Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
The Main Pulmonary Artery to the Ascending Aorta Diameter Ratio (PA/A) as a Predictor of Worse Outcomes in Hospitalized Patients with AECOPD
Authors Cheng Y, Li L, Tu X, Pei R
Received 10 January 2022
Accepted for publication 30 April 2022
Published 16 May 2022 Volume 2022:17 Pages 1157—1165
DOI https://doi.org/10.2147/COPD.S357696
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Yusheng Cheng,1,* Lingling Li,1,* Xiongwen Tu,1 Renguang Pei2
1Department of Respiratory Medicine, Yijishan Hospital, Wannan Medical College, Wuhu, People’s Republic of China; 2Department of Interventional Therapy, Yijishan Hospital, Wannan Medical College, Wuhu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yusheng Cheng, Department of Respiratory Medicine, Yijishan Hospital, Wannan Medical College, Wuhu, People’s Republic of China, Email [email protected] Renguang Pei, Department of Interventional Therapy, Yijishan Hospital, Wannan Medical College, Wuhu, 241001, People’s Republic of China, Email [email protected]
Purpose: The main pulmonary artery (PA) to ascending aorta diameter ratio (PA/A) greater than one is a promising indicator of pulmonary hypertension (PH) in acute exacerbation (AE) of chronic obstructive pulmonary disease (COPD) (AECOPD). This study aims to disclose the associations between the PA/A ratio and clinical outcomes in hospitalized patients with AECOPD.
Patients and Methods: Consecutive AECOPD patients admitted to the Department of Respiratory Medicine from September 2017 to July 2021 were reviewed. The treatment success of AECOPD patients was defined as improvement in the clinical condition when discharged from the hospital. Conversely, treatment failure was considered to be an event of in-hospital death or deterioration of the clinical condition prior to discharge.
Results: A total of 118 individuals were ultimately reviewed in this study: 74 individuals with a PA/A ratio < 1 and 44 individuals with a PA/A ratio ≥ 1. The outcomes of 21 patients were treatment failure, and 97 patients were considered successes. Patients with a PA/A ratio ≥ 1 had significantly higher PaCO2, red cell distribution width, brain natriuretic peptide, PA diameters, RICU admission rates, and proportions of treatment failure than patients with PA/A ratios < 1 (P < 0.05). The PA diameter and PA/A ratio were significantly increased in the treatment failure group compared with the success group (P < 0.05). A survival analysis indicated that patients with a PA/A ratio ≥ 1 had worse outcomes than patients with a PA/A ratio < 1 during hospitalization (P < 0.05). A multivariate analysis showed that a PA/A ratio ≥ 1 was an independent risk factor for treatment failure in patients with AECOPD.
Conclusions: AECOPD patients with a PA/A ratio ≥ 1 may have worse outcomes during hospitalization. A PA/A ratio ≥ 1 may be a promising predictor of treatment failure in patients with AECOPD.
Keywords: pulmonary hypertension, chronic obstructive pulmonary disease, PA/A ratio
Introduction
Chronic obstructive pulmonary disease (COPD) is a clinical syndrome that features chronic respiratory symptoms and structural pulmonary abnormalities leading to lung function impairment with persistent airflow limitation.1 A recent study indicated that the overall prevalence of spirometry defined for COPD was 8.6% of adults in China, including 11.9% of men aged 40 years or older. The acute exacerbation of COPD (AECOPD) is a key factor that affects the disease prognosis and leads to hospitalization. Thus, AECOPD-related morbidity and mortality should be given more attention.2,3 Pulmonary hypertension (PH) is a common and severe comorbidity of COPD that results in an increased risk of hospitalization, reduced exercise capacity, and shorter survival. Right-heart catheterization (RHC) is the “gold standard” for the diagnosis of PH. However, RHC related significant risks and its difficulty of placement limits this procedure in patients with PH. Echocardiography is a noninvasive method that is widely used to assess PH in patients with AECOPD.4 A tricuspid regurgitant jet ≥3 m/s tested by echocardiography is diagnosed as PH, which may lead to underdetermined diagnoses of PH.5 Moreover, pulmonary artery systolic pressure detected by echocardiography is poorly correlated with the mean pulmonary artery pressure (mPAP) in severe COPD. A main pulmonary artery to ascending aorta diameter ratio (PA/A) of greater than one has been reported to be a promising indicator for revealing PH.6,7 Furthermore, an increased ratio of PA/A was closely associated with the poor survival of patients with COPD, particularly in individuals with moderate-to-severe cases.8 Nevertheless, the impact of the PA/A ratio in AECOPD remains to be elucidated. In this present study, we aim to disclose the associations between the PA/A ratio and clinical outcomes in hospitalized patients with AECOPD.
