Back to Journals » Journal of Inflammation Research » Volume 16
The Management of Diabetes with Hyperuricemia: Can We Hit Two Birds with One Stone?
Received 2 August 2023
Accepted for publication 31 October 2023
Published 27 December 2023 Volume 2023:16 Pages 6431—6441
DOI https://doi.org/10.2147/JIR.S433438
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Yunyang Wang,1 Jie Lu1– 3
1Department of Endocrinology and Metabolism, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 2Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China; 3Shandong Provincial Clinical Research Center for Immune Diseases and Gout, the Affiliated Hospital of Qingdao University, Qingdao, People’s Republic of China
Correspondence: Jie Lu, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, the Affiliated Hospital of Qingdao University, 16 Jiangsu Road, Qingdao, Shandong, People’s Republic of China, 266003, Tel +86 17853297395, Fax +86 0532-82912019, Email [email protected]
Abstract: Serum urate (SU) is an independent predictor for the incidence of diabetes. In current diabetes treatment regimens, there is insufficient appreciation of the importance of hyperuricemia (HU) in disease control and prevention. To summarize the updated knowledge on the effects of SU on β-cell function, insulin resistance and chronic diabetic complications, as well as to evaluate the management of patients with both HU and diabetes, we searched the MEDLINE PubMed database, and included 285 journal articles. An inverted U-shaped relationship between fasting plasma glucose and SU levels was established in this review. Elevated SU levels may enhance the development of chronic diabetic complications, including macrovascular and microvascular dysfunction. Diet and exercise are essential parts of the lifestyle changes necessary for HU and diabetes management. Glucose- and urate-lowering drug selection and combination should be made with the principle of ameliorating, and at least not deteriorating, diabetes and HU. Medical artificial intelligence technology and monitoring systems can help to improve the effectiveness of long-term management of HU and diabetes through digital healthcare. This study comprehensively reviews and provides a scientific and reliable basis for and viewpoints on the clinical management of diabetes and HU.
Keywords: diabetes, hyperuricemia, U-shaped relationship, urate-lowering treatment, management
Introduction
A large body of recent evidence suggests that hyperuricemia (HU) may play a role in the development and pathogenesis of a number of metabolic, hemodynamic and systemic pathological diseases, including diabetes mellitus (DM).1,2 Serum urate (SU) is a strong and independent risk factor for type 2 diabetes (T2D), and the incidence of HU increases in diabetic patients compared with their unaffected counterparts.3,4 In the past few decades, a great many clinical, epidemiological and experimental studies have revealed that HU mediates all stages of diabetes as well as diabetic chronic complications,5,6 from initiation to progression. In addition, uric-acid based metabolic indices are associated with T2D7 and its complications, including diabetic kidney disease.8 Moreover, conditions that are related to T2D are also associated with uric acid elevations, such as hypertension,9 metabolic syndrome10 and hepatosteatosis.11 However, in current diabetes treatment regimens, there is insufficient appreciation of the importance of HU in disease control and prevention. The management of hyperuricemic patients demands more attention and specific features in diagnosis and the therapeutic approach.
We searched the MEDLINE PubMed database with the terms “((urate) OR (uric acid)) OR (hyperuricemia)) AND ((diabetes [MeSH])) AND ((epidemiology) OR (mechanism) OR (treatment))” and found 285 journal articles that had published experimental results (ie, not reviews) in English between 2012 and October 2023. This article summarizes the most recently published evidence on the relationship between diabetes and HU, and adds some updated knowledge, with an emphasis on how to improve the management of diabetic patients with HU.
Epidemiology
HU is defined as an SU level greater than 420 μmol/L (or 7.0 mg/dL). Increased life expectancy and changes in diet and lifestyle have resulted in rising incidences and prevalences of both HU and diabetes worldwide, and especially in China. A nationally representative epidemiological survey by Li et al indicated that the overall prevalence of diabetes in mainland China in 2017 was 12.8%, using the American Diabetes Association (ADA) diagnostic criteria.12 Although there is an absence of nationwide epidemiological data for HU, a meta-analysis published in 2020 showed that the pooled prevalence in Chinese adults was 11.7% in rural areas and 16.8% in urban areas.13 A cross-sectional survey by our team indicated a dramatic increase in prevalence of 25.4% among Chinese adolescents.14
Some prospective studies found that SU is an independent predictor for the incidence of T2D in middle-aged and elderly Chinese people.15–17 Dose–response analysis showed the risk of T2D increased by 6% per 1 mg/dL increment in SU (multivariate adjusted relative risk: 1.06; 95% confidence interval: 1.04–1.07).15 Another meta-analysis of 11 combined cohort studies found a significant relationship between elevated SU level and risk of developing T2D, indicating a 17% increment in the risk of diabetes per 1 mg/dL increase in SU level.18 Uricase (Uox) catalyzes the first reaction of oxidative uricolysis, eliminating purine nitrogen through a water-soluble compound in hominids. It has been postulated that the loss of uricase in humans may have raised hepatic uric acid levels, thereby stimulating hepatic glucose production and serum glucose levels.19 Thus, the loss of uricase may have functioned as a “thrifty gene”, as proposed by James Neel,20 which would have improved survival during food scarcity but in modern societies may predispose to diabetes.
Regarding the association between fasting blood glucose and SU levels, we analyzed the data from an epidemic survey of 1367 male participants in Shandong Province, China (Supplementary Methods, sTable 1). An inverted U-shaped relationship between fasting plasma glucose (FPG) and SU levels was found in Chinese populations aged 18–94 years, showing an upward trend of SU with increased FPG and then a downward slope of the relationship (Figure 1). After adjusting for age, body mass index, triglycerides and fasting plasma insulin, the same trend was found, with the FPG threshold decreasing from 7.01 mmol/L (Figure 1A) to 6.63 mmol/L (Figure 1B).
