulation of proteomic and inflammatory signals by Bradykinin in podocytes. J Adv Res. 2020;24:409–422. doi:10.1016/j.jare.2020.05.021
72. Lytvyn Y, Bjornstad P, van Raalte DH, Heerspink HL, Cherney DZI. The new biology of diabetic kidney disease-mechanisms and therapeutic implications. Endocr Rev. 2020;41(2):202–231. doi:10.1210/endrev/bnz010
73. Gui D, Huang J, Guo Y, et al. Astragaloside IV ameliorates renal injury in streptozotocin-induced diabetic rats through inhibiting NF-κB-mediated inflammatory genes expression. Cytokine. 2013;61(3):970–977. doi:10.1016/j.cyto.2013.01.008
74. Wang Y, Lin C, Ren Q, Liu Y, Yang X. Astragaloside effect on TGF-β1, SMAD2/3, and α-SMA expression in the kidney tissues of diabetic KKAy mice. Int J Clin Exp Pathol. 2015;8(6):6828–6834.
75. Zhan H, Han P, Wang M, et al. Combination of astragaloside IV and ACEi ameliorates renal injuries in db/db mice. Int J Clin Exp Pathol. 2020;13(5):827–836.
76. Lei X, Zhang BD, Ren JG, Luo FL. Astragaloside suppresses apoptosis of the podocytes in rats with diabetic nephropathy via miR-378/TRAF5 signaling pathway. Life Sci. 2018;206:77–83. doi:10.1016/j.lfs.2018.05.037
77. Chen Y, Gui D, Chen J, He D, Luo Y, Wang N. Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol Biochem. 2014;33(6):1975–1987. doi:10.1159/000362974
78. Wang ZS, Xiong F, Xie XH, Chen D, Pan JH, Cheng L. Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress. BMC Nephrol. 2015;16:44. doi:10.1186/s12882-015-0031-7
79. Yao XM, Liu YJ, Wang YM, et al. Astragaloside IV prevents high glucose-induced podocyte apoptosis via downregulation of TRPC6. Mol Med Rep. 2016;13(6):5149–5156. doi:10.3892/mmr.2016.5167
80. Menne J, Eulberg D, Beyer D, et al. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol Dial Transplant. 2017;32(2):307–315. doi:10.1093/ndt/gfv459
81. Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–1449. doi:10.1172/JCI38019
82. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21(2):212–222. doi:10.1681/ASN.2008121226
83. Chen J, Gui D, Chen Y, Mou L, Liu Y, Huang J. Astragaloside IV improves high glucose-induced podocyte adhesion dysfunction via alpha3beta1 integrin upregulation and integrin-linked kinase inhibition. Biochem Pharmacol. 2008;76(6):796–804. doi:10.1016/j.bcp.2008.06.020
84. Sun L, Li W, Li W, Xiong L, Li G, Ma R. Astragaloside IV prevents damage to human mesangial cells through the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under high glucose conditions. Int J Mol Med. 2014;34(1):167–176. doi:10.3892/ijmm.2014.1741
85. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–222. doi:10.1038/s41581-019-0234-4
86. Chung S, Overstreet JM, Li Y, et al. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight. 2018;3(21):e123563. doi:10.1172/jci.insight.123563
87. Chen X, Yang Y, Liu C, Chen Z, Wang D. Astragaloside IV ameliorates high glucose-induced renal tubular epithelial-mesenchymal transition by blocking mTORC1/p70S6K signaling in HK-2 cells. Int J Mol Med. 2019;43(2):709–716. doi:10.3892/ijmm.2018.3999
88. Wang YN, Zhao SL, Su YY, et al. Astragaloside IV attenuates high glucose-induced EMT by inhibiting the TGF-β/Smad pathway in renal proximal tubular epithelial cells. Biosci Rep. 2020;40(6):BSR20190987. doi:10.1042/BSR20190987