Patients and Methods
Study Population
This retrospective observational study was conducted at the Yijishan Hospital affiliated with the Wannan Medical College and was approved by the Research Ethics Committee of Yijishan Hospital. The clinical data of patients was maintained with confidentiality and in compliance with the Declaration of Helsinki. Written informed consent from patients was waived due to the retrospective nature of this study. Consecutive AECOPD patients admitted to the Department of Respiratory Medicine and Respiratory Intensive Care Units (RICU) were reviewed from September 2017 to July 2021. Patients with advanced lung cancer, pneumothorax, stroke, pneumonia, diffuse interstitial lung disease, hemodialysis, or left-heart failure, as well as those who only accepted palliative therapy, or had a lack of chest computed tomography (CT) images, were excluded from the final analysis.
AECOPD is defined as COPD with an acute worsening of respiratory symptoms (typically cough, dyspnea, increased sputum volume, and/or sputum purulence) requiring additional treatments.9 Indications for RICU admission were made according to the expert consensus released in 2014 on AECOPD in China.10 In brief, these consisted of a significant increase in symptom intensity (severe dyspnea, changes in mental status, moderate or severe hypoxemia with or without hypercapnia), failure of an exacerbation to respond to initial medical management, hemodynamic instability, and a patient requiring mechanical ventilation (MV). The treatment success of AECOPD patients was defined as improvement in the clinical condition when discharged from the hospital. Conversely, treatment failure was thought to occur as an event of in-hospital death or deterioration of the clinical condition prior to discharge.
Demographic characteristics, including gender, age, the age-adjusted Charlson Comorbidity Index (aCCI), length of stay, body mass index (BMI) and in-hospital death, were collected. Laboratory tests, including an arterial blood gas analysis (pH value, oxygenation index, the ratio of arterial partial pressure of oxygen to the fraction of inspired oxygen), PaCO2, and the blood lactate level), hemoglobin, blood red cell distribution width (RDW), D-dimer, brain natriuretic peptide (BNP), fibrinogen (Fib), and blood platelet (PLT), were initially recorded after admission. The percentage of ICU admissions requiring invasive MV (IMV) was also calculated. A chest CT was performed when the patient was admitted to the hospital. The procedure for measuring the pulmonary artery (PA) diameter and PA/A ratio determined by the chest CT conformed to a previous study.6 Briefly, the PA diameter and ascending aorta diameter were averaged from two perpendicular measurements at the PA bifurcation level collected from the same chest CT images, as shown in Figure 1.
 |
Figure 1 Diameters of the PA and A were determined by CT scan at the PA bifurcation. (A) PA/A ratio < 1; (B) PA/A ratio > 1. Abbreviations: A, aorta; PA, pulmonary artery. |
Statistical Analysis
Continuous data were analyzed using a normal distribution test prior to further analysis. Continuous data are indicated as the mean (standard deviation [SD]) or median (inter-quartile range [25,75]). Categorical variables are presented as the number (n) or percentage. Continuous variables were analyzed using the independent t-test or the Mann-Whitney U-test, and categorical variables were analyzed using a Chi-square test. The logistic regression model was used as a multivariate analysis to reveal the independent risk factors of in-hospital worst outcomes in patients with AECOPD. The Kaplan–Meier survival method was used to analyze the effect of the PA/A ratio on outcomes of AECOPD patients. A Log rank test was applied to appraise the statistical differences between the two survival curves. A receiver operating characteristic (ROC) curve analysis was conducted to evaluate factors predicting an in-hospital worst outcome. A P value less than 0.05 was considered statistically significant. The statistical analyses were performed using SPSS for Windows (release 22.0, IBM Corporation, USA).
