Back to Journals » International Journal of General Medicine » Volume 16
The Kynurenine/Tryptophan Ratio as a Promising Metabolomic Biomarker for Diagnosing the Spectrum of Tuberculosis Infection and Disease
Authors Fadhilah F , Indrati AR, Dewi S, Santoso P
Received 2 September 2023
Accepted for publication 11 November 2023
Published 28 November 2023 Volume 2023:16 Pages 5587—5595
DOI https://doi.org/10.2147/IJGM.S438364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Fitri Fadhilah,1 Agnes Rengga Indrati,2 Sumartini Dewi,3 Prayudi Santoso3
1Doctorate in Medicine Program, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 2Clinical Pathology Department, Hasan Sadikin General Hospital, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia; 3Internal Medicine Department, Hasan Sadikin General Hospital, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia
Correspondence: Agnes Rengga Indrati, Clinical Pathology Department, Hasan Sadikin Hospital, Faculty of Medicine, Universitas Padjadjaran, Jl. Prof Eckyman No. 38, Bandung, West Java, 40161, Indonesia, Tel +62-8112257928, Email [email protected]
Abstract: The metabolic system and immunology used to be seen as distinct fields of study. Recent developments in our understanding of how the immune system operates in health and disease have connected these fields to complex systems. An effective technique for identifying probable abnormalities of metabolic homeostasis brought on by disease is metabolomics, which is defined as the thorough study of small molecule metabolic intermediates within a biological system that collectively make up the metabolome. A prognostic metabolic biomarker with adequate prognostic accuracy for tuberculosis progression has recently been created. The rate-limiting host enzyme for the conversion of tryptophan to kynurenine, indoleamine 2,3-dioxygenase (IDO), is greatly elevated in the lungs of tuberculosis disease patients. Targeted study on tryptophan in tuberculosis disease indicates that such decreases may also resembled this upregulation. Although tuberculosis diagnosis has improved with the use of interferon release assay and tuberculosis nucleic acid amplification, tuberculosis control is made difficult by the lack of a biomarker to diagnose active tuberculosis disease. We hope that the reader of this work can develop an understanding of the advantages of metabolomics testing, particularly as a sort of testing that can be used for both diagnosing and monitoring a patient’s response to treatment for tuberculosis.
Keywords: biomarker, K/T ratio, metabolomic study, tuberculosis spectrum
Introduction
Metabolomics was originally part of the science of biology and was introduced there. After going through improvements in both analytical and bioinformatic terms, metabolomics is now a popular and widely recognized method and is even used for diagnostic approaches, such as in the clinical or biomedical fields. Metabolomics measurement is part of a biological system that is often related to a person’s physiological processes, where these processes are closely related to the initial process of disease. This approach allows metabolomics as a research method to assist in the diagnosis or prognosis of various body disorders caused by organisms, or it can be used to look for certain biomarkers as markers of a disease.1–3
Metabolomics has been recognized as a highly effective assessment technique in recent years, particularly for examining diverse disorders. Liquid chromatography (LC), gas chromatography (GC), nuclear magnetic resonance (NMR) spectroscopy, capillary electrophoresis, and mass spectrometry are just several examples of separation methods used in metabolomics. Some of those techniques are combined, such as tandem mass spectrometry (MS/MS), which uses ultra-high performance liquid chromatography (UHPLC) for recognizing smaller compounds across a larger range.4–7
Target and nontarget are two types of metabolomics categories. Clinical laboratory tests such as the detection of amino acids or the analysis of very long-chain fatty acids are examples of the use of targeted metabolomics. For medical research, commonly used samples may be plasma, cerebrospinal fluid (CSF), or urine samples.8–11 In this review, we will discuss several studies related to targeted metabolomics, namely kynurenine and tryptophan, and their changes during infection, especially in tuberculosis.
The keywords “kynurenine/tryptophan ratio” and “TB” were combined to discover publications in online databases like PubMed, ScienceDirect, Scopus, and Clinical Key. Full-text English articles published within the last ten years met the inclusion requirements. After extensive research, the exclusion criteria were literature reviews, the research subjects are human and papers that had no direct relevance to our purpose. Six papers were objectively picked as the most essential for this review topic after searches were done with the phrases “tuberculosis’ and either ‘kynurenine/tryptophan ratio” either “IDO activity” or “metabolomic”.
