Back to Journals » Cancer Management and Research » Volume 13
The Incidence, Risk Factors, and Patterns of Peripherally Inserted Central Catheter-Related Venous Thrombosis in Cancer Patients Followed Up by Ultrasound
Authors Li X , Wang G, Yan K, Yin S, Wang H , Wang Y, Bai X, Shen Y
Received 13 January 2021
Accepted for publication 14 April 2021
Published 1 June 2021 Volume 2021:13 Pages 4329—4340
DOI https://doi.org/10.2147/CMAR.S301458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Xiang Li,1,* Guodong Wang,2,* Kun Yan,1 Shanshan Yin,1 Hongzhi Wang,2 Yanjie Wang,1 Xiumei Bai,1 Yanfen Shen2
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Ultrasound, Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China; 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), ICU, Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kun Yan
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Ultrasound, Peking University Cancer Hospital & Institute, No. 52 Fucheng Road, Haidian District, Beijing, 100042, People’s Republic of China
Tel + 86 13611176073
Fax +86 10-88196195
Email [email protected]
Hongzhi Wang
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), ICU, Peking University Cancer Hospital & Institute, No. 52 Fucheng Road, Haidian District, Beijing, 100142, People’s Republic of China
Tel/Fax +86 10-88196112
Email [email protected]
Purpose: A peripherally inserted central catheter (PICC) is associated with venous thromboembolism (VTE) especially in patients suffering from cancer. We analyzed the incidence, risk factors, and patterns of PICC-related VTE in cancer patients.
Patients and Methods: Patients with cancer who underwent PICC placement were evaluated retrospectively. Routine, prospective ultrasound post-PICC placement was used for asymptomatic and symptomatic patients to identify VTE. Multivariable logistic regression models with odds ratios (ORs) were used to examine VTE risk factors.
Results: Of 2353 PICCs placed, 165 patients (7.01%) developed PICC-related VTE with a median thrombosis time of 12 days. After adjustment of multivariable analysis, patients with PICC-related VTE were more likely to have a ratio of PICC diameter:vein diameter > 0.35 (adjusted OR, 1.689; 95% CI, 1.023– 2.789) and high level of triglycerides (1.561; 1.096– 2.223). The prevalence of A (adjusted OR, 1.680; 95% CI, 1.009– 2.798), B (1.835; 1.137– 2.961), and AB (3.275; 1.840– 5.829) blood group was significantly higher than that of the O blood group in VTE patients. Venous recanalization was observed in 44.8% (74/165) patients after anticoagulation therapy, and more often in patients with combined deep VTE than in patients with isolated superficial VTE (OR, 17.942; 95% CI, 5.427– 59.316). The recanalization time was 20± 5 (range, 10– 31) days.
Conclusion: The non-O blood group, larger ratio of PICC diameter:vein diameter, and high level of triglycerides were significantly associated with PICC-related VTE. Almost half of cases of PICC-related deep VTE could be reversed by anticoagulation treatment.
Keywords: recanalization, ultrasound, venous thromboembolism
Introduction
A peripherally inserted central catheter (PICC) is essential in the treatment and nursing of patients requiring intensive care, or for patients suffering from cancer. A PICC is especially important for the chemotherapy and nutritional support of cancer patients. The infusion of chemotherapy drugs through a PICC not only reduces the risk of drug extravasation and the stimulation of peripheral blood vessels, it also satisfies the demand of having a catheter during the interval of chemotherapy in the outpatient setting.