Results
As indicated in Figure 2, a total of 229 patients with AECOPD were reviewed. According to the inclusion criteria and exclusion criteria, 111 patients were excluded due to the condition being combined with advanced lung cancer (n = 10), pneumothorax (n = 4) stroke (n = 5), pneumonia (n = 29), diffuse interstitial lung disease (n = 7), hemodialysis (n = 6), left-heart failure (n = 19), palliative therapy (n = 23), and a lack of CT images (n = 10). Ultimately, 118 eligible individuals were reviewed in this study: 74 individuals with a PA/A ratio <1 and 44 individuals with PA/A ratio ≥1. The outcomes of 21 patients were treatment failures, and 97 patients were treatment successes when discharged from the hospital.
 |
Figure 2 A flowchart of this study. |
Characteristics of the AECOPD Patients with a PA/A Ratio <1 or a PA/A Ratio ≥1
The pH value in the PA/A ratio ≥1 group was significantly lower than that in the PA/A ratio <1 group (p = 0.026). Remarkably, the PA/A ratio ≥1 group had a significantly higher value of PaCO2, RDW, BNP, PA diameter, and RICU admissions, as well as worse outcomes than the PA/A ratio <1 group (P < 0.05). However, there were no significant statistical differences for the other indicators between the two groups (Table 1).
 |
Table 1 Characteristics of AECOPD Patients with Different PA/A Ratio |
Clinical Features of the AECOPD Patients with Treatment Failure
As indicated in Table 2, compared to the treatment success group, the treatment failure group had a much lower pH value (7.34 ± 0.11 vs 7.28 ± 0.13, respectively, p = 0.040) and less count of PLT (median 167 × 109/L vs 130 × 109/L, respectively, p = 0.018). The treatment failure group had higher levels of D-dimer and BNP compared with the improved group (P < 0.05). In addition, the percentage of RDW, rate of RICU admissions, and the proportion of IMV in the treatment failure group were significantly higher than that in the improved group (P < 0.05). Notably, the PA diameter and PA/A ratio were significantly increased in the treatment failure group than in the improved group (mean PA diameter: 3.71 vs 3.22, p = 0.001; mean PA/A ratio: 1.09 vs 0.89, p < 0.001).
 |
Table 2 Characteristics of Treatment Success Group and Treatment Failure Group in Severe AECOPD |
A PA/A Ratio ≥1 Was an Independent Risk Factor for Treatment Failure in AECOPD
The multivariate analysis indicated that the PA/A ratio ≥1 (OR value = 6.129, 95% CI: 1.665–22.565, p = 0.006) and IMV (OR value = 10.798, 95% CI: 2.072–56.261, p = 0.005) were two independent risk factors for treatment failure in patients with AECOPD. Although the RDW, D-dimer, PLT, and RICU admissions had observed significant differences between the two groups according to the univariate analysis, they did not reach significant statistical differences according to the multivariate analysis (Table 3). Additionally, the Kaplan–Meier survival analysis indicated that patients with a PA/A ratio ≥1 had worse outcomes than patients with a PA/A ratio <1 during hospitalization (HR = 5.277, 95% CI: 2.178–12.78, p < 0.001) (Figure 3).
 |
Table 3 Multivariate Analysis for Risk Factors of Treatment Failure in AECOPD |
Predictors of Treatment Failure in Hospitalized Patients with AECOPD
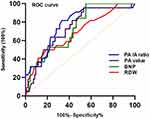
Figure 4 displays the diverse ROC curve of the PA/A ratio, the PA value, the BNP, and the RDW for predicting treatment failure in hospitalized patients with AECOPD. Even though there were no significant statistical differences observed, the area under the curve (AUC) value of the PA/A ratio was numerically larger than that of the other indicators. The best cut-off value of the PA/A ratio for predicting treatment failure was 0.925. The sensitivity was 81.82%, and the specificity was 66.67% (Table 4).
