Back to Journals » Cancer Management and Research » Volume 15
The Incidence and Management of Cancer-Related Anorexia During Treatment with Vascular Endothelial Growth Factor Receptor-Tyrosine Kinase Inhibitors
Authors Takahashi S, Matsumoto K, Ohba K, Nakano Y, Miyazawa Y, Kawaguchi T
Received 21 April 2023
Accepted for publication 7 September 2023
Published 22 September 2023 Volume 2023:15 Pages 1033—1046
DOI https://doi.org/10.2147/CMAR.S417238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ahmet Emre Eşkazan
Shunji Takahashi,1 Koji Matsumoto,2 Kojiro Ohba,3 Yasuhiro Nakano,4 Yasushi Miyazawa,5 Takumi Kawaguchi6
1Department of Medical Oncology, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan; 2Department of Medical Oncology, Hyogo Cancer Center, Akashi, Japan; 3The Department of Urology and Renal Transplantation, Nagasaki University Hospital, Nagasaki, Japan; 4Department of Pharmacy, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan; 5Department of Clinical Nutrition, Tokyo Medical University Hospital, Tokyo, Japan; 6Division of Gastroenterology, Department of Medicine, Kurume University School of Medicine, Kurume, Japan
Correspondence: Shunji Takahashi, Department of Medical Oncology, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, 3-8-31 Ariake, Koto, Tokyo, 135-8550, Japan, Tel +81-3-3520-0111, Fax +81-3-3520-0141, Email [email protected]
Abstract: Cancer-related anorexia is a common complication and frequently occurs in cancer patients treated with vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFR-TKIs). Anorexia contributes to malnutrition, body weight loss, and cachexia in affected patients. Furthermore, patients who experience anorexia have worse outcomes than those who maintain their appetite, highlighting the importance of managing anorexia and related symptoms. However, as the causes of anorexia are both diverse and interconnected, there have been challenges in evaluating and implementing effective interventions. In this review, we described the contributing factors to cancer-related anorexia and reviewed recent literature for the frequency of anorexia symptoms in patients treated with VEGFR-TKIs. Additionally, we evaluated the evidence for current interventions and the potential benefits of multimodal and multidisciplinary approaches to care. The frequency of anorexia symptoms in patients who received VEGFR-TKIs ranged from 14%– 58% for all-grade anorexia and 0%– 6% for grade 3 or 4 anorexia. While many of the interventions for cancer-related anorexia have minimal benefit or adverse events, recent advances in our understanding of cancer-related anorexia suggest that multimodal therapy with multidisciplinary care is a promising avenue of investigation. Several studies currently underway are anticipated to further assess the effectiveness of multimodal approaches.
Keywords: appetite, cancer-related anorexia-cachexia syndrome, gastrointestinal symptoms, malnutrition, VEGFR-TKI
Introduction
Cancer-related anorexia (defined as a loss of appetite) is a common complication of cancer and is estimated to affect between 40%–80% of patients with advanced cancer, with considerable impacts on their mood, daily activities, and overall quality of life.1,2 Anorexia in these patients contributes to weight loss and cachexia, which leads to sarcopenia in affected patients.3,4 There are a wide variety of factors that can contribute to anorexia, including nausea, dysgeusia, dysphagia, mouth ulcers, early satiety, constipation, diarrhea, general malaise, pain, and depression.5
Anorexia and the associated weight loss are serious symptoms in patients with cancer, and both are independently associated with poor prognosis and cancer progression.5–7 Although there are other known causes of weight loss and cachexia in patients with cancer,3,4,8 in this review, we have focused on weight loss associated with cancer-related anorexia. Patients with cancer-related anorexia have a decreased quality of life9 and often experience other related effects including weight loss, malnutrition, and a decrease in muscle and fat.10 Furthermore, patients with anorexia have a significantly shorter survival than patients who do not experience loss of appetite.11 Given the poor outcomes associated with anorexia, effective management of anorexia and weight loss symptoms is necessary to improve outcomes for patients with cancer. However, there have been challenges in implementing these management strategies in clinical practice: first, a lack of widely accepted methodology for assessing cancer-related anorexia means that the identification of affected patients is inconsistent and the evaluation of interventions is difficult.5,6 Second, the multiple causes and pathogenesis of cancer-related anorexia means that a single approach may be unlikely to work for all patients; anorexia can be induced by the tumor, cancer treatment, or both together.12 Third, pain associated with cancer, depression, nausea, and other symptoms can also contribute to anorexia.13 Therefore, a multimodal therapy that addresses both symptoms of anorexia and the associated malnutrition, weight loss, and increased cachexia is desirable and is currently being investigated.14
Tyrosine kinase inhibitors (TKIs) have recently become important in the treatment of cancer, regardless of the type of carcinoma. Unfortunately, patients treated with TKIs can develop anorexia, sarcopenia, and cachexia, leading to treatment discontinuation. In this paper, we reviewed the mechanisms of cancer-related anorexia and the frequency of anorexia and related symptoms in patients treated with TKIs. Because there is a lack of evidence for managing TKI-related anorexia, we evaluated the evidence for the current interventions for chemotherapy-related anorexia and examined the effectiveness of multidisciplinary care in clinical practice. Furthermore, as anorexia and cachexia are interrelated, cancer-related cachexia is included in this review.