However, studies have shown that the incidence of venous thromboembolism (VTE) of the upper extremity in patients with PICC is significantly higher than that in patients with a central venous catheter.1 Especially for patients with cancer (whose blood is in hypercoagulable state or vascular endothelium is injured by long-term chemotherapy), the incidence of PICC-related VTE has increased significantly to 5.6–12%,2,3 and has become an important cause of PICC obstruction. In rare cases, the thrombus falls off and flows in blood to the superior vena cava to enter the pulmonary artery, after which a pulmonary embolism may form to endanger life.4
Studies have revealed several risk factors for PICC-related VTE: previous history of deep venous thrombosis (DVT); large diameter of the PICC; abnormal location of the PICC tip; high body mass index (BMI); hyperlipidemia, cancer type.2,5,6 Other studies have found that the ABO blood group is closely related to thrombosis, but Haddad et al7 indicated that the non-O blood group does not increase the thrombosis risk after PICC placement. Therefore, the association between blood group and PICC-related VTE is not clear. PICC-related VTE includes superficial venous thrombosis (SVT) and DVT according to the extension of the affected vein.8 However, few studies have compared the clinical symptoms, degree of occlusion, and risk factors between PICC-related SVT and PICC-related DVT. We recruited patients who underwent PICC in our center, and explored the incidence, risk factors and patterns of PICC-related VTE.
Patients and Methods
Patients, Study Outcome and Data Collection
This study was a retrospective analysis with data collected prospectively using a hospital database. Patients with cancer who underwent the first PICC placement between January 2018 to December 2019 in our hospital were enrolled consecutively.
Patients were included if they had 1) a documented active malignancy; 2) PICC placement for systemic chemotherapy; 3) were followed up by ultrasound until PICC removal or VTE occurrence.
The excluded criteria were patients 1) with a communication disorder; 2) with contraindications to anticoagulation therapy; 3) suffering from hematopathy; 4) with expected survival <1 month; 5) lost to follow-up.
The primary study outcome was time to PICC-related VTE and PICC removal. All patients were divided into a “VTE group” and “normal group” according to ultrasound results.
The characteristics of patients and their laboratory data were obtained from the electronic-medical records system of our hospital. These data were as follows:age; sex; BMI, more than one attempt at PICC placement; previous VTE; comorbidities of diabetes mellitus and hypertension; tobacco smoking; alcohol consumption; triglycerides (defined as “normal” [<1.7 mmol/L] and “high” [≥1.7 mmol/L]); low-density lipoprotein cholesterol (defined as “normal” [<3.4 mmol/L] and “high” [≥3.4 mmol/L]); total cholesterol (defined as “normal” [<5.2 mmol/L] and “high” [≥5.2 mmol/L]),9 activated partial thromboplastin time (APTT), white blood cell count; platelet count; ABO blood group.
All patients provided written informed consent before PICC placement. The study protocol was approved by the ethics committee of Peking University Cancer Hospital (Beijing, China), and this study was conducted in accordance with the Declaration of Helsinki.
PICC Placement and Nursing Care
PICC placements were undertaken by a nurse at our Venous Access Center, who had inserted a PICC>300 times previously. We wished to avoid the complications caused by PICC with a larger diameter. Hence, the same type of single-lumen 4-Fr PICC (Groshong®, Bard Access Systems, Salt Lake City, UT, USA), inserted with an improved Seldinger technique under ultrasound guidance, was used in all patients.10 Ultrasound was undertaken routinely at the upper mid-arm before PICC placement to identify a suitable vein for insertion. The first choice was the basilic vein, followed by the brachial vein and cephalic vein. After insertion, the PICC-tip position was confirmed by chest radiography and adjusted to ensure that it was located at the middle or distal-third of the superior vena cava.11 During hospitalization, all PICC lumina were flushed with 10 mL of physiologic (0.9%) saline and 5 mL of heparin daily to ensure that the PICC remained unobstructed. After PICC placement, patients were observed every day to ascertain if the affected limb had edema, pain/ache, or cyanosis of the skin. Care and assessment of the PICC was carried out every week after hospital discharge by a nurse specializing in vascular access. The arm circumference (5 cm above the upper margin of the elbow on the affected side) was measured by dedicated staff. The average value was used for those without VTE; the value on the day of the thrombosis was used for those with VTE.