 |
Table 4 ROC Curve Analysis for Factors Predicting Treatment Failure |
Discussion
The strengths of this study were its primary findings. First, patients with a PA/A ratio ≥1 had significantly higher PaCO2, RDW, BNP, PA diameters, RICU admission rates, and proportions of treatment failure. Second, the PA diameter and PA/A ratio were significantly increased in the treatment failure group compared with the treatment success group. Third, a PA/A ratio ≥1 was an independent risk factor for treatment failure in patients with AECOPD. The Kaplan–Meier survival analysis indicated that patients with a PA/A ratio ≥1 had worse outcomes than patients with a PA/A ratio <1 during hospitalization. Finally, the PA/A ratio may be a promising factor for predicting treatment failure in hospitalized AECOPD patients.
A previous study indicated that the relative pulmonary arterial enlargement (PA/A ratio >1 on CT scanning) predicted hospitalization for AECOPD, and a PA/A ratio >1 with increased blood troponin levels shared close associations with increased respiratory failure, ICU admission, and in-hospital mortality.11 Iliaz et al reported that the PA/A ratio was related to the frequency of hospitalizations and exacerbations due to COPD in one year after hospital discharge.12 However, the relationships between a PA/A ratio >1 alone and ICU admission or in-hospital mortality are still unclear. In the present study, we found that AECOPD patients with a PA/A ratio ≥1 had a decreased pH value and increased PaCO2 compared with patients with a PA/A ratio <1, implicating increased type II respiratory failure in patients with a PA/A ratio ≥1. A decreased pH value and increased PaCO2 may contribute directly to pulmonary vasoconstriction leading to a rise in pulmonary vascular resistance and pulmonary arterial pressure.13 In addition, we also disclosed a higher percentage of RICU admissions and a markedly increased rate of treatment failure in hospitalized AECOPD patients with a PA/A ratio ≥1. Thus, an increased PA/A ratio was associated with severity and worse outcomes in inpatients with AECOPD. Many studies have revealed that the RDW is a valuable biomarker for predicting pulmonary hypertension and its associated prognosis.14–16 In a previous study performed by our group, we indicated that the RDW shared positive relationships with the PA/A ratio in patients with pH secondary to COPD.17 Similar to previous studies, we found an increase in the RDW in AECOPD patients with a PA/A ratio ≥1. Likewise, the serum level of BNP was drastically elevated. BNP is an important indicator for identifying risk categories in PH. Increased BNP is related to a worse outcome of PH.18
In this study, we demonstrated that there was a decreased pH value, lower number of PLTs, and increases in the RDW, D-dimer, BNP, PA diameter, and PA/A ratio in AECOPD patients with treatment failure compared with the improved group. Patients with treatment failure also required more IMV supports and intensive care. It was reported that lower pH values were associated with short or long mortality in hospitalized AECOPD patients.19,20 RDW is an indicator that reflects the heterogeneity of red blood cell volume. Recently, RDW was found to be an independent negative prognostic factor closely associated with adverse outcomes in hospitalized AECOPD patients.21,22 Dysregulation of erythrocyte homeostasis and metabolic imbalance may account for significant changes in the RDW in AECOPD patients. However, the underlying pathophysiological mechanisms remain unknown.23 A hypercoagulable state is a feature of hospitalized AECOPD patients. An increased D-dimer level is not only an important independent risk factor for pulmonary embolism in inpatients with AECOPD but also a predictor of higher mortality in stable COPD patients.24,25 Cardiac failure is a frequent complication of AECOPD, deeply affecting exercise tolerance and life span in patients with COPD. BNP is widely used to evaluate heart function. BNP can be used to risk-stratify, and an elevated BNP is associated with a higher MV use and worse outcomes in AECOPD patients.26 An increased PA/A ratio is positively correlated with COPD severity. Previous studies have reported that pulmonary artery enlargement detected by CT is a risk predictor for a severe exacerbation of COPD.27,28 Intriguingly, the PA/A ratio is an important determinant of mortality in moderate-to-severe COPD.8 In our present study, we found that a PA/A ratio ≥1 was a strong independent risk-factor of in-hospital treatment failure in patients with AECOPD. In addition, the PA/A ratio might be a better predictor of in-hospital treatment failure compared with other indicators including the PA value, BNP, and RDW. Taken together, the results of the present study provide additional evidence for a close association between the PA/A ratio and the outcome of AECOPD.