89. Wang Q, Shao X, Xu W, et al. Astragalosides IV inhibits high glucose-induced cell apoptosis through HGF activation in cultured human tubular epithelial cells. Ren Fail. 2014;36(3):400–406. doi:10.3109/0886022X.2013.867798
90. Wang T, Zhang Z, Song C, et al. Astragaloside IV protects retinal pigment epithelial cells from apoptosis by upregulating miR-128 expression in diabetic rats. Int J Mol Med. 2020;46(1):340–350. doi:10.3892/ijmm.2020.4588
91. Ding Y, Yuan S, Liu X, et al. Protective effects of astragaloside IV on db/db mice with diabetic retinopathy. PLoS One. 2014;9(11):e112207. doi:10.1371/journal.pone.0112207
92. Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93(6):1296–1313. doi:10.1016/j.neuron.2017.02.005
93. Yu J, Zhang Y, Sun S, et al. Inhibitory effects of astragaloside IV on diabetic peripheral neuropathy in rats. Can J Physiol Pharmacol. 2006;84(6):579–587. doi:10.1139/y06-015
94. Stino AM, Rumora AE, Kim B, Feldman EL. Evolving concepts on the role of dyslipidemia, bioenergetics, and inflammation in the pathogenesis and treatment of diabetic peripheral neuropathy. J Peripher Nerv Syst. 2020;25(2):76–84. doi:10.1111/jns.12387
95. Singh M, Kapoor A, McCracken J, Hill B, Bhatnagar A. Aldose reductase (AKR1B) deficiency promotes phagocytosis in bone marrow derived mouse macrophages. Chem Biol Interact. 2017;265:16–23. doi:10.1016/j.cbi.2017.01.012
96. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: a Cellular Perspective. Physiol Rev. 2019;99(1):665–706. doi:10.1152/physrev.00067.2017
97. Boniakowski AM, denDekker AD, Davis FM, et al. SIRT3 regulates macrophage-mediated inflammation in diabetic wound repair. J Invest Dermatol. 2019;139(12):2528–2537.e2. doi:10.1016/j.jid.2019.05.017
98. Liu D, Yang P, Gao M, et al. NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci. 2019;133(4):565–582. doi:10.1042/CS20180600
99. Salmaninejad A, Valilou SF, Soltani A, et al. Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell Oncol. 2019;42(5):591–608. doi:10.1007/s13402-019-00453-z
100. Luo X, Huang P, Yuan B, et al. Astragaloside IV enhances diabetic wound healing involving upregulation of alternatively activated macrophages. Int Immunopharmacol. 2016;35:22–28. doi:10.1016/j.intimp.2016.03.020
101. Zhang D, Huang Q. Encapsulation of astragaloside with matrix metalloproteinase-2-responsive hyaluronic acid end-conjugated polyamidoamine dendrimers improves wound healing in diabetes. J Biomed Nanotechnol. 2020;16(8):1229–1240. doi:10.1166/jbn.2020.2971
102. Huang SF, Xu L. Preparation of astrageloside IV nanomicelles and transport study in Caco-2 cell mode. Xibei Med J. 2022;37(2):94–98. Chinese.
103. Lv S, Zhang W, Yuan P, Lu C, Dong J, Zhang J. QiShenYiQi pill for myocardial collagen metabolism and apoptosis in rats of autoimmune cardiomyopathy. Pharm Biol. 2022;60(1):722–728. doi:10.1080/13880209.2022.2056206
104. Zhou Y, Wu C, Qian X, et al. Multitarget and multipathway regulation of zhenqi fuzheng granule against non-small cell lung cancer based on network pharmacology and molecular docking. Evid Based Complement Alternat Med. 2022;17(2022):5967078.
105. Lin X, Wang Q, Sun S, et al. Astragaloside IV promotes the eNOS/NO/cGMP pathway and improves left ventricular diastolic function in rats with metabolic syndrome. J Int Med Res. 2020;48(1):300060519826848. doi:10.1177/0300060519826848
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.