Hyperuricemia, Diabetes and Chronic Diabetic Complications
Effects of Urate on β-Cell Function and Insulin Sensitivity
Insulin resistance and β-cell failure are regarded as two key events in T2D development. The association of HU with insulin resistance and β-cell dysfunction has already been well demonstrated, but whether there is a causal relationship is still inconclusive.
Wan et al21 found that urate directly induces hepatic insulin resistance in diet-induced HU mice. Mice fed a high-fat and purine-rich diet showed more impaired glucose metabolism compared with those fed a high-fat diet (HFD) alone. Their study also indicated Nod-like receptor protein 3 (NLRP3) as the modulator between urate and insulin resistance.21 However, in our more recent research performed on genetically modified HU mouse models, HFD-fed Uox deficiency (Uox-KO) male mice only displayed glucose intolerance, without changed insulin sensitivity.22 We presume that the relationship between urate and insulin resistance would be detected in sugar-/fructose-fed Uox-KO mice, as urate-dependent insulin resistance occurred in models of sugar/fructose induction.23 Moreover, several clinical trials have suggested that urate-lowering therapy in hyperuricemic patients does improve insulin resistance or fasting glucose concentrations.24,25 Although Han et al26 presented a cross-lagged path analysis in China and found that urate was likely to be causal for insulin resistance, three Mendelian randomization studies found that genes that predict SU levels do not predict the risk of T2D.27–29 Another clinical study, on 299 women with recent gestational diabetes, showed that SU does not track with changes over time in insulin sensitivity, β-cell function or glycemia, adding to evidence suggesting that HU does not directly contribute to the development of diabetes.30
The effects of HU on pancreatic islet β-cells are also controversial. In vivo data suggest that an elevated level of urate causes β-cell injury directly via the nuclear factor-kappa B–inducible isoform nitric oxide synthase–nitric oxide (NF-κB-iNOS-NO) signaling axis.31 Lu et al22 reported that urate participates in the transition from impaired glucose tolerance to diabetes via the action of prompting β-cell apoptosis. In this study, the Uox-KO mouse models received the HFD accompanied by multiple low-dose streptozotocin injections, and eventually developed diabetes, accompanied by increased β-cell apoptosis and hypoinsulinemia, which are pathophysiological determinants of diabetes.22 However, substantial short-term urate-lowering therapy did not enhance β-cell survival,22 which could be explained by the hypothesis in Figure 2. Tang et al32 report that subjects with higher levels of SU had higher levels of insulin secretion, including the early-phase and total insulin secretion. Although subjects with higher SU secrete more insulin, this does not mean that high SU is beneficial to β-cell function.
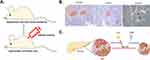 |
Figure 2 Hypothesis of the effects of urate on the streptozotocin (STZ)-induced diabetic mouse model. (A) Establishment of the hyperuricemic and diabetic mouse model based on uricase-knockout mice. (B) Urate-lowering therapy reversed few pancreatic β-cell deaths stimulated by multiple low-dose STZ. Pancreatic sections were immunohistochemically stained for insulin (data from Lu et al, 2020).22 (C) Schematic figure showing that STZ is the main driver in the toxic attack on β-cells, while urate just plays a contributory role in diabetes, explaining why urate-lowering therapy could only partially reverse β-cell apoptosis. |
Although investigations on the effects of urate on inflammatory cell activation initially focused on the effects of urate crystal, more recent studies have found soluble urate to have many pro-inflammatory and pro-oxidative effects in the intracellular environment. Zhang et al33 showed that HU induced oxidative stress and increased reactive oxygen species (ROS) levels in cultured rat pancreatic β-cells, which activated adenosine monophosphate-activated protein kinase (AMPK) and the extracellular signal-regulated kinase (ERK) signaling pathway, and ultimately decreased cell growth and insulin secretion. Another study showed that HU impaired mitochondrial function and reduced insulin secretion through the IRS2/Akt signaling pathway in pancreatic β-cells.34 Since T2D is also characterized by an increased burden of inflammation,35 SU elevation may be a reflection of the inflammatory burden. Compelling evidence suggests that activation of the NLRP3 inflammasome has a central role in both soluble urate36 and T2D.37 Therefore, NLRP3 inflammasome could be a possible therapeutic target for attenuating HU- and T2D-induced inflammation.