Tryptophan Metabolism
One of the essential amino acids that we get from our daily protein intake is tryptophan. There are 20 types of amino acids that make up proteins, one of which is a relatively small amount, namely tryptophan, which can be found in the proteins that make up plasma. A distinctive feature of this amino acid is the extremely low amount found in the body, but it is known to be involved in a wide variety of physiological functions throughout. Tryptophan is known to be a precursor for the synthesis of serotonin as well as niacin. With various physiological conditions in a person and low levels of tryptophan, of course, it can lead to an increase in the situation of unbalanced tryptophan homeostasis.12–14 The role of tryptophan in physiological and pathological conditions is certainly unique and extraordinary.
In cells such as fat cells, macrophages, antigen-presenting cells, fibroblasts, and placental trophoblasts tryptophan degradation can be detected. Inflammatory cytokines will be released through activated immune cells, stimulating the expression and activity of an enzyme called Indoleamine Dioxygenase (IDO). Tryptophan degradation can be induced by the immune system, such as interferon α and β, as well as tumor necrosis factor a, but interferon gamma plays an important role. In a recent study, it was stated that dendritic cells (DC) can also express IDO. Among DCs, there is a position to categorize SSIs into certain subsets, either constitutively or after induction.14–16
From several theories and studies that have been carried out, there is a theory that tryptophan metabolism via the kynurenine pathway has a role in regulating the immune response to maintain autoimmunity.17–19 An essential amino acid called tryptophan is utilized to make proteins and gives rise to a number of bioactive substances with crucial physiological roles, including serotonin, tryptamine, indoles, kynurenines, and nicotinamide adenine dinucleotide (NAD+).20 Tryptophan cannot be synthesized by humans by any of the biochemical pathways. The process of absorption of tryptophan by erythrocytes occurs in the intestine; the hepatic portal system transports tryptophan into the liver; less than 1% of ingested tryptophan is used for protein synthesis; and 95% is metabolized via the kynurenine pathway in the liver.21,22 The rest of the tryptophan is utilized by peripheral tissue cells, including vascular endothelial cells, fibroblasts, and cells of innate immunity acquired by secretion in the bloodstream.
In experimentally developed viral, bacterial, or parasite inside the cell or inflammation, plasma tryptophan levels have been shown to decrease or to raise kynurenine-tryptophan levels.23 When the immune system is chronically activated, as in autoimmune disorders,18,24 inflammatory diseases,2,25 cancer,14,26 and major trauma, tryptophan use has been reported to increase. The increase of metabolic intermediates including kynurenine and quinolinic acid in almost all of these investigations suggests tryptophan breakdown pathway participation. A high level of IDO raises the possibility that IDO plays a role in the degradation of tryptophan when taken as an entire substance.14,16,19,24
Kynurenine
Kynurenine (KYN) is a direct precursor of kynurenic acid, anthranilic acid, 3-hydroxykynurenine (3HK), and tryptophan metabolites, as well as various downstream metabolites formed along the kynurenine pathway. More than 95% of tryptophan degradation in mammals occurs in this pathway.