“PICC-related VTE” was defined as the end point event and was confirmed by ultrasound. In accordance with American College of Chest Physicians Guideline,12 patients diagnosed with VTE had anticoagulation therapy. Initially, this was administration of low-molecular-weight heparin, followed by vitamin-K antagonists or direct oral anticoagulants in the first 3 months. The PICC was removed only if it could not be used or because chemotherapy was complete. After 2–4 weeks of treatment, ultrasound was undertaken to ascertain if the affected veins had recanalized. Patients with acute hypoxia, chest pain, or other clinical symptoms were examined by contrast-enhanced computed tomography (CT) for a suspected pulmonary embolism. The “indwelling time of the PICC” was defined as the time from the insertion to removal of the PICC. The “time to PICC-related VTE” was defined as the time from PICC placement to first identification of a thrombotic complication.
Ultrasound Examination and Follow-Up
PICC placement was guided by ultrasound using the LOGIQ Book system (General Electric, Piscataway, NJ, USA) and followed up by ultrasound using the IU22 system (Philips, Amsterdam, the Netherlands). The depth (from the subcutaneous to the anterior wall of the vein) and diameter of the vein for catheter indwelling were measured by ultrasound before puncture. The ratio of the PICC diameter:vein diameter was calculated as 0.144 mm/vein diameter (mm).
After PICC placement, all patients had follow-up ultrasound irrespective of whether they were symptomatic or were suspected to have PICC-related VTE. The examination was done by two physicians (with >5 years’ experience in vascular ultrasound) at 1 week, 2 weeks, as well as 1, 3, 6 and 12 months. If the patient had clinical symptoms related to upper-extremity thrombosis, ultrasound could be carried out at any time.
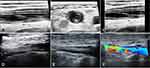
Patients with PICC-related VTE were divided into two groups according to the thrombosis extension: isolated SVT (“SVT” group), and SVT combined with DVT (“SVT+DVT” group). SVT was established if thrombosis involved a superficial vein of the upper extremity, including basilic and cephalic veins. DVT was established if a deep vein, such as the brachial vein, axillary vein, or subclavian vein, were affected. According to the guideline of relating to the diagnostic criteria of VTE by ultrasound,13 we classified a PICC-related upper-extremity VTE into two types: 1) “complete occlusion” (non-compressibility of the vein with a visible intraluminal thrombus and absence of a color signal in the vein lumen); 2) “partial occlusion” (partial closure after compression with a residual color signal in the vein lumen). Vein recanalization was determined by ultrasound, with thrombus resolution allowing for the vein to change from complete occlusion to partial/complete compression, or from partial occlusion to complete compression.14
Statistical Analyses
Statistical analyses were undertaken using SPSS 22.0 (IBM, Armonk, NY, USA). Continuous variables (means ± standard error) were analyzed with a two-tailed Student’s t-test. Variables with a non-normal distribution were analyzed using the Mann–Whitney rank sum test. Categorical variables were compared using the chi-square test and Fisher’s exact test. Kaplan–Meier curves were used to estimate the cumulative probability of PICC-related VTE. Predictors of VTE were searched by binary logistic regression after univariate analysis. P < 0.05 was considered significant.