The Mechanisms of Cancer-Related Anorexia
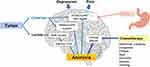
The physiological causes of cancer-related anorexia follow several pathways (Figure 1).3 Appetite is controlled by two types of neurons in the central hypothalamus that form two complementary pathways: pro-opiomelanocortin (POMC)/cocaine and amphetamine-regulated transcript (CART) neurons that reduce appetite, and neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons that increase appetite.15–17 Tumor cells can influence appetite through the production of inflammatory cytokines; these cytokines modulate neurotransmitter pathways by increasing the activity of the POMC/CART neurons and inhibiting the activity of NPY/AgRP neurons, leading to a suppression of appetite. Additionally, the cytokines are involved in a dysfunction of the melanocortin system, which contributes to anorexia.5,12 Increased inflammatory cytokines are also associated with depression in patients with cancer.18 Tumor cells produce lactate, which can suppress appetite via 5-adenosine monophosphate (AMP) kinase/malonyl-coenzyme A (CoA) signaling in the hypothalamus.19–21 Central effects (such as depression and pain) can contribute to anorexia by impacting these neurotransmitter pathways.12
The hormone ghrelin is secreted by the stomach; it increases the expression of NPY/AgRP and suppresses POMC/CART, thereby increasing appetite.22–25 In addition, ghrelin promotes the secretion of gastric acid and emptying of the stomach.22 Patients with cancer tend to have higher ghrelin levels,25 which may function as a compensatory response to weight loss.26 Patients with cachexia may also have increased ghrelin levels yet experience no increase in appetite, which indicates that such patients start developing resistance to ghrelin.26 However, this may not be the case for all patients with cancer, as reports have shown that ghrelin levels are significantly lower in patients with gastric or pancreatic cancer27 and that these levels are further decreased following chemoradiotherapy.28 Patients who receive chemotherapy may also experience dysgeusia and other gastrointestinal symptoms such as vomiting, abdominal cramps, and mucositis.12 Approximately 39% of patients who receive chemotherapy will experience dysgeusia,29 which can contribute to a decrease in appetite.30
Development of Anorexia Following TKI Treatment
In recent years, vascular endothelial growth factor receptor (VEGFR)-TKIs have played an increasingly important role in the treatment of cancer.31,32 Several meta-analyses have shown that patients experience an increased frequency of gastrointestinal side effects, including a decrease in appetite, following VEGFR-TKI therapy.33,34 Patients who received TKI therapy had a significantly increased risk of all-grade anorexia compared with those in the control group (36.8% vs 24.3%; p < 0.00001).33 When grouped by the type of carcinoma, the relative risk of all-grade anorexia was significantly higher for patients with colorectal cancer, thyroid cancer, and renal cell carcinoma (RCC), and grade ≥3 anorexia was significantly higher for those with thyroid cancer and RCC, which may reflect the relatively longer progression-free survival duration. Furthermore, increases in the incidence of nausea and vomiting were observed following TKI therapy, and for some patients, gastrointestinal adverse events (AEs) prevented the continuation of treatment.