Effects of Urate on Chronic Diabetic Complications
Effects of Urate on Macrovascular Dysfunction
Resl et al38 conducted a prospective observational study in which 494 patients with diabetes were followed for 12.8 months, and underscored the importance of SU as a cardiovascular risk marker in patients with diabetes. SU is related to known risk factors such as hypertension and cerebral infarction. Further, Bjornstad et al39 indicated a positive association between SU and systolic blood pressure (SBP) in adults with diabetes over a 6-year follow-up period. More recently, it was demonstrated that HU strongly predicts the onset of hypertension in adolescents with T2D.40 A Mendelian randomization study among a Chinese population of females with diabetes supported a causal effect of SU on diabetic macrovascular disease through a genetic risk score, which was calculated using 17 selected single-nucleotide polymorphisms and the strength of their effects on SU levels.41 The possible mechanism of SU and diabetic macrovascular dysfunction could be the stimulation of the renin–angiotensin–aldosterone system (RAAS), thereby promoting ROS production and resulting in inflammation.42
Effects of Urate on Microvascular Dysfunction
Diabetic nephropathy is one of the most common diabetic microvascular complications. HU was found to be associated with an increased rate of progression of chronic kidney disease (CKD) in a prospective cohort of 422 individuals with an average of 15 years of T2D and a follow-up period of 43-months.43,44 Of patients with diabetic nephropathy, a large proportion of patients with SU levels higher than 378 μmol/L will have a poor prognosis.45 Other research reported that a doubling in the SU level is an independent risk factor for the loss of the kidney function in patients with T1D, including a decline in the estimated glomerular filtration rate (eGFR) of ≥30% and an increase in the urine albumin creatine ratio.46 A Japanese cohort study of 1802 patients with T2D found that an elevated SU level and male gender are risk factors for albuminuria, but not a decrease in eGFR.25 However, there was no evidence of a causal relationship between SU and the development of diabetic nephropathy in a Mendelian randomization study.47 Moreover, SU levels were higher in patients with peripheral arterial disease (PAD) than in those without PAD (345.0±95.2 vs 309.3±89.2 µmol/L).48 PAD in hyperuricemic patients may be caused by elevated purine oxidation, which leads to increased ROS, accompanied by reduced NO, and subsequent vascular injuries.
Management of Diabetes with Hyperuricemia
Diet
Carbohydrates
For individuals with HU and diabetes, carbohydrate intake is encouraged, with a focus on fresh vegetables, legumes, fruit and dairy products, and especially foods with a high insoluble fiber content and low glycemic load. Given the contrary effects between whole grains and refined grains, owing to differences in their purine content and glycemic index (GI), patients should carefully balance their daily grain intake. Although fructose has a low GI, there is evidence to suggest that visceral adiposity and increased cardiometabolic risk are linked to the metabolism of fructose, which produces urate as a byproduct.49 Although the intake of fruit is limited in patients with HU and diabetes,50 we also note that the fiber, vitamin C and flavonoids in fruit can block urate production.51–53
Protein
Avoidance of purine-rich animal protein (eg, red meat, seafood, poultry and visceral organs) is helpful for hyperuricemic patients. In addition to animal products, some vegetable protein sources (eg, legumes, soy products and sea vegetables) contain a high purine load as well, although their protective elements could prevent the elevation of SU levels and reduce the risk of gout attacks.51,54 Choosing diets containing high-quality protein is the common principle for both hyperuricemic and diabetic patients.
Fats
Diets high in unsaturated fatty acids increase the level of high-density lipoprotein (HDL) cholesterol, which increases the risk of HU.55 A Mediterranean-style eating pattern, which emphasizes less animal protein and less saturated fatty acid, is recommended for both hyperuricemic and diabetic patients.56,57 Multiple randomized controlled trials including patients with T2D have reported that a Mediterranean-style dietary pattern, rich in polyunsaturated and monounsaturated fats, can benefit both glucose and lipid control.58
Micronutrients
Higher plasma vitamin C is associated with a lower risk of HU, by promoting uricosuria in the renal proximal tubules and resisting the pro-oxidative effects of urate.59 Folic acid therapy was shown to have a urate-lowering effect in hypertensive patients in the China Stroke Primary Prevention Trial.60 A randomized, double-blind, placebo-controlled study observed that treatments combined with glycine and tryptophan increase the solubility of urate and alkalinize the urine, thus helping to reduce the SU concentrations.61 Dietary minerals such as zinc and magnesium have been found to be inversely associated with HU among US adults in a cross-sectional study.62,63 The dietary suggestions are listed in Table 1.
 |
Table 1 Dietary Recommendations for Diabetic and Hyperuricemic Patients |
Physical Activity
Exercise is a double-edged sword for gout patients, with moderate exercise helping to lower SU and excessive exercise leading to gout flare. Avoiding extended sedentary periods, and undertaking regular resistance exercise or moderate-to-vigorous intensity aerobic activity are recommended for diabetic patients.64 Randomized controlled trials show that intensity training improves β-cell function and decreases pancreatic fat.65 However, slow-motion and non-weight-bearing activities, such as swimming, walking or taiji, are recommended for HU patients. With regard to exercise time, 20–60 minutes daily or 150–300 minutes weekly may be recommended. It is critical to pay attention to exercise-induced hypoglycemia and dehydration.
Pharmacotherapy
Glycemic Control
Accumulating data have proved that diabetes and hypoglycemic agents have an impact on renal urate excretion. Hypoglycemic agents with identified urate-lowering effects include α-glucosidase inhibitors, insulin-sensitizing agents, thiazolidinediones (TZDs), sodium–glucose co-transporter-2 inhibitors (SGLT2i), dipeptidyl peptidase-4 inhibitors (DPP-4i) and biguanides, which would be the first choice for patients with diabetes and HU. Insulin initiators are not recommended as they increase the levels of SU via the regulation of urate transporter-1 (URAT1) and ATP-binding cassette subfamily G member-2 (ABCG2).66 If insulin initiators are required, one strategy is to combine them with insulin-sensitizing drugs or α-glucosidase inhibitors to neutralize the increase in SU.