Results
After reviewing 2681 PICCs inserted in patients with cancer, we finally enrolled 2353 patients (Figure 1). In most instances, the PICC was placed in the right upper-extremity (72.5%, 1706/2535). The vein into which the PICC was inserted was the basilic vein (83.8%, 1972/2353), brachial vein (13.9%, 327/2353) or cephalic vein (2.3%, 54/2353). The incidence of PICC-related VTE and symptomatic VTE was 7.01% in 165 patients and 3.4% in 88 patients, respectively, and the median indwelling time was 129 (range, 5–370) days (Figure 2). Ultrasound detected 77 (46.7%, 77/165) instances of asymptomatic VTE. Eighty-eight patients (53.3%, 88/165) were symptomatic with swelling of the upper extremity (65.9%, 58/88), pain/ache (29.6%, 26/88), or cyanosis of the skin (4.5%, 4/88). The indwelling time of the PICC in the VTE group was significantly shorter than that in the normal group (median 72 days vs 131 days). The median interval between PICC placement and thrombosis onset was 12 (range, 2–160) days, and 64.2% (106/165) instances of VTE occurred within 2 weeks after insertion. The characteristics of patients at baseline are summarized in Table 1. The univariate analysis showed that BMI >25 kg/m2, ABO blood group, and a high level of triglycerides were related to VTE, and that the APTT in the VTE group was significantly shorter than that in the normal group (30.0 ± 5.0 s vs 32.7 ± 6.5 s, P < 0.001). The arm circumstance of patients with VTE was significantly larger than that of normal patients (27.2 ± 3.0 vs 26.6 ± 3.0, P = 0.006). A comparison of vascular and PICC-insertion factors is shown in Table 2. The vein diameter in the VTE group was significantly smaller than that in the normal group (0.39 ± 0.07 mm vs 0.41 ± 0.08 mm, P = 0.001), whereas the ratio of the PICC diameter:vein diameter was larger (0.35 ± 0.06 vs 0.33 ± 0.06, P < 0.001). After the patients were grouped according to values of vein diameter (0.40 mm) and the ratio of the PICC diameter:vein diameter (0.35), we found that the PICC-related VTE happened more often when vein diameter ≤ 0.40mm (P = 0.019) and the ratio of the PICC diameter:vein diameter > 0.35 (P = 0.004).
 |
Table 1 Univariate Analysis of Clinical, Vascular and Technical Characteristics of Patients with and without PICC-Related VTE |
 |
Table 2 Univariate Analysis of Vascular and Technical Characteristics of Patients with and without PICC-Related VTE |
 |
Figure 1 Flowchart showing the final study cohort. |
After adjustment of the APTT, and arm circumference in the binary regression model, a ratio of PICC diameter:vein diameter >0.35 (odds ratio (OR), 1.689; 95% confidence interval (CI), 1.023–2.789, P = 0.041) and high level of triglycerides (1.561; 1.096–2.223, 0.014) were found to be independently associated with the risk of PICC-related VTE. The prevalence of A (OR, 1.680; 95% CI, 1.009–2.798, P = 0.046), B (1.835; 1.137–2.961, 0.013), and AB (3.275; 1.840–5.829, P<0.001) blood groups in VTE patients was significantly higher than that of the O blood group (Table 3, Figure 3).
 |
Table 3 Multivariable Logistic Regression of Risk Factors Related to PICC-Related Venous Thromboembolism |
 |
Figure 3 Forest plot showing the adjusted odd ratios for risk factors of PICC-related VTE. PICC, peripherally inserted central catheter; VTE, venous thromboembolism. |
In patients with PICC-related VTE, there were 35.8% (59/165) instances of SVT and 64.2% (106/165) instances of SVT+DVT. Also, only one patient had a pulmonary embolism (0.6%) with the symptoms of dyspnea and chest pain, and he was diagnosed by contrast-enhanced CT. Compared with the SVT group in the multivariable analysis model, the indwelling time was decreased and thrombosis time was increased in the SVT+DVT group (OR, 0.981; 95% CI, 0.970–0.992, P < 0.001, and 1.031; 1.013–1.050, 0.001, respectively), and the APTT was significantly shorter (0.868; 0.778–0.968, 0.011). Patients in the SVT+DVT group had more opportunity to have symptoms than those in the SVT group (OR, 3.109; 95% CI, 1.602–6.034, P = 0.001). The arm circumferences in the SVT+DVT group were larger than those in the SVT group (P = 0.028). In addition, the incidence of complete occlusion of veins in the SVT group (91.5%) was higher than that in the SVT+DVT group (50.9%) (OR, 0.158; 95% CI, 0.039–0.649, P = 0.01) (Table 4).