We reviewed the frequency and risk of decreased appetite when treated with VEGFR-TKIs in a variety of carcinomas, focusing on phase III clinical trials (Table 1). The frequency of decreased appetite varied by the specific drug used, with the occurrence of all-grade anorexia ranging from 14%–58%, and between 0%–6% for grade 3 and above. Many treatment-emergent AEs (TEAEs) that occur with VEGFR-TKI treatment can lead to anorexia, including nausea and stomatitis; additionally, diarrhea and mucositis contribute to malnutrition (Figure 2). These AEs can lead to weight loss and contribute to cachexia,3,13,30,35,36 necessitating the careful management of these symptoms as well as management of anorexia. A systematic review stated that the incidence of stomatitis is between 20%–35% with TKI treatment (35.2% with sunitinib, 34.21% with cabozantinib, 20.63% with axitinib, and 20.52% with sorafenib), although the majority of these cases were low-grade (grade 1 or 2).37 We reviewed the frequency of both anorexia and other AEs related to malnutrition associated with VEGFR-TKI therapy in patients with RCC for whom the frequency of anorexia and cachexia is relatively high. The incidence of AEs related to anorexia and malnutrition ranged from 20%–62% for nausea, 16%–39% for vomiting, 43%–74% for diarrhea, and 14%–30% for stomatitis (Table 2). Early satiety, a symptom that is highly associated with anorexia, is not included as an AE for VEGFR-TKI therapy. Patients with cancer-related anorexia-cachexia syndrome often experience early satiety,38 which can contribute to weight loss.9 Early satiety is reported to be more common in patients with severe anorexia.39 However, standard questionnaires often do not capture information on early satiety, potentially leading to underreporting of this important symptom.40 These high rates of anorexia and other AEs highlight the importance of careful monitoring of patients treated with TKIs.
 |
Table 1 Frequency of Anorexiaa with TKI Treatment |
 |
Table 2 The Frequency of Anorexia and Related Adverse Events in Patients with Renal Cell Carcinoma Treated with VEGF/VEGFR TKIs |
 |
Figure 2 Chemotherapy-induced adverse events contributing to malnutrition. |
Management of Cancer-Related Anorexia
The development of cancer-related anorexia and cancer-related anorexia-cachexia syndrome can be the result of the tumor, pain, and depression, and can also be a treatment-related AE (Figure 2).30,59,60 These factors are interrelated and can occur simultaneously, making it difficult for clinicians to easily identify the cause of anorexia. Importantly, there is currently no effective treatment for cachexia,59,60 contributing to the challenge of managing symptoms in these patients. Therefore, it is necessary to understand the patient’s experience to ascertain the factors contributing to anorexia and develop a management plan that addresses each factor. For patients who experience anorexia as a TEAE, a reduction in dose or drug withdrawal are the primary measures for those experiencing AEs of grade 3 and above. However, less severe symptoms, such as those below grade 2, may act synergistically and result in an inadequate nutritional intake, weight loss, and cachexia. Therefore, interventions such as medication and nutritional therapy should be considered to improve the patient’s appetite and prevent malnutrition.61 Other suggested approaches include exercise therapy, dietary counseling, progesterone analogs, and corticosteroids (Table 3).62 As there is a high frequency of AEs associated with TKI therapy that are directly related to appetite and eating, such as nausea, vomiting, and stomatitis, a comprehensive response that considers these symptoms is likely to improve the appetite of affected patients and support a healthy nutritional intake.
 |
Table 3 The Currently Approved Treatments for Cancer Anorexia-Cachexia Syndrome in Japan and the American Society of Clinical Oncology Guideline Recommendations62 |
Treatment of Associated Symptoms
As anorexia often occurs with other symptoms such as stomatitis, nausea, and vomiting, management of these related symptoms is crucial when treating patients with cancer-related anorexia. Stomatitis is common in patients with metastatic breast cancer who are treated with mammalian target of rapamycin inhibitors; however, treatment options are limited to educating patients about good oral health care and prophylactic use of a dexamethasone mouth rinse.63 Although stomatitis experienced by patients treated with TKI therapy is often mild (grade 1 or 2),37 the use of appropriate prophylaxis is recommended because untreated stomatitis can lead to a loss of appetite. Similarly, dysgeusia can also alter appetite by making normally appetizing foods unappealing. Treatment with monosodium glutamate has been recently shown to increase the expression of the taste receptor subunit T1R3 and increase energy intake in patients with advanced head and neck cancer.64
Oral mucositis is a common side effect of both chemotherapy and radiotherapy.65 Glutamine has been studied as a treatment for chemotherapy- and radiotherapy-induced oral mucositis: a recent systematic review of several clinical trials showed that patients treated with glutamine had a significantly reduced incidence of severe (grade 3 or 4) oral mucositis.66,67 Conversely, an earlier systematic review reported that glutamine had no effect on the incidence and severity of radiotherapy-induced oral mucositis in patients with head and neck cancer.68 As stomatitis and oral mucositis experienced by patients treated with TKIs is often mild, the use of glutamine for these patients requires further investigation.