The main pathway of tryptophan metabolism is the kynurenine pathway. Initially, tryptophan is converted to N-formylkynurenine via indoleamine 2.3-dioxygenase (IDO) and tryptophan 2.3- dioxygenase (TDO), and then to kynurenine (Kyn) via N-formylkynurenine formamidase (FAM).20,27 TDO mediates tryptophan under normal conditions. TDO is a determinant of the amount of extrahepatic tryptophan and is induced by tryptophan, estrogen, and glucocorticoids. In abnormal conditions where there is an excessive increase in cortisol concentrations and the presence of inflammation, TDO expression is suppressed in the liver, while IDO1 expression occurs in cells of the immune system as part of negative feedback, which aims to control the inflammatory response.20,22
Metabolomic, Immune Systems, and Tuberculosis
Tuberculosis diagnosis and biomarkers are popular and widely discussed types of research. Tuberculosis endemic areas have their own complex problems, so an appropriate and validated method has not been found to become a specific biomarker. One of the widely studied and proposed biomarkers is the enzymatic activity of indoleamine 2, 3-dioxygenase (IDO). IDO is a cytosolic interferon γ– induced enzyme that catalyzes the degradation of tryptophan (Trp) to kynurenine (Kyn).14,15,19,28 T cell proliferation and function are suppressed by IDO, by degrading tryptophan and producing immunomodulatory metabolites, which lead to immune suppression and tolerance. Physiological functions, such as pregnancy, modulate the pathogenesis of various pathological conditions, such as cancer and infectious diseases, that can be regulated by IDO.16,23
Two purposes, microbial amino acid deprivation and immunological tolerance, may be served by the IDO-induced removal of tryptophan through host immune activation. A strategy for preventing the spread of bacteria, viruses, and parasites would be the local tryptophan depletion brought on by IDO activation in macrophages.12 By depleting tryptophan via IDO, interferon-stimulated lung cells can stop the growth of bacteria in in vitro models of T. gondii and S. aureus infections.20,29 According to this claim, host tryptophan modification in the parasites’ microenvironment inhibits microbial production, giving the host a competitive edge.30 As a result, in immunocompetent cells, tryptophan depletion was once thought to represent a defense mechanism brought on by immunological activation. However, the increased consumption of tryptophan during long-term immune activation from non-pathogenic sources suggests that IDO has a wider range of actions.26
During pregnancy, IDO plays a protective role by preventing rejection of the fetus by maternal T lymphocyte cells, so it is known that IDO can regulate T cell activity.31,32 T-cell proliferation by IDO is carried out through different mechanisms. The first mechanism, prevention of lymphocyte proliferation by tryptophan depletion in the cell environment by IDO-expressing macrophages, inhibits the G1 phase of the cellular cycle of activated T cells.33 In addition, catabolites such as kynurenine, 3-hydroxyanthranilic acid, and quinolinic acid can induce apoptosis by conferring cytotoxic properties on T cells.34 In addition to macrophages, DCs also express IDO, which is associated with the acquisition of a regulatory phenotype, leading to immune tolerance. This leads to the induction of the maturation of T cells so that they become regulatory T cells or activate resting memory T cells that have a regulatory phenotype. In the absence of tryptophan, IDO is still able to initiate tolerogenesis via DC due to downstream enzymes of the kynurenine pathway.35
IDO is essentially expressed in many immune system cells, including regulatory B cells, eosinophils, neutrophils, macrophages, monocytes, DCs, and a number T-cell subsets.14,26 Antigen-presenting cells (APCs), such as DCs and monocyte-derived macrophages, as well as other components of the innate immune system (NK cells, eosinophils, and neutrophils), are all affected in a variety of ways by the activation of IDO expression and activity.
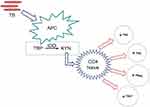
APC is an IDO-expressing cell, APC that is activated by M. tuberculosis infection causes increased IDO activity which will result in tryptophan depletion and upregulation of kynurenine as its metabolite, kynurenine formed will affect naive CD4 cells in response to the immune system (Figure 1).
Trp cleavage inhibits T-cell proliferation by substantially reducing the local tissue amino acid pool. Trp-deficient T cells are unable to synthesize sufficient protein to proliferate following antigen presentation by antigen-presenting cells (APCs). IDO1-based Trp depletion in CD4+ T cells activates the general control sensor amino acid non-depressible kinase 2 (GCN2K), which regulates the programs involved in transcription and translation which consider cell growth and the availability of amino acids into factor. IDO-1 can inhibit the enzymes responsible for CD4+ T cells’ fatty acid production by promoting GCN2K. With T-cell activation, fatty acid production increases and is necessary to avoid growing cells from dying. In this way, IDO1-dependent GCN2K activation and the purification of the acid-leaf syncytia inhibit cell proliferation while making CD4+ differentiation the primary effector of cell differentiation. It has been demonstrated that TRP depletion and the primary TRP metabolites Kyn, 3-HKyn, and 3-HAA can prevent cytotoxin-induced TCR zeta complex chain downregulation in CD8+ T cells. Nowadays, the process by which CD4+ CD25-T cells develop a Treg phenotype involves GCN2K, the suppression of IL-2 production, and elevated IL-10 and TGF-β.19