 |
Table 4 Multivariable Analysis of Related Variables in SVT and SVT+DVT Groups |
After treatment for 2–4 weeks, venous recanalization was observed in 74 (44.8%, 74/165) patients, and more often in patients in the SVT+DVT group (61.3%) than in patients in the SVT group (15.3%) (OR, 17.942; 95% CI, 5.427–59.316, P < 0.001) (Table 4, Figure 4). The recanalization time was 20 ± 5 (range, 10–31) days, and there was no significant difference in the recanalization time between the SVT+DVT group (20±5 days) and SVT group (18±4 days) (P = 0.186) (Table 4, Figure 5).
 |
Figure 5 The recanalization time of PICC-related VTE. The blue line denotes all patients with VTE; the purple line denotes the SVT group; the yellow line denotes the SVT+DVT group. |
Discussion
One meta-analysis pointed out that PICC increased the incidence of upper extremity VTE compared with central venous catheters, especially for patients who were critically ill or suffering from cancer (OR, 6.67; 95% CI, 4.69–8.64). The incidence of PICC-related VTE in patients with cancer was from 1.9% to 27%,1,2,11,15 whereas the incidence of asymptomatic VTE was <54%.16 This discrepancy in incidence may have resulted from the different sample sizes, clinical manifestations of patients, follow-up methods, size and location of catheters, and operators’ techniques in those studies. Therefore, all PICCs were placed by skilled nurses in the venous access center in our study. To reduce the risk of complications, 4-Fr PICCs were selected and chest radiography was done to confirm that the PICC-tip was located at the middle or lower segment of the inferior vena cava.17 Moreover, ultrasound was used for follow-up and to confirm asymptomatic VTE and symptomatic VTE. Finally, we found that the incidence of PICC-related VTE was 7.01%, the median time to VTE was 12 (range, 2–160) days, and 64.2% (106/165) VTE events occurred within 2 weeks after catheterization. Those data are consistent with results from other studies.1,16
Patients with PICC-related VTE may present with corresponding clinical symptoms and have an increased risk of PICC obstruction, which may shorten the survival time of the PICC (the median time decreased from 131 days to 72 days) significantly. We used ultrasound as a routine method and examined veins at regular intervals. We found that 46.7% of VTE cases were asymptomatic, which can be missed readily. We recommended that ultrasound be used to screen VTE within 2 weeks after PICC placement irrespective of whether symptoms are present.
A meta-analysis by Dentali et al18 revealed that the risk of thrombosis in a patient with the non-O blood group was twofold higher than that in a patient with the O blood group (OR, 2.08; 95% CI, 1.83–2.37). Hence, the non-O blood group may be a genetic risk factor for VTE. However, Haddad et al7 conducted a case–control study and pointed out that the ABO blood group was not a risk factor for PICC-related DVT, but the different result may have been due to the small sample size. In the present study, compared with patients with the O blood group, the risk of PICC-related VTE was significantly higher in patients with A (OR, 1.680; 95% CI, 1.009–2.798), B (1.835; 1.137–2.961), and AB (3.275, 1.840–5.829) blood groups. How the ABO blood group is associated with VTE is not clear. However, it has been postulated that the levels of von Willebrand factor (vWF) and factor VIII, which have been shown to be a risk factor for thrombosis, are significantly lower in patients with the O blood group.19 The plasma clearance rate of vWF may be affected by the ABO blood group, and the vWF level is increased significantly in cancer patients.20 Therefore, we wonder if vWF plays an important role in PICC-related VTE in cancer patients, and further research is needed.
Several studies have found that BMI >25 kg/m2 is an independent risk factor for catheter-related thrombosis.21,22 The univariate analysis in our study also revealed that BMI >25 kg/m2 to be associated with PICC-related VTE, but was not an independent risk factor (P = 0.382). Catheterization in obese patients can be difficult and repeated puncture can damage the vessel walls, which can result in an increased risk of thrombosis. Moreover, patients with a high BMI may have an increased level of triglycerides related to blood hypercoagulability. We found that a high level of triglycerides was 1.5-times more common in patients with VTE than that in normal patients (adjusted OR, 1.561; 95% CI, 1.096–2.223). Few studies have analyzed the relationship between the level of triglycerides and thrombosis. However, one meta-analysis23 revealed that the triglycerides level in people with VTE was significantly higher than that in a control group (the average difference was 21.0mg/dL). Those results were based on the hypothesis that the concentration of triglycerides is positively correlated with the response intensity of vascular endothelial cells to inflammatory factors, and initiate the inflammation of endothelium.24 In addition, triglycerides are related to the increased levels of coagulation factors VII, VIII, IX and fibrinogen, which can cause a hypercoagulable state and thrombus formation. Our results showing a significant correlation between a high level of triglycerides and thrombosis could guide the treatment strategy for patients with VTE (which is statins to improve vascular endothelial and coagulation functions and reduce the VTE risk).