Pharmacologic Approaches to Cancer-Related Anorexia
The progesterone analog megestrol acetate has been shown to improve appetite, weight gain, and quality of life in patients with anorexia in a Cochrane review,69 and is recommended for the treatment of cancer-related anorexia in the American Society of Clinical Oncology (ASCO) guidelines.62 However, as this drug has not been approved for the treatment of anorexia in Japan (Table 3), pharmacologic approaches to managing cancer-related anorexia in Japan rely on corticosteroids and the recently approved ghrelin receptor agonist anamorelin. Here, we describe the evidence for these two classes of drugs for the management of cancer-related anorexia. Other pharmacologic approaches are expected to be available in the future: for example, olanzapine has recently shown promising results in patients with locally advanced or metastatic gastric, hepatopancreatobiliary, and lung cancers.70 However, these newer treatments are outside of the scope of this review.
Corticosteroids
Corticosteroids are widely used to manage cancer-related anorexia as they inhibit the release of inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin-1, thereby increasing appetite and weight in patients with cachexia.71 However, the benefits are transient and corticosteroids are not suitable for long-term use because of potential AEs such as hyperglycemia, hypertension, infection, bone fractures, and gastrointestinal bleeding.72,73 Furthermore, steroids used in combination with immune checkpoint inhibitors (ICIs) may decrease the efficacy of ICIs and lead to worse patient outcomes, though this has not been conclusively shown.74–76 Thus, corticosteroids may not be appropriate for all patients, and the dose must be carefully managed to maximize the benefits while preventing unwanted side effects.
Anamorelin
The ghrelin receptor agonist anamorelin was recently approved in Japan for the treatment of cachexia in patients with colorectal cancer, gastric cancer, non-small-cell lung cancer, and pancreatic cancer.77 In a meta-analysis of patients with cancer, those treated with anamorelin had an increased appetite as measured by the Anderson Symptom Assessment Scale score of 8.05 (95% confidence interval [CI]: 5.97–10.12, p < 0.00001) and improved symptom relief and quality of life.78 Another meta-analysis also reported that treatment with anamorelin increases lean body mass and quality of life compared with placebo (mean difference: 1.10; 95% CI: 0.35–1.85, p = 0.004; and mean difference: 0.19; 95% CI: 0.08–0.30, p = 0.0006, respectively).79 The largest phase III clinical trials included in the meta-analysis, the ROMANA 1 and ROMANA 2 trials, found an increase in lean body mass following anamorelin treatment compared with placebo.80 However, no improvement in hand grip strength was obtained with anamorelin treatment. When treatment was extended for a further 12 weeks (the ROMANA 3 trial), patients showed improvement in anorexia-cachexia symptoms compared with placebo, but no improvement in hand grip strength.81 Thus, anamorelin has not been approved for use in the United States for the treatment of cancer cachexia and is not recommended by the ASCO guidelines.62
In Japan, anamorelin at a dose of 100 mg was approved based on the results of two studies: a study of 174 patients with advanced non-small-cell lung cancer and cachexia, who showed improvements in lean body mass, performance status, and quality of life with anamorelin treatment;82 and a study in patients with advanced gastrointestinal cancer, who showed improvements in anorexia symptoms, nutritional status, lean body mass, and body weight following anamorelin treatment.83 Thus, anamorelin is often used in Japanese clinical practice. However, some patients may not benefit from anamorelin treatment, such as those with low performance status, and more evidence is needed to assess potential biomarkers and identify which patients will benefit the most. Newer multimodal interventions, that combine nutritional and exercise therapy, may work synergistically with anamorelin and lead to better outcomes when anamorelin alone is not sufficient, and are currently being explored.84 Other pharmacologic options (including thalidomide, olanzapine, and mirtazapine) are not currently indicated for the treatment of cachexia and anorexia (Table 3).