Immunological tolerance and the regulation of the Th1/Th2 ratios are both significantly influenced by IDO-1. Dendritic cells express IDO-1, which inhibits the proliferation of human T cells and produces local immunity. Dendritic cell IDO-1 activity limits the growth of Th2 cells while inhibiting the production of cytotoxic T lymphocytes (CTLs), naive CD4+ and CD8+ T cells, and Th1 cells. Both Kyn and its downstream metabolites have an impact on the Th17/Treg system’s equilibrium when this balance is out of whack and support immunosuppressive Tregs.19
Spectrum of Tuberculosis Infection
Due in part to a lack of understanding on the clinical pathogenic range of infections and diseases associated with tuberculosis (TB), it is the most common infectious cause of mortality worldwide. Researchers and health workers are currently struggling to eradicate tuberculosis; unfortunately, they are more focused on two types of TB, namely latent TB and active TB disease. Based on recent research, tuberculosis in humans changes from latent to active through several stages that cause changes in the metabolic spectrum of bacterial activity and an opposing immunological response. Between these two spectrums, there are several stages, including incipient and subclinical TB. If we can distinguish these various spectrums, it will certainly help us determine the right therapy and develop an appropriate way of diagnosing to prevent the development of latent tuberculosis germs to become active and prevent their spread.36
The TB clinical spectrum is a classification that describes the degradation of the severity of TB disease. The TB spectrum classification is based on the pathogenesis of TB disease, which is known through examination of clinical symptoms of TB, X-rays, and microscopic sputum examination. The clinical spectrum of TB is used as an operational basis for TB case management programs in the community.23,36,37 In addition, this TB clinical spectrum classification can provide a basis for doctors to describe the severity of TB disease so that it can be used as a basis for providing appropriate treatment according to the level of TB infection, as can be seen in Table 1. Spectrum determination, especially in Latent TB, subclinical TB, and Active TB, requires further understanding, and research still needs to be carried out to determine the possibility of a change from latent TB to subclinical TB and even active TB in vulnerable groups such as HIV patients. Based on this approach, several researchers have found a method to help provide an overview regarding the differences between latent TB and active TB, namely through a metabolomics approach, especially in terms of the kynurenine/tryptophan ratio as described in Table 2.
 |
Table 1 Categorical States of Tuberculosis |
 |
Table 2 Kynurenine/Tryptophan (IDO Activity) in TB |
Research by Adu Gyamfi38 in 2017 and Olsson27 in 2022 showed similar results where there was an increase in Tryptophan levels in HIV co-infected TB patients, where in Adu Gyamfi’s study it was expressed in the form of IDO activity which is a ratio of kynurenine/tryptophan, even IDO activity was found to be higher high in people who later turned into active TB, after TB treatment IDO activity decreased in line with the reduction in TB germs until the results resembled controls without TB, whereas in Olsson’s study it was described based on each parameter such as tryptophan which increased in HIV co-infected TB patients compared to patients HIV without TB, while the kynurenine levels of the two groups were not much different, but the kynurenine/tryptophan ratio still showed a significant difference.
In another study by Shi,14 IDO activity was measured in various TB comorbid conditions, ranging from DS-TB and MDR-TB to patients with lung cancer, and IDO activity was found to be higher in MDR-TB patients than other groups. This is in line with research by Collins,23 who also examined the quinuronium/tryptophan ratio in various TB groups, including MDR-TB and DS-TB. The tryptophan pathway plays a crucial role in the host response to TB infection and disease along with chemotherapy-mediated bacterial clearance, according to metabolic pathway examinations. An increase in tryptophan catabolism to kynurenine, which was seen in both silent LTBI patients and those with active TB disease, was the main factor contributing to changes in tryptophan metabolism. They also demonstrate that rises tryptophan catabolism is reversible with effective treatment of active TB disease and LTBI at a rate of this closely reflects the way the body reacts to treatment. IDO-1 transcript levels are much higher in populations with LTBI and in those with active TB disease, suggesting that IDO-1 induction is a mechanism through which tryptophan catabolism is increased. These findings point to IDO-1-mediated catabolism of tryptophan is a metabolic process.