A significant urate-lowering effect of pioglitazone (30 mg/day for 24 weeks) has been observed in patients with urate kidney stones.67 Pioglitazone users present a dose-dependent decrease in gout incidence as well.68 These two reports indicate that pioglitazone assists the management of HU/gout in diabetes, without changes in renal urate excretion.67 A meta-analysis of 62 clinical trials showed that SGLT2i reduce circulating urate by inducing uricosuria, and contribute to cardiorenal benefits.69
DPP-4 inhibitors decrease SU; an in vivo experiment proved that DPP-4 inhibitors mediate multiple beneficial effects by inhibiting the adenosine deaminase–xanthine oxidase–urate pathway, improving insulin resistance, and inhibiting oxidative stress and hepatic triglyceride accumulation.70 Considering the weight-loss effect of glucagon-like peptide-1 (GLP-1) receptor agonists, there should be a parallel urate-lowering effect. However, the available clinical evidence indicates that liraglutide and exenatide have no effects on SU levels.71
Urate-Lowering Therapy (ULT)
Allopurinol is the first xanthine oxidase inhibitor (XOI) to be prescribed in patients with HU and gout. Despite its remarkable efficacy and lower price, the risk of allopurinol hypersensitivity syndrome (AHS) is the main concern, especially in patients of Asian and African origin.72 Thus, it is a priority to test for the HLA-B*5801 allele before starting allopurinol treatment. For patients with accompanying diabetes, allopurinol was shown to increase the risk of hypersensitive reactions compared with those without diabetes.73 Febuxostat is a specific XOI that is especially suitable for patients with chronic renal insufficiency. However, considering the cost and the potential adverse cardiovascular effects, the European and American guidelines generally recommend febuxostat as an option for patients who are intolerant to allopurinol or present signs of poor efficacy.74
Benzbromarone decreases urate reabsorption by inhibiting URAT-1 in the proximal renal tubules, and is suitable for patients with reduced excretion of renal urate. Increased water intake and urine alkalization will protect against the deposition of sodium urate crystals in the kidney. In a retrospective cohort study on a population with gout, the incidence of newly developed diabetes was lower in benzbromarone users than in non-users.75 An in vivo study showed that benzbromarone dependently reduced blood glucose levels and rectified insulin resistance in db/db mice, and the authors also reported that the glucose-lowering effect is due to the inhibition of fatty acid-binding protein-4 (FABP4), which plays an important role in maintaining glucose homeostasis, making benzbromarone a potential drug candidate for the treatment of diabetes.26 In patients taking benzbromarone, one should pay attention to the liver function, as benzbromarone causes serious hepatotoxicity in Caucasians, although this is rare in Asian populations.
Although accumulating data suggest that higher SU levels have a detrimental effect on renal function, the potential benefits of ULT in individuals with asymptomatic HU are still under debate. Evidence from diabetic rat models indicates the advantages of XOI, including rectification of glucose intolerance and the insulin resistance state,48 amelioration of albuminuria and renal oxidative stress,76 and different degrees of improvement in glomerular sclerosis and tubulointerstitial fibrosis.77 Clinical trials also suggest that allopurinol can decrease albumin excretion and slow the decline in eGFR in adults with diabetes.78 However, a multicenter, randomized, double-blind, placebo-controlled study showed that patients receiving febuxostat had no significant benefit or deterioration in renal function after 12 months of follow-up.79 Consistently, no detectable effects of febuxostat on the eGFR were observed in patients with T2D and diabetic nephropathy in another double-blinded randomized controlled trial.80 The Preventing Early Renal function Loss (PERL) trial found no evidence of clinically meaningful benefits of allopurinol on kidney outcomes among patients with T1D and early-to-moderate diabetic kidney disease.81
Given the discrepancies between studies and the potential adverse cardiovascular and cutaneous events induced by allopurinol or febuxostat, the risk/benefit ratio of ULT in this indication is unclear. Every effort should be made to avoid prescribing ULT for inappropriate indications.
Blood Pressure Control
A prospective study on Japanese men without hypertension showed that HU may have a longitudinal association with the development of hypertension after 9 years’ follow-up.82 About 40–60% of hyperuricemic patients were complicated with clinical hypertension. Losartan and calcium channel blockers have been found to have a urate-lowering effect and to reduce the risk of gout attacks.83 Potassium diuretics, beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers (except for losartan) should be avoided as they have an elevating effect on SU.
Antilipemic and Anticoagulant Agents
HU was found to be associated with dyslipidemia and atherosclerosis. Our previous research demonstrated that allopurinol can alleviate atherosclerosis inflammatory cytokines and neointimal lesions which were possibly induced by HU in mice.84 A large randomized controlled trial demonstrated that fenofibrate reduced SU by 20% after 6 weeks’ follow-up and halved the incidence of a first gout attack over 5 years.85 Therefore, fenofibrate is recommended as the first choice of antilipemic drug in HU patients complicated with hypertriglyceridemia. Besides, atorvastatin calcium was recommended as the first-line drug for HU patients with high levels of cholesterol owing to its role in promoting renal urate excretion.86 Aspirin is a widely used antiplatelet agent used to prevent thromboembolic events, but the effect on SU levels is still controversial.87 The medical management principles are listed in Table 2.
 |
Table 2 Principles of Medical Management of Diabetes with Hyperuricemia |
Monitoring Technology
Lifestyle monitoring technology for the detection of health deterioration in long-term conditions has been applied in diseases such as heart failure88 and dementia,89 as well as in diabetes. A 3-month follow-up study, including 1354 participants, showed that the higher the levels of patient activation and engagement with remote patient monitoring technology, the better the outcomes of glycemic control.90 Wearable sensor technologies help to realize personalized medicine through continuous monitoring, and have been applied to the detection of glucose91 and uric acid levels.92 The development of artificial intelligence technology could be important in improving the effectiveness of the management of HU and diabetes.