Multimodal Therapy
As noted throughout the previous sections of this review, each individual therapy for cancer-related anorexia on its own often shows either inconsistent benefits or potential unwanted side effects. This is also true for exercise therapy: the ASCO Exercise, Diet, and Weight management guidelines state that exercise therapy during cancer treatment led to improvements in fatigue, muscle strength, and quality of life of patients and recommend both aerobic and resistance exercise.85 However, the ASCO guidelines for cachexia state that there is no evidence for the benefit of exercise therapy,62 and a Cochrane review also found no evidence for the use of nutritional therapy to treat cachexia.86 As anorexia and cachexia often involve interrelated symptoms and diverse causes, a combined approach that brings together different treatments may offer an effective management of anorexia. While either nutritional or exercise therapy alone may have limited efficacy, combinations of these therapies should be considered.14,36 Combined approaches that address the multiple factors contributing to the condition are termed multimodal therapy and are likely to be the next step in cancer-related anorexia therapy. Taking a multimodal approach involves identifying the treatable causes and symptoms for each individual patient and developing a personalized plan involving a combination of therapies for reducing inflammation, improving metabolism, and restoring appetite, with the aim to increase skeletal muscle mass and physical function.40,85
Current multimodal therapies that combine exercise and nutritional therapy have shown promising results for the treatment of cachexia, anorexia, and weight loss. A systematic review that evaluated the use of combined exercise and nutritional therapy for the rehabilitation of patients with refractory cancer found that this type of multimodal therapy improved physical endurance and reduced depression scores.87 A study of patients with advanced tumors (n = 58) showed that nutrition counseling with an exercise program resulted in reduced nausea and vomiting, and improved protein intake compared to standard care.88 A phase II study of patients with refractory lung or pancreatic cancer found that the multimodal therapy (including omega-3 fatty acid nutritional supplements, exercise, and the anti-inflammatory drug celecoxib) cohort tended to maintain their body weight while the standard care cohort did not; however, this study had a small sample size, and no further effects were identified.89 A larger phase III clinical trial is currently underway to investigate the multimodal therapy described above for cachexia.90 Another study found that patients who received multimodal therapy (involving fish oil supplements, nutritional counseling, and exercise therapy) had increased skeletal muscle compared with the control group.91 Although the evidence for these multimodal therapies is so far limited to small clinical trials, the results are promising and warrant further investigation. The Japanese phase II NEXTAC-TWO clinical trial of multimodal therapy has been planned,92 which will further contribute to our knowledge regarding multimodal therapy.
Multidisciplinary Care
Coordination and collaboration among multiple disciplines, including nurses, pharmacists, dietitians, physical therapists, and psychotherapists, is important for successful implementation of multimodal therapy.93 It is also important to collect information from multiple perspectives to best understand the patient’s experience, and difficulties, and to provide an appropriate diagnosis and treatment.61 Several studies reported that the use of multimodal therapy based on a multidisciplinary program leads to improvements in quality-of-life measures.94,95 Additionally, a study that investigated multimodal therapy in an interdisciplinary clinic reported that the therapy contributed to both weight gain and increased functional strength in patients with cancer.96 A phase II clinical trial that examined the efficacy of multimodal treatment from a multidisciplinary team (an oncologist, a nutritionist, and a psychotherapist) for treating patients with metastatic esophagogastric cancer showed that patients treated with multimodal therapy had significantly increased median overall survival compared with the standard care cohort (14.8 months vs 11.9 months, p = 0.021).97 Patients treated with multimodal therapy also showed improvements in their emotional and cognitive functions, but no significant difference in anorexia was measured between the two cohorts. Similar results were reported from a feasibility study of a multidisciplinary care program including nutrition counseling, exercise therapy, and psychological support; the study reported high patient satisfaction, although non-attendance rates increased as the study progressed.98
Early detection of cachexia is important for improving outcomes in affected patients, as cachexia is interrelated with anorexia. Nurses are important for the identification of cachexia and assessment of a patient’s nutritional state.99 However, a survey of Japanese nurses showed that while nurses were aware of the importance of identifying cachexia, they were often not confident in their abilities to assess cachexia.100 Thus, further training and education of nurses in assessing cachexia is important for the early identification and treatment of affected patients.101 Other key roles include dietitians, who work with the wider clinical team to understand the patient’s condition, symptoms, quality of life, and needs, to safely incorporate a nutrition plan that will improve anorexia and cachexia.102 In addition, the dietitian can play an integral role in the identification and management of cancer cachexia, focusing on aspects of nutrition screening and intervention.103
The use of complementary supplements and alternative medicine has been met with concern, as they are often accompanied by reduced adherence to standard therapy, significant reduction in 5-year overall survival, and increased risk of death.104 Similarly, the use of alternative medicines by patients with nonmetastatic breast, prostate, lung, and colorectal cancer is associated with an increased risk of death compared with standard care.105 A multicenter prospective cohort study of patients with early-stage breast cancer revealed that patients who used complementary medicines were less likely to start chemotherapy than those who did not use complementary medicines.106 It is important for the wider team, including dietitians and pharmacists, to build a trusting relationship with the patient to ensure that appropriate education is available and that effective treatments are provided.