Pregnancy is one of the causes of increased tryptophan levels, so Adu Gyamfi31 also conducted research on this matter. The patients studied were pregnant women with TB, and it was found that the Plasma K/T ratio was elevated in women with active TB at time of diagnosis compared to those without TB. This strengthens the hypothesis that IDO activity is directly proportional to the activation of TB germs. Adu Gyamfi28 also conducted research to compare two methods for measuring IDO activity, including MS and ELISA, where these two tools showed results that were not significantly different but still gave results discovered that people with HIV with active tuberculosis had higher K/T ratios than HIV-uninfected individuals with active tuberculosis, who also had higher K/T ratios than those with latent tuberculosis or individuals without TB.
Discussion
Pulmonary TB patients are under the spotlight in various studies, especially because of an increase in kynurenine and a decrease in tryptophan or also known as an increase in IDO activity which can be measured by the kynurenine/tryptophan ratio.23,39 The potential of the kynurenine/tryptophan ratio as a biomarker for the diagnosis of TB has actually been proposed by many researchers, of course the results have also been associated with the effect of treatment. Induction of the immunoregulatory enzyme indoleamine 2.3-dioxygenase (IDO-1) associated with tryptophan depletion in which tryptophan is broken down into its metabolite, kynurenine, thereby suppressing the immune response through changes in macrophage metabolism in response to tuberculosis germs, induction of T-cell energy and apoptosis.16,24 In cases of multidrug-resistant TB, cavitary disease, and extrapulmonary TB, metabolic changes are also detected, as evidenced by low tryptophan levels.14,23 Interestingly, tryptophan can return to normal within a certain time after TB treatment, which indicates that changes in tryptophan catabolism respond to treatment. The Kynurenine/Tryptophan (K/T) ratio and IDO-1 performance have been associated with TB diagnosis and response to treatment in other susceptible populations of concern, such as HIV- positive individuals.28,32,40
Indoleamine 2.3-dioxygenase 1 (IDO-1) is at the central of immune synapses that link innate and adaptive immune responses involving antigen-presenting cells (APCs) such as dendritic cells (DCs), macrophages (MPs) and lymphocytes, effector T cells (Teff), natural killer (NK) cells and regulatory T cells (Treg). Induction of Indoleamine 2.3-dioxygenase 1 (IDO1) in cells belonging to innate immunity causes a decrease in tryptophan levels, formation of kynurenine and its metabolites which are crucial regulators of adaptive immune system. Patients with active TB who had low tryptophan/kynurenine ratio (high IDO activity) progressed more slowly than those with low IDO activity.
Physiological differences in tuberculosis patients are influenced by the activity of bacteria in the body; of course, in susceptible groups, TB bacteria that were initially latent can become active. Metabolic changes are expected to be a special characteristic in determining the spectrum of TB because if there is a change in the activity of TB germs from latent to active, a different treatment is required. Decreased immunity in patients with HIV or other immune disorders can encourage the activation of TB germs, so that preventive therapy can no longer prevent TB disease, but appropriate TB drugs need to be given. It is hoped that a clearer diagnosis of various TB spectrums can reduce the incidence of TB, especially in tuberculosis-endemic countries.
Conclusion
Given these data, it is essential to recognize how amino acids or metabolomic changes contribute to immune cell characteristic following Mtb infection spectrum, interesting research can be done by evaluate at the different types of tuberculosis coinfection on amino acid levels especially the kynurenine/tryptophan ratio or even various types of amino acids, both essential and non-essential. Further research needs to be carried out to determine the usefulness of the kynurenine/tryptophan ratio, especially to establish it as a biomarker in various TB spectrums.
Acknowledgments
Financial support for the study was provided by Padjadjaran University, The Indonesia Endowment Fund for Education (LPDP) and Center for Higher Education Funding (BPPT) Kemendikbudristek.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bujak R, Struck-Lewicka W, Markuszewski MJ, Kaliszan R. Metabolomics for laboratory diagnostics. J Pharm Biomed Anal. 2015;113:108–120. doi:10.1016/j.jpba.2014.12.017
2. Hanna LE, Babu H, Sperk M, et al. Plasma metabolic signature and abnormalities in hiv-infected individuals on long-term successful antiretroviral therapy. Metabolites. 2019;9(10):1–16.