Conclusion
An inverted U-shaped relationship between FPG and SU levels was established in this review. SU has been recognized as a predictor of DM development, with effects on chronic diabetic complications, both macrovascular dysfunction (hypertension and cerebral infarction) and microvascular dysfunction (chronic kidney disease and peripheral arterial disease). Furthermore, the association of HU with insulin resistance and β-cell dysfunction has already been well demonstrated, but whether there is a causal relationship remains inconclusive. We have outlined the recent ideas on HU and diabetes management, including diet, physical exercise and medicine. Choosing medicines balancing the SU and blood glucose levels is critical in therapeutic management. Medical artificial intelligence technology and monitoring systems can help to improve the effectiveness of long-term management of HU and diabetes through digital healthcare. To summarize, in light of the preceding discussion on the relationship between HU and diabetes and their effects on complications, we can reconsider the optimal approach to the management of hyperuricemic and diabetic patients.
Acknowledgments
We really appreciate Prof. Changgui Li’s team’s support and help in our work; they are affiliated to the Shandong Provincial Clinical Research Center for Immune Diseases and Gout, Qingdao, China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the final submitted manuscript.
Funding
This work was sponsored by the Taishan Scholar Programme of Shandong Province (#tsqn202211377) and Shandong Provincial Science Foundation for Outstanding Youth Scholars (#ZR2021YQ56). No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fatima T, Iftikhar S, Qureshi IH. Association between hyperuricemia and ischemic stroke: a case-control study. J Coll Physicians Surg Pak. 2020;30(8):853–856. doi:10.29271/jcpsp.2020.08.853
2. Chien KL, Chen MF, Hsu HC, et al. Plasma uric acid and the risk of type 2 diabetes in a Chinese community. Clin Chem. 2008;54(2):310–316. doi:10.1373/clinchem.2007.095190
3. Stack AG, Dronamraju N, Parkinson J, et al. Effect of intensive urate lowering with combined verinurad and febuxostat on albuminuria in patients with type 2 diabetes: a randomized trial. Am J Kidney Dis. 2021;77(4):481–489. doi:10.1053/j.ajkd.2020.09.009
4. Tanaka A, Taguchi I, Teragawa H, et al. Febuxostat does not delay progression of carotid atherosclerosis in patients with asymptomatic hyperuricemia: a randomized, controlled trial. PLoS Med. 2020;17(4):e1003095. doi:10.1371/journal.pmed.1003095
5. Hu F, Zhang T. Study on risk factors of diabetic nephropathy in obese patients with type 2 diabetes mellitus. Int J Gen Med. 2020;13:351–360. doi:10.2147/ijgm.S255858
6. Kocak MZ, Aktas G, Duman TT, Atak BM, Savli H. Is Uric Acid elevation a random finding or a causative agent of diabetic nephropathy? Rev Assoc Med Bras. 2019;65(9):1155–1160. doi:10.1590/1806-9282.65.9.1156
7. Aktas G, Kocak MZ, Bilgin S, Atak BM, Duman TT, Kurtkulagi O. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. Aging Male. 2020;23(5):1098–1102. doi:10.1080/13685538.2019.1678126
8. Aktas G, Yilmaz S, Kantarci DB, et al. Is serum uric acid-to-HDL cholesterol ratio elevation associated with diabetic kidney injury? Postgrad Med. 2023;135(5):519–523. doi:10.1080/00325481.2023.2214058
9. Aktas G, Khalid A, Kurtkulagi O, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgrad Med. 2022;134(3):297–302. doi:10.1080/00325481.2022.2039007
10. Kocak MZ, Aktas G, Erkus E, Sincer I, Atak B, Duman T. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Rev Assoc Med Bras. 2019;65(1):9–15. doi:10.1590/1806-9282.65.1.9
11. Kosekli MA, Kurtkulagii O, Kahveci G, et al. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: the abund study. Rev Assoc Med Bras. 2021;67(4):549–554. doi:10.1590/1806-9282.20201005
12. Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American diabetes association: national cross sectional study. BMJ. 2020;369:m997. doi:10.1136/bmj.m997
13. Dong X, Zhang H, Wang F, et al. Epidemiology and prevalence of hyperuricemia among men and women in Chinese rural population: the Henan rural cohort study. Mod Rheumatol. 2020;30(5):910–920. doi:10.1080/14397595.2019.1660048
14. Lu J, Sun W, Cui L, et al. A cross-sectional study on uric acid levels among Chinese adolescents. Pediatr Nephrol. 2020;35(3):441–446. doi:10.1007/s00467-019-04357-w
15. Lv Q, Meng X, He F, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8(2):e56864. doi:10.1371/journal.pone.0056864
16. Bhole V, Choi JW, Kim SW, de Vera M, Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med. 2010;123(10):957–961. doi:10.1016/j.amjmed.2010.03.027
17. Wang T, Bi Y, Xu M, et al. Serum uric acid associates with the incidence of type 2 diabetes in a prospective cohort of middle-aged and elderly Chinese. Endocrine. 2011;40(1):109–116. doi:10.1007/s12020-011-9449-2
18. Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32(9):1737–1742. doi:10.2337/dc09-0288
19. Cicerchi C, Li N, Kratzer J, et al. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J. 2014;28(8):3339–3350. doi:10.1096/fj.13-243634
20. Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”. Am J Hum Genet. 1962;14(4):353–362.