Studies have demonstrated that the size of a PICC is positively correlated with VTE, whereas the vein diameter is negatively correlated with VTE.6 However, few studies have focused on the relationship between the veinpuncture diameter, or the ration of the PICC diameter:vein diameter and VTE. We found that a smaller diameter of venipuncture and larger ratio of PICC diameter: vein diameter were closely related to VTE. In the risk model, although a vein diameter > 0.4mm was not an independent risk factor, a ratio of PICC diameter:vein diameter >0.35was significantly associated with VTE (OR, 1.689; 95% CI, 1.023–2.789). Therefore, we recommended PICC placement under ultrasound guidance, because it is useful for selecting vein with a large diameter to puncture, and reducing the risk of thrombotic complications. In addition, we found that the APTT in the VTE group, which may predict VTE after PICC, was significantly shorter than that in the normal group.
VTE occurrence must be judged according to the clinical symptoms of patients because follow-up ultrasound is not commonly used for timely examination. We showed that the average arm circumference in the VTE group was 27.2 cm, which was higher than that in the normal group. Furthermore, the average arm circumference of patients in SVT group was smaller than that of patients in SVT+DVT group, because the affected limb may be swollen due to disturbance in vein drainage when the thrombosis involves a deep vein. Thus, we found that most instances of asymptomatic PICC-related VTE occurred in a superficial vein, which may be missed without ultrasound examination. According to the literature,1 61% cases of VTE start from a superficial vein and <50% of them spread to a deep vein, whereas the remaining 39% cases of VTE are located only in a deep vein. However, our study cohort comprised cancer patients in a hypercoagulable state, a thrombus could be formed readily from a punctured superficial vein to deep vein. Thus, most patients had SVT combined with DVT (64.2%, 106/165), and 35.8% (59/165) had isolated SVT. A pulmonary embolism was found in one patient who had SVT combined with DVT, which may have been caused by lower-extremity VTE besides PICC-related VTE due to the hypercoagulability found in cancer patients.
The incidence of complete occlusion in patients with SVT combined with DVT was significantly lower than that in patients who had SVT only (OR, 0.158; 95% CI, 0.039–0.649). Most of the veins (91.5%) were totally occluded if the thrombosis involved only a superficial vein, but almost half of the veins (49.1%) were partially occluded if the thrombosis extended to a deep vein. After PICC placement, thrombosis usually starts from a superficial vein, which has a relatively small diameter and low volume of blood flow. However, catheterization using a PICC occupies a large intraluminal space and can cause complete vascular occlusion because it is a long foreign object.1 Compared with a superficial vein, a deep vein has a larger diameter and more blood flow to prevent thrombus aggregation. The blood flow can pass through the space between vessel wall and PICC, and the thrombus only attaches on the PICC to result in partial occlusion.