3. Tounta V, Liu Y, Cheyne A, Larrouy-Maumus G. Metabolomics in infectious diseases and drug discovery. Mol Omi. 2021;17(3):376–393. doi:10.1039/D1MO00017A
4. Gowda GAN, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics: a review. Expert Rev Mol Diagn. 2008;8(5):617. doi:10.1586/14737159.8.5.617
5. Crook AA, Powers R. Quantitative NMR-based biomedical metabolomics: current status and applications. Molecules. 2020;25(21):5128. doi:10.3390/molecules25215128
6. Baidoo EEK, Teixeira Benites V. Mass spectrometry-based microbial metabolomics: techniques, analysis, and applications. Methods Mol Biol. 2019;1859:11–69.
7. Odom JD, Sutton VR. Metabolomics in clinical practice: improving diagnosis and informing management. Clin Chem. 2021;67(12):1606–1617. doi:10.1093/clinchem/hvab184
8. Isa F, Collins S, Lee MH, et al. Mass spectrometric identification of urinary biomarkers of pulmonary tuberculosis. EBioMedicine. 2018;31:157. doi:10.1016/j.ebiom.2018.04.014
9. van Laarhoven A, Dian S, Aguirre-Gamboa R, et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: an observational cohort study. Lancet Infect Dis. 2018;18(5):526–535. doi:10.1016/S1473-3099(18)30053-7
10. Cui L, Lu H, Lee YH. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom Rev. 2018;37(6):772–792. doi:10.1002/mas.21562
11. Liebenberg C, Luies L, Williams AA. Metabolomics as a tool to investigate HIV/TB co-infection. Front Mol Biosci. 2021;8(October):1–19. doi:10.3389/fmolb.2021.692823
12. Floc’h N L, Otten W, Merlot E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids. 2011;41(5):1195–1205. doi:10.1007/s00726-010-0752-7
13. Moriska M, Lubis G, Herman RB, Moriska M, Lubis G, Herman RB. Hubungan antara Kadar Total Triptofan Plasma dan Indeks Massa Tubuh dengan Gejala Depresi dan Skor [Association between Total Plasma Tryptophan Levels and Body Mass Index with Depression Symptoms and Scores]. Sari Pediatri.2016;17(5):373–378.
14. Shi W, Wu J, Tan Q, et al. Plasma indoleamine 2,3-dioxygenase activity as a potential biomarker for early diagnosis of multidrug-resistant tuberculosis in tuberculosis patients. Infect Drug Resist. 2019;12:1265–1276. doi:10.2147/IDR.S202369
15. Adu-Gyamfi CG, Savulescu D, George JA, Suchard MS. Indoleamine 2, 3-dioxygenase- mediated tryptophan catabolism: a leading star or supporting act in the tuberculosis and HIV Pas-de-Deux? Front Cell Infect Microbiol. 2019;9(October):1–12. doi:10.3389/fcimb.2019.00372
16. Suchard MS, Adu-Gyamfi CG, Cumming BM, Savulescu DM. Evolutionary views of tuberculosis: indoleamine 2,3-dioxygenase catalyzed nicotinamide synthesis reflects shifts in macrophage metabolism: indoleamine 2,3-dioxygenase reflects altered macrophage metabolism during tuberculosis pathogenesis. BioEssays. 2020;42(5):1–10. doi:10.1002/bies.201900220
17. Bo L, Guojun T, Li G. An expanded neuroimmunomodulation axis: sCD83-indoleamine 2,3-dioxygenase-kynurenine pathway and updates of kynurenine pathway in neurologic diseases. Front Immunol. 2018;9(JUN). doi:10.3389/fimmu.2018.01363
18. Mondanelli G, Iacono A, Carvalho A, et al. Amino acid metabolism as drug target in autoimmune diseases. Autoimmun Rev. 2019;18(4):334–348. doi:10.1016/j.autrev.2019.02.004
19. Krupa A, Kowalska I. The kynurenine pathway—new linkage between innate and adaptive immunity in autoimmune endocrinopathies. Int J Mol Sci. 2021;22(18):9879. doi:10.3390/ijms22189879
20. Badawy AAB. Tryptophan availability for kynurenine pathway metabolism across the life span: control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology. 2017;112(Pt B):248–263. doi:10.1016/j.neuropharm.2015.11.015
21. Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19(1):55–61. doi:10.1097/MCO.0000000000000237
22. Marszalek-Grabska M, Walczak K, Gawel K, et al. Kynurenine emerges from the shadows – current knowledge on its fate and function. Pharmacol Ther. 2021;225:107845. doi:10.1016/j.pharmthera.2021.107845