21. Wan X, Xu C, Lin Y, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol. 2016;64(4):925–932. doi:10.1016/j.jhep.2015.11.022
22. Lu J, He Y, Cui L, et al. Hyperuricemia predisposes to the onset of diabetes via promoting pancreatic β-cell death in uricase-deficient male mice. Diabetes. 2020;69(6):1149–1163. doi:10.2337/db19-0704
23. Xu C, Wan X, Xu L, et al. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: one stone hits two birds. J Hepatol. 2015;62(6):1412–1419. doi:10.1016/j.jhep.2015.01.019
24. Meng J, Li Y, Yuan X, Lu Y. Effects of febuxostat on insulin resistance and expression of high-sensitivity C-reactive protein in patients with primary gout. Rheumatol Int. 2017;37(2):299–303. doi:10.1007/s00296-016-3612-2
25. Takagi M, Babazono T, Uchigata Y. Differences in risk factors for the onset of albuminuria and decrease in glomerular filtration rate in people with Type 2 diabetes mellitus: implications for the pathogenesis of diabetic kidney disease. Diabet Med. 2015;32(10):1354–1360. doi:10.1111/dme.12793
26. Han T, Lan L, Qu R, et al. Temporal relationship between hyperuricemia and insulin resistance and its impact on future risk of hypertension. Hypertension. 2017;70(4):703–711. doi:10.1161/hypertensionaha.117.09508
27. Pfister R, Barnes D, Luben R, et al. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia. 2011;54(10):2561–2569. doi:10.1007/s00125-011-2235-0
28. Keenan T, Zhao W, Rasheed A, et al. Causal assessment of serum urate levels in cardiometabolic diseases through a Mendelian randomization study. J Am Coll Cardiol. 2016;67(4):407–416. doi:10.1016/j.jacc.2015.10.086
29. Sluijs I, Holmes MV, van der Schouw YT, et al. A Mendelian randomization study of circulating uric acid and type 2 diabetes. Diabetes. 2015;64(8):3028–3036. doi:10.2337/db14-0742
30. Volpe A, Ye C, Hanley AJ, Connelly PW, Zinman B, Retnakaran R. Changes over time in uric acid in relation to changes in insulin sensitivity, beta-cell function, and glycemia. J Clin Endocrinol Metab. 2020;105(3):e651–9. doi:10.1210/clinem/dgz199
31. Jia L, Xing J, Ding Y, et al. Hyperuricemia causes pancreatic β-cell death and dysfunction through NF-κB signaling pathway. PLoS One. 2013;8(10):e78284. doi:10.1371/journal.pone.0078284
32. Tang W, Fu Q, Zhang Q, et al. The association between serum uric acid and residual β -cell function in type 2 diabetes. J Diabetes Res. 2014;2014:709691. doi:10.1155/2014/709691
33. Zhang Y, Yamamoto T, Hisatome I, et al. Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells. Mol Cell Endocrinol. 2013;375(1–2):89–96. doi:10.1016/j.mce.2013.04.027
34. Hu Y, Zhao H, Lu J, et al. High uric acid promotes dysfunction in pancreatic β cells by blocking IRS2/AKT signalling. Mol Cell Endocrinol. 2021;520:111070. doi:10.1016/j.mce.2020.111070
35. Kocak MZ, Aktas G, Erkus E, et al. Neuregulin-4 is associated with plasma glucose and increased risk of type 2 diabetes mellitus. Swiss Med Wkly. 2019;149:w20139. doi:10.4414/smw.2019.20139
36. Braga TT, Forni MF, Correa-Costa M, et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci Rep. 2017;7:39884. doi:10.1038/srep39884
37. Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism. 2017;74:1–9. doi:10.1016/j.metabol.2017.06.002
38. Resl M, Clodi M, Neuhold S, et al. Serum uric acid is related to cardiovascular events and correlates with N-terminal pro-B-type natriuretic peptide and albuminuria in patients with diabetes mellitus. Diabet Med. 2012;29(6):721–725. doi:10.1111/j.1464-5491.2011.03515.x
39. Bjornstad P, Paul Wadwa R, Sirota J, et al. Serum uric acid and hypertension in adults: a paradoxical relationship in type 1 diabetes. J Clin Hypertens. 2014;16(4):283–288. doi:10.1111/jch.12305
40. Bjornstad P, Laffel L, Lynch J, et al. Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care. 2019;42(6):1120–1128. doi:10.2337/dc18-2147
41. Yan D, Wang J, Jiang F, et al. A causal relationship between uric acid and diabetic macrovascular disease in Chinese type 2 diabetes patients: a Mendelian randomization analysis. Int J Cardiol. 2016;214:194–199. doi:10.1016/j.ijcard.2016.03.206
42. Lytvyn Y, Perkins B, Cherney D. Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes. 2015;39(3):239–246. doi:10.1016/j.jcjd.2014.10.013
43. Bartáková V, Kuricová K, Pácal L, et al. Hyperuricemia contributes to the faster progression of diabetic kidney disease in type 2 diabetes mellitus. J Diabetes Complications. 2016;30(7):1300–1307. doi:10.1016/j.jdiacomp.2016.06.002
44. Chen Z, Ding Z, Fu C, Yu C, Ma G. Correlation between serum uric Acid and renal function in patients with stable coronary artery disease and type 2 diabetes. J Clin Med Res. 2014;6(6):443–450. doi:10.14740/jocmr1909w
45. Pilemann-Lyberg S, Hansen T, Persson F, et al. Uric acid is not associated with diabetic nephropathy and other complications in type 1 diabetes. Nephrol Dial Transplant. 2019;34(4):659–666. doi:10.1093/ndt/gfy076
46. Pilemann-Lyberg S, Hansen T, Tofte N, et al. Uric acid is an independent risk factor for decline in kidney function, cardiovascular events, and mortality in patients with type 1 diabetes. Diabetes Care. 2019;42(6):1088–1094. doi:10.2337/dc18-2173