In this study, AECOPD patients with a PA/A ratio ≥1 had markedly higher values of PaCO2, RDW, BNP, the PA diameter, ICU admission rates, and proportions of treatment failure and had worse outcomes during hospitalization. A PA/A ratio ≥1 was an independent risk factor for treatment failure in patients with AECOPD. The PA/A ratio may be a promising predictor for treatment failure. It is worth noting that there are several limitations in this study. First, the sample size was small, and this might lead to an interpretation bias in the final analysis. Further work is required to validate the initial conclusion for a larger sample size. Second, the PA/A ratio partially reflects a change in the pulmonary artery pressure. However, the association between the PA/A ratio and the pulmonary artery pressure was not assessed in this study. Finally, to reduce the chance of radioactive exposure, a dynamic change in the PA/A ratio during hospitalization was unclear.
Acknowledgments
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The design of the study and collection, analysis, and interpretation of data were supported by the Anhui Provincial Key projects of the Natural Science Foundation for Colleges and Universities (KJ2021A0834).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381:1257–1266. doi:10.1056/NEJMra1900500
2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391:1706–1717. doi:10.1016/S0140-6736(18)30841-9
3. Garcia-Sanz MT, Canive-Gomez JC, Senin-Rial L, et al. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis. 2017;9:636–645. doi:10.21037/jtd.2017.03.34
4. Nakayama S, Chubachi S, Sakurai K, et al. Characteristics of chronic obstructive pulmonary disease patients with pulmonary hypertension assessed by echocardiography in a three-year observational cohort study. Int J Chron Obstruct Pulmon Dis. 2020;15:487–499. doi:10.2147/COPD.S230952
5. Carpio AM, Goertz A, Kelly C, et al. Unrecognized pulmonary arterial hypertension in hospitalized patients. Int J Cardiovasc Imaging. 2021;37:1237–1243. doi:10.1007/s10554-020-02108-9
6. Iyer AS, Wells JM, Vishin S, et al. CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest. 2014;145:824–832. doi:10.1378/chest.13-1422
7. Schneider M, Ran H, Pistritto AM, et al. Pulmonary artery to ascending aorta ratio by echocardiography: a strong predictor for presence and severity of pulmonary hypertension. PLoS One. 2020;15(7):e235716. doi:10.1371/journal.pone.0235716
8. Terzikhan N, Bos D, Lahousse L, et al. Pulmonary artery to aorta ratio and risk of all-cause mortality in the general population: the Rotterdam Study. Eur Respir J. 2017;49:1602168. doi:10.1183/13993003.02168-2016
9. Zeng Y, Cai S, Chen Y, et al. Current status of the treatment of COPD in China: a multicenter prospective observational study. Int J Chron Obstruct Pulmon Dis. 2020;15:3227–3237. doi:10.2147/COPD.S274024
10. Cai BQ, Cai SX, Chen RC, et al. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People’s Republic of China. Int J Chron Obstruct Pulmon Dis. 2014;9:381–395. doi:10.2147/COPD.S58454
11. Wells JM, Morrison JB, Bhatt SP, et al. Pulmonary artery enlargement is associated with cardiac injury during severe exacerbations of COPD. Chest. 2016;149:1197–1204. doi:10.1378/chest.15-1504
12. Iliaz S, Tanriverdio E, Chousein E, et al. Importance of pulmonary artery to ascending aorta ratio in chronic obstructive pulmonary disease. Clin Respir J. 2018;12:961–965. doi:10.1111/crj.12612
13. Morray JP, Lynn AM, Mansfield PB. Effect of pH and PCO2 on pulmonary and systemic hemodynamics after surgery in children with congenital heart disease and pulmonary hypertension. J Pediatr. 1988;113:474–479. doi:10.1016/S0022-3476(88)80631-0
14. Zuk M, Migdal A, Dominczak J, et al. Usefulness of Red Cell Width Distribution (RDW) in the assessment of children with Pulmonary Arterial Hypertension (PAH). Pediatr Cardiol. 2019;40:820–826. doi:10.1007/s00246-019-02077-4
15. Ulrich A, Wharton J, Thayer TE, et al. Mendelian randomisation analysis of red cell distribution width in pulmonary arterial hypertension. Eur Respir J. 2020;55:1901486.