We demonstrated that 74 cases (44.8%, 74/165) of venous recanalization started early after initiation of therapeutic anticoagulation in PICC-related VTE, and that partial or complete recanalization was achieved. The recanalization time was 20±5 (range, 10–31) days, which emphasized that VTE should be treated as quickly as possible if observed by ultrasound. Anticoagulation therapy is recommended to be used for ≥3 months, but some studies have shown that acute thrombi can be removed completely after 2 weeks and older thrombi can be completely recanalized after 4 weeks.25,26 Furthermore, all of our patients were examined by ultrasound routinely to detect acute thrombi, which can resolve completely after anticoagulation therapy. Meanwhile, the incidence of recanalization in the SVT+DVT group was significantly higher than that in the SVT group after anticoagulation treatment (OR, 17.942; 95% CI, 5.427–59.316). Patients in the SVT+DVT group may have benefited from incomplete occlusion, early diagnosis of thrombosis due to symptoms, and more blood flow in a larger lumen. Therefore, checking for PICC-related VTE by ultrasound in a timely manner is useful for anticoagulation therapy, which can reverse the thrombosis process and reduce the risk of complications, especially for patients with acute DVT.
Our study had four main limitations. First, although this study had a prospective design, the results of a retrospective analysis may be subjected to biases or incomplete information. For example, the time to thrombosis may have been inaccurate because ultrasound may not have been done in a timely manner to identify VTE due to non-obvious symptoms, an absence of daily visits by a nurse, and lack of observation after hospital discharge. Second, the accuracy of PICC placement was strictly controlled by using a small-diameter PICC to reduce thrombosis risk, so our research results may not be applicable in other centers or for other sizes of PICC. Third, potential confounding variables, such as the arm circumstance, and APPT, could not be evaluated in our logistic regression model, and the cancer type (as a possible factor affecting VTE) should be investigated in future research. These limitations notwithstanding, our study had important strengths: a relatively large sample size and analyses of multiple variables. In addition, we classified PICC-related VTE as SVT and SVT associated with DVT, which differ in terms of patterns of thrombosis and requirement of anticoagulation therapy.
Conclusions
The incidence of PICC-related VTE in cancer patients was 7.01%. A non-O blood group, a ratio of PICC diameter:vein diameter >0.35 and high level of triglycerides were significantly associated with VTE. The latter two factors may suggest PICC placement under ultrasound guidance to select a larger vein to puncture, and use of statin to improve vascular endothelial and coagulation functions, to reduce the VTE risk. Patients with isolated SVT had complete occlusion of veins, whereas about half of the patients with DVT had partial occlusion, which was more readily reversed after anticoagulation treatment. More than forty percent veins recanalized at 2–4 weeks after beginning therapeutic anticoagulation. Early and routine ultrasound examination could have an active role in detecting thrombi and guiding aggressive treatment.
Funding
The study was supported by the Beijing Municipal Science & Technology Commission (No. Z151100004015186).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bonizzoli M, Batacchi S, Cianchi G, et al. Peripherally inserted central venous catheters and central venous catheters related thrombosis in post-critical patients. Intensive Care Med. 2011;37:284–289. doi:10.1007/s00134-010-2043-x
2. Aw A, Carrier M, Koczerginski J, et al. Incidence and predictive factors of symptomatic thrombosis related to peripherally inserted central catheters in chemotherapy patients. Thromb Res. 2012;130:323–326.