47. Ahola A, Sandholm N, Forsblom C, Harjutsalo V, Dahlström E, Groop P. The serum uric acid concentration is not causally linked to diabetic nephropathy in type 1 diabetes. Kidney Int. 2017;91(5):1178–1185. doi:10.1016/j.kint.2016.11.025
48. Mizuno Y, Yamamotoya T, Nakatsu Y, et al. Xanthine oxidase inhibitor febuxostat exerts an anti-inflammatory action and protects against diabetic nephropathy development in KK-ay obese diabetic mice. Int J Mol Sci. 2019;20(19):4680. doi:10.3390/ijms20194680
49. Sangüesa G, Roglans N, Montañés J, et al. Chronic liquid fructose, but not glucose, supplementation selectively induces visceral adipose tissue leptin resistance and hypertrophy in female Sprague-Dawley rats. Mol Nutr Food Res. 2018;62(22):e1800777. doi:10.1002/mnfr.201800777
50. Murphy R, Thornley S, de Zoysa J, Stamp L, Dalbeth N, Merriman T. Sugar sweetened beverage consumption among adults with gout or type 2 diabetes. PLoS One. 2015;10(5):e0125543. doi:10.1371/journal.pone.0125543
51. Sun Y, Sun J, Zhang P, Zhong F, Cai J, Ma A. Association of dietary fiber intake with hyperuricemia in U.S. adults. Food Funct. 2019;10(8):4932–4940. doi:10.1039/c8fo01917g
52. Žuvela P, David J, Yang X, Huang D, Wong MW. Non-linear quantitative structure⁻activity relationships modelling, mechanistic study and in-silico design of flavonoids as potent antioxidants. Int J Mol Sci. 2019;20(9):2328. doi:10.3390/ijms20092328
53. Choi HK, Gao X, Curhan G. Vitamin C intake and the risk of gout in men: a prospective study. Arch Intern Med. 2009;169(5):502–507. doi:10.1001/archinternmed.2008.606
54. Teng G, Pan A, Yuan J, Koh W. Food sources of protein and risk of incident gout in the Singapore Chinese health study. Arthritis Rheumatol. 2015;67(7):1933–1942. doi:10.1002/art.39115
55. Liu X, Wu Q, Chen Z, et al. Elevated triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio increased risk of hyperuricemia: a 4-year cohort study in China. Endocrine. 2020;68(1):71–80. doi:10.1007/s12020-019-02176-5
56. Yokose C, McCormick N, Rai SK, et al. Effects of low-fat, Mediterranean, or low-carbohydrate weight loss diets on serum urate and cardiometabolic risk factors: a secondary analysis of the dietary intervention randomized controlled trial (DIRECT). Diabetes Care. 2020;43(11):2812–2820. doi:10.2337/dc20-1002
57. Bergia RE, Giacco R, Hjorth T, et al. Differential glycemic effects of low- versus high-glycemic index Mediterranean-style eating patterns in adults at risk for type 2 diabetes: the medgi-carb randomized controlled trial. Nutrients. 2022;14(3):706. doi:10.3390/nu14030706
58. Kontogianni M, Chrysohoou C, Panagiotakos D, et al. Adherence to the Mediterranean diet and serum uric acid: the ATTICA study. Scand J Rheumatol. 2012;41(6):442–449. doi:10.3109/03009742.2012.679964
59. Kobylecki C, Afzal S, Nordestgaard B. Genetically high plasma vitamin C and urate: a Mendelian randomization study in 106 147 individuals from the general population. Rheumatology. 2018;57(10):1769–1776. doi:10.1093/rheumatology/key171
60. Qin X, Li Y, He M, et al. Folic acid therapy reduces serum uric acid in hypertensive patients: a substudy of the China Stroke Primary Prevention Trial (CSPPT). Am J Clin Nutr. 2017;105(4):882–889. doi:10.3945/ajcn.116.143131
61. Oshima S, Shiiya S, Nakamura Y. Serum uric acid-lowering effects of combined glycine and tryptophan treatments in subjects with mild hyperuricemia: a randomized, double-blind, placebo-controlled, crossover study. Nutrients. 2019;11(3). doi:10.3390/nu11030564
62. Zhang Y, Qiu H. Dietary magnesium intake and hyperuricemia among US adults. Nutrients. 2018;10(3). doi:10.3390/nu10030296
63. Zhang Y, Liu Y, Qiu H. Association between dietary zinc intake and hyperuricemia among adults in the United States. Nutrients. 2018;10(5). doi:10.3390/nu10050568
64. American Diabetes A. 4. lifestyle management: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl1):S38–S50. doi:10.2337/dc18-S004
65. Nieuwoudt S, Fealy C, Foucher J, et al. Functional high-intensity training improves pancreatic β-cell function in adults with type 2 diabetes. Am J Physiol Endocrinol Metab. 2017;313(3):E314–E320. doi:10.1152/ajpendo.00407.2016
66. Toyoki D, Shibata S, Kuribayashi-Okuma E, et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am J Physiol Renal Physiol. 2017;313(3):F826–F834. doi:10.1152/ajprenal.00012.2017
67. Maalouf N, Poindexter J, Adams-Huet B, Moe O, Sakhaee K. Increased production and reduced urinary buffering of acid in uric acid stone formers is ameliorated by pioglitazone. Kidney Int. 2019;95(5):1262–1268. doi:10.1016/j.kint.2018.11.024
68. Niu S, Chang K, Lin H, et al. Decreased incidence of gout in diabetic patients using pioglitazone. Rheumatology. 2018;57(1):92–99. doi:10.1093/rheumatology/kex363
69. Zhao Y, Xu L, Tian D, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(2):458–462. doi:10.1111/dom.13101