16. Liu J, Yang J, Xu S, et al. Prognostic impact of red blood cell distribution width in pulmonary hypertension patients: a systematic review and meta-analysis. Medicine. 2020;99:e19089. doi:10.1097/MD.0000000000019089
17. Yang J, Liu C, Li L, et al. Red blood cell distribution width predicts pulmonary hypertension secondary to chronic obstructive pulmonary disease. Can Respir J. 2019;2019:3853454. doi:10.1155/2019/3853454
18. Hoeper MM, Pausch C, Olsson KM, et al. COMPERA 2.0: a refined 4-strata risk assessment model for pulmonary arterial hypertension. Eur Respir J. 2021;2102311. doi: 10.1183/13993003.02311-2021
19. Gayaf M, Karadeniz G, Guldaval F, et al. Which one is superior in predicting 30 and 90 days mortality after COPD exacerbation: DECAF, CURB-65, PSI, BAP-65, PLR, NLR. Expert Rev Respir Med. 2021;15:845–851. doi:10.1080/17476348.2021.1901584
20. Chen L, Chen L, Zheng H, et al. Emergency admission parameters for predicting in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease with hypercapnic respiratory failure. BMC Pulm Med. 2021;21:258. doi:10.1186/s12890-021-01624-1
21. Hu GP, Zhou YM, Wu ZL, et al. Red blood cell distribution width is an independent predictor of mortality for an acute exacerbation of COPD. Int J Tuberc Lung Dis. 2019;23:817–823. doi:10.5588/ijtld.18.0429
22. Epstein D, Nasser R, Mashiach T, et al. Increased red cell distribution width: a novel predictor of adverse outcome in patients hospitalized due to acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2018;136:1–7. doi:10.1016/j.rmed.2018.01.011
23. Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: a simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi:10.3109/10408363.2014.992064
24. Wang J, Ym D. Prevalence and risk factors of pulmonary embolism in acute exacerbation of chronic obstructive pulmonary disease and its impact on outcomes: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25:2604–2616. doi:10.26355/eurrev_202103_25424
25. Husebo GR, Gabazza EC, D’Alessandro GC, et al. Coagulation markers as predictors for clinical events in COPD. Respirology. 2021;26:342–351. doi:10.1111/resp.13971
26. Vallabhajosyula S, Haddad TM, Sundaragiri PR, et al. Role of B-type natriuretic peptide in predicting in-hospital outcomes in acute exacerbation of chronic obstructive pulmonary disease with preserved left ventricular function: a 5-year retrospective analysis. J Intensive Care Med. 2018;33:635–644. doi:10.1177/0885066616682232
27. Yang T, Chen C, Chen Z. The CT pulmonary vascular parameters and disease severity in COPD patients on acute exacerbation: a correlation analysis. BMC Pulm Med. 2021;21:34. doi:10.1186/s12890-020-01374-6
28. Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–921. doi:10.1056/NEJMoa1203830
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.