3. Bertoglio S, Faccini B, Lalli L, et al. Peripherally Inserted Central Catheters (PICCs) in cancer patients under chemotherapy: a prospective study on the incidence of complications and overall failures. J Surg Oncol. 2016;113(6):708–714. doi:10.1002/jso.24220
4. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311–325. doi:10.1016/S0140-6736(13)60592-9
5. Chopra V, Fallouh N, McGuirk H, et al. Patterns, risk factors and treatment associated with PICC-DVT in hospitalized adults: a nested case–control study. Thromb Res. 2015;135(5):829–834. doi:10.1016/j.thromres.2015.02.012
6. Saber W, Moua T, Williams EC, et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319. doi:10.1111/j.1538-7836.2010.04126.x
7. Haddad RA, Alnimer Y, Abdalla A, et al. Is peripherally inserted central catheter–related thrombosis associated with ABO blood group? A case–control pilot study. Clin Appl Thromb Hemost. 2018;24(8):1297–1300. doi:10.1177/1076029618770289
8. Menéndez JJ, Verdú C, Calderón B, et al. Incidence and risk factors of superficial and deep venous thrombosis associated with peripherally inserted central catheters in children. J Thromb Haemost. 2016;14:2158–2168. doi:10.1111/jth.13478
9. Stone NJ, Robinson JG, Lichtenstein AH, et al. American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi:10.1016/j.jacc.2013.11.002
10. Li J, Fan YY, Xin MZ, et al. A randomised, controlled trial comparing the long-term effects of peripherally inserted central catheter placement in chemotherapy patients using B-mode ultrasound with modified Seldinger technique versus blind puncture. Eur J Oncol Nurs. 2014;18:94–103. doi:10.1016/j.ejon.2013.08.003
11. Kang J, Chen W, Sun W, et al. Peripherally inserted central catheter-related complications in cancer patients: a prospective study of over 50,000 catheter days. J Vasc Access. 2017;18:153–157. doi:10.5301/jva.5000670
12. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi:10.1016/j.chest.2015.11.026
13. Needleman L, Cronan JJ, Lilly MP, et al. Ultrasound for lower extremity deep venous thrombosis multidisciplinary recommendations from the society of radiologists in ultrasound consensus conference. Circulation. 2018;137:1505–1515. doi:10.1161/CIRCULATIONAHA.117.030687
14. Jezovnik MK, Poredos P. Factors influencing the recanalisation rate of deep venous thrombosis. Int Angiol. 2012;31:169–175.
15. Al-Asadi O, Almusarhed M, Eldeeb H. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single Centre cohort study. Thromb J. 2019;17:2. doi:10.1186/s12959-019-0191-y
16. Liu Y, Gao Y, Wei L, et al. Peripherally inserted central catheter thrombosis incidence and risk factors in cancer patients: a double-center prospective investigation. Ther Clin Risk Manag. 2015;11:153–160. doi:10.2147/TCRM.S73379
17. Zochios V, Umar I, Simpson N, et al. Peripherally inserted central catheter (PICC)-related thrombosis in critically ill patients. J Vasc Access. 2014;15:329–337. doi:10.5301/jva.5000239
18. Dentali F, Sironi AP, Ageno W, et al. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Semin Thromb Hemost. 2012;38:535–548. doi:10.1055/s-0032-1315758
19. Ward SE, O’Sullivan JM, O’Donnell JS. The relationship between ABO blood group, von Willebrand factor and primary hemostasis. Blood. 2020;136:2864–2874. doi:10.1182/blood.2020005843
20. Garam N, Maláti É, Sinkovits G, et al. Platelet count, ADAMTS13 activity, von willebrand factor level and survival in patients with colorectal cancer: 5-year Follow-up Study. Thromb Haemost. 2018;118(01):123–131. doi:10.1160/TH17-07-0548
21. Yi XL, Chen J, Li J, et al. Risk factors associated with PICC-related upper extremity venous thrombosis in cancer patients. J Clin Nurs. 2014;23:837–843. doi:10.1111/jocn.12227
22. Shi Y, Wen L, Zhou Y, et al. Thrombotic risk factors in patients undergoing chemotherapy via peripherally inserted central catheter. J Int Med Res. 2014;42:863–869. doi:10.1177/0300060514527061
23. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism A meta-analysis. Circulation. 2008;117(1):93–102. doi:10.1161/CIRCULATIONAHA.107.709204
24. Ting HJ, Stice JP, Schaff UY, et al. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-alpha. Circ Res. 2007;100:381–390. doi:10.1161/01.RES.0000258023.76515.a3
25. Antignani PL, Allegra C, Fareed J. Treatment of deep vein thrombosis with rivaroxaban and its potential to prevent the post-thrombotic syndrome. Int Angiol. 2019;38:17–21. doi:10.23736/S0392-9590.18.04031-2
26. Aguiar de Sousa D, Lucas Neto L, Arauz A, et al. Early recanalization in patients with cerebral venous thrombosis treated with anticoagulation. Stroke. 2020;51:1174–1181. doi:10.1161/STROKEAHA.119.028532
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.