23. Collins JM, Siddiqa A, Jones DP, et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight. 2020;5(10):1.
24. Yeung AWS, Terentis AC, King NJC, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci. 2015;129(7):601–672.
25. Seishima M, Yamamoto Y, Sakurai M, et al. Serum profiles of tryptophan-kynurenine pathway metabolites in psoriasis. Explor Immunol. 2021;1:258–268.
26. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34(3):137–143. doi:10.1016/j.it.2012.10.001
27. Olsson O, Skogmar S, Tesfaye F, Mulleta D, Jansson M, Björkman P. Kynurenine/tryptophan ratio for detection of active tuberculosis in adults with HIV prior to antiretroviral therapy. AIDS. 2022;36(9):1245–1253. doi:10.1097/QAD.0000000000003235
28. Adu-Gyamfi CG, Snyman T, Makhathini L, et al. Diagnostic accuracy of plasma kynurenine/tryptophan ratio, measured by enzyme-linked immunosorbent assay, for pulmonary tuberculosis. Int J Infect Dis. 2020;99:441–448. doi:10.1016/j.ijid.2020.08.028
29. Blumenthal A, Nagalingam G, Huch JH, et al. M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS One. 2012;7(5):e37314. doi:10.1371/journal.pone.0037314
30. Shaun Lott J. The tryptophan biosynthetic pathway is essential for Mycobacterium tuberculosis to cause disease. Biochem Soc Trans. 2020;48(5):2029–2037. doi:10.1042/BST20200194
31. Adu-Gyamfi C, Savulescu D, Mikhathani L, et al. Plasma kynurenine-to-tryptophan ratio, a highly sensitive blood-based diagnostic tool for tuberculosis in pregnant women living with Human Immunodeficiency Virus (HIV). Clin Infect Dis. 2021;73(6):1027–1036. doi:10.1093/cid/ciab232
32. Schnittman SR, Byakwaga H, Boum Y, et al. Changes in immune activation during pregnancy and the postpartum period in treated HIV infection. Open Forum Infect Dis. 2021;8(6):1–9. doi:10.1093/ofid/ofab245
33. Bantug GR, Galluzzi L, Kroemer G, Hess C. The spectrum of T cell metabolism in health and disease. Nat Rev Immunol. 2018;18(1):19–34. doi:10.1038/nri.2017.99
34. Byakwaga H, Hunt PW, Laker-Oketta M, et al. The kynurenine pathway of tryptophan catabolism and AIDS-associated kaposi sarcoma in Africa. J Acquir Immune Defic Syndr. 2015;70(3):296–303. doi:10.1097/QAI.0000000000000747
35. Abrahem R, Chiang E, Haquang J, Nham A, Ting YS, Venketaraman V. The role of dendritic cells in TB and HIV infection. J Clin Med. 2020;9(8):1–15. doi:10.3390/jcm9082661
36. Drain PK, Bajema KL, Dowdy D, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. 2018;31(4). doi:10.1128/CMR.00021-18
37. Haas MK, Belknap RW. Diagnostic tests for latent tuberculosis infection. Clin Chest Med. 2019;40(4):829–837. doi:10.1016/j.ccm.2019.07.007
38. Adu-Gyamfi CG, Snyman T, Hoffmann CJ, et al. Plasma indoleamine 2, 3-dioxygenase, a biomarker for tuberculosis in human immunodeficiency virus-infected patients. Clin Infect Dis. 2017;65(8):1356–1363. doi:10.1093/cid/cix550
39. Suzuki Y, Suda T, Asada K, et al. Serum indoleamine 2,3-dioxygenase activity predicts prognosis of pulmonary tuberculosis. Clin Vaccine Immunol. 2012;19(3):436–442. doi:10.1128/CVI.05402-11
40. Wang X, Mehra S, Kaushal D, Veazey RS, Xu H. Abnormal tryptophan metabolism in HIV and Mycobacterium tuberculosis Infection. Front Microbiol. 2021;12(June):1–9.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.