70. Omolekulo T, Michael O, Olatunji L. Dipeptidyl peptidase-4 inhibition protects the liver of insulin-resistant female rats against triglyceride accumulation by suppressing uric acid. Biomed Pharmacother. 2019;110:869–877. doi:10.1016/j.biopha.2018.12.036
71. Tonneijck L, Muskiet M, Smits M, et al. Effect of immediate and prolonged GLP-1 receptor agonist administration on uric acid and kidney clearance: post-hoc analyses of four clinical trials. Diabetes Obes Metab. 2018;20(5):1235–1245. doi:10.1111/dom.13223
72. Vargas-Santos A, Peloquin C, Zhang Y, Neogi T. Association of chronic kidney disease with allopurinol use in gout treatment. JAMA Intern Med. 2018;178(11):1526–1533. doi:10.1001/jamainternmed.2018.4463
73. Singh J, Cleveland J. Hypersensitivity reactions with allopurinol and febuxostat: a study using the Medicare claims data. Ann Rheum Dis. 2020;79(4):529–535. doi:10.1136/annrheumdis-2019-216917
74. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American college of rheumatology guideline for the management of gout. Arthritis Rheumatol. 2020;72(6):879–895. doi:10.1002/art.41247
75. Niu S, Chang K, Ta A, et al. Decreased incidence of diabetes in patients with gout using benzbromarone. Rheumatology. 2018;57(9):1574–1582. doi:10.1093/rheumatology/key138
76. Komers R, Xu B, Schneider J, Oyama T. Effects of xanthine oxidase inhibition with febuxostat on the development of nephropathy in experimental type 2 diabetes. Br J Pharmacol. 2016;173(17):2573–2588. doi:10.1111/bph.13527
77. Yisireyili M, Hayashi M, Wu H, et al. Xanthine oxidase inhibition by febuxostat attenuates stress-induced hyperuricemia, glucose dysmetabolism, and prothrombotic state in mice. Sci Rep. 2017;7(1):1266. doi:10.1038/s41598-017-01366-3
78. Pilemann-Lyberg S, Persson F, Frystyk J, Rossing P. The effect of uric acid lowering treatment on albuminuria and renal function in Type 1 diabetes: a randomized clinical trial. Diabet Med. 2018;35(3):392–393. doi:10.1111/dme.13577
79. Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L. Impact of Febuxostat on Renal Function in Gout Patients With Moderate-to-Severe Renal Impairment. Arthritis Rheumatol. 2016;68(8):2035–2043. doi:10.1002/art.39654
80. Beddhu S, Filipowicz R, Wang B, et al. A randomized controlled trial of the effects of febuxostat therapy on adipokines and markers of kidney fibrosis in asymptomatic hyperuricemic patients with diabetic nephropathy. Can J Kidney Health Dis. 2016;3:2054358116675343. doi:10.1177/2054358116675343
81. Doria A, Galecki AT, Spino C, et al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med. 2020;382(26):2493–2503. doi:10.1056/NEJMoa1916624
82. Tomiyama H, Shiina K, Vlachopoulos C, et al. Involvement of arterial stiffness and inflammation in hyperuricemia-related development of hypertension. Hypertension. 2018;72(3):739–745. doi:10.1161/hypertensionaha.118.11390
83. Choi H, Soriano L, Zhang Y, Rodríguez L. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ. 2012; 344:d8190. doi:10.1136/bmj.d8190
84. Lu J, Sun M, Wu X, et al. Urate-lowering therapy alleviates atherosclerosis inflammatory response factors and neointimal lesions in a mouse model of induced carotid atherosclerosis. FEBS J. 2019;286(7):1346–1359. doi:10.1111/febs.14768
85. Waldman B, Ansquer J, Sullivan D, et al. Effect of fenofibrate on uric acid and gout in type 2 diabetes: a post-hoc analysis of the randomised, controlled FIELD study. Lancet Diabetes Endocrinol. 2018;6(4):310–318. doi:10.1016/s2213-8587(18)30029-9
86. Derosa G, Maffioli P, Reiner Ž, Simental-Mendía L, Sahebkar A. Impact of statin therapy on plasma uric acid concentrations: a systematic review and meta-analysis. Drugs. 2016;76(9):947–956. doi:10.1007/s40265-016-0591-2
87. Zhang P, Wang H, Chen X, Liang W, Liu W, Liu M. Effect of low-dose aspirin on serum uric acid levels in Chinese individuals over 60: subanalysis of a multicentre randomized clinical trial. Eur Rev Med Pharmacol Sci. 2020;24(5):2719–2724. doi:10.26355/eurrev_202003_20544
88. Hargreaves S, Hawley M, Haywood A, Enderby P. Informing the design of “lifestyle monitoring” technology for the detection of health deterioration in long-term conditions: a qualitative study of people living with heart failure. J Med Internet Res. 2017;19(6):e231. doi:10.2196/jmir.6931
89. Dugstad J, Eide T, Nilsen E, Eide H. Towards successful digital transformation through co-creation: a longitudinal study of a four-year implementation of digital monitoring technology in residential care for persons with dementia. BMC Health Serv Res. 2019;19(1):366. doi:10.1186/s12913-019-4191-1
90. Su D, Michaud T, Estabrooks P, et al. Diabetes management through remote patient monitoring: the importance of patient activation and engagement with the technology. Telemed J E Health. 2019;25(10):952–959. doi:10.1089/tmj.2018.0205
91. Gao W, Emaminejad S, Nyein H, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529(7587):509–514. doi:10.1038/nature16521
92. Yang Y, Song Y, Bo X, et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat Biotechnol. 2020;38(2):217–224. doi:10.1038/s41587-019-0321-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