Future Research: Nutritional Therapy
There have been several studies that have examined the potential use of nutritional therapy for treating anorexia. However, the lack of established evaluation methods and the differences within and between study populations has resulted in inconsistent evidence for the use of vitamins, minerals, and other dietary supplements. Therefore, the ASCO guidelines do not recommend their use by patients with cancer experiencing anorexia.62 However, nutrition counseling by a registered dietitian for advanced cancer patients with decreased appetite and weight loss has a moderate recommendation in the ASCO guidelines.62 The European Society for Clinical Nutrition and Metabolism guidelines also recommend that appropriately trained professionals provide nutritional guidance.107 As noted earlier in this review, nutritional therapy may play an important role in multimodal therapies, working synergistically with other therapies.14,36 Here, we discuss the emerging evidence for the use of omega-3 fatty acids and royal jelly for the management of cancer-related anorexia.
Eicosapentaenoic (EPA) and docosapentaenoic acid are both omega-3 fatty acids that have been extensively studied in nutritional therapy for cachexia, and have been shown to improve cachexia, promote weight gain, reduce inflammation, improve neuropathy, and improve quality of life measures.108 The ASCO guidelines evaluated three systematic reviews and meta-analyses in 2011, 2015, and 2018, and found an effect of omega-3 fatty acids on improving weight gain,62,109 but found insufficient evidence for treating cachexia, and thus the ASCO guidelines do not recommend their use for managing cachexia. Similarly, the European Society for Medical Oncology guidelines state that reports of omega-3 fatty acids have shown some benefits on body weight, lean body mass, and quality of life measures, but lacked sufficient evidence for their benefit.14 In a 2022 systematic review, EPA treatment did not show any clear effect on weight gain.110 Another recent systematic review of the use of omega-3 fatty acids found that patients gained weight in some trials, but the sample sizes were small and not supported by sufficient evidence.111 In contrast, an earlier systematic review that examined the effectiveness of nutritional interventions for anorexia reported that increased EPA from oral nutritional supplements, dietary counseling, and fish oil supplements, improved appetite and nutrition outcomes in patients with cancer.112 Thus, there is inconsistent evidence for the use of omega-3 fatty acids for the treatment of anorexia or cachexia.
Royal jelly, a substance collected from honeybees, has been investigated for interactions with chemotherapeutic agents and potential anti-inflammatory effects,113–115 and a previous study examining royal jelly revealed insights into the mechanism of TKI-induced anorexia.116 Royal jelly affects the regulation of cytokines such as TNF-α and TGF-β;117,118 and it has recently been considered for its potential to prevent TKI-induced anorexia.116,118 Patients with RCC who received pre-treatment with royal jelly before TKIs had a reduction in TNF-α levels and an increase in TGF-β and macrophage colony-stimulating factor levels; however, the effect was transient and did not last beyond 12 weeks.116 In another study, patients with RCC who received royal jelly had significantly lower rates of TKI-induced anorexia compared with those receiving placebo,118 and fewer patients who received royal jelly required TKI dose reductions. Considering these findings, royal jelly is a promising candidate for its potential in preventing anorexia in patients with cancer.
Conclusion
In this review, we have discussed the causes and mechanisms of cancer-related anorexia, including the interconnected symptoms experienced by affected patients. In addition, we have described the current management approaches, ranging from pharmacologic therapies to the recent advances in multimodal and multidisciplinary care and the emerging evidence for nutritional therapy. The frequency of all-grade anorexia was evaluated in clinical trials of patients treated with VEGFR-TKIs and was found to be between 14%–58%. Anorexia can severely impact quality of life, resulting in weight loss and contributing to cachexia, which can lead to treatment discontinuations. Therefore, early detection and effective management of anorexia are crucial in patients with cancer. A comprehensive approach, encompassing not only pharmacotherapy but also exercise and nutritional therapy, may be necessary to effectively treat anorexia. Effective collaboration among healthcare professionals is essential to ensure successful multidisciplinary management.
Acknowledgments
The authors wish to thank Hannah Read, PhD, of Edanz (www.edanz.com) for providing medical writing support, which was funded by Eisai Co., Ltd., in accordance with the 2022 Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022). We acknowledge Mr. Hiromasa Sasaki and Dr. Michiko Sugawara (Eisai Co., Ltd.) for their support as publication coordinators.
Disclosure
Although authors received payment for advisory board services, this present manuscript was prepared independently with funding for medical writing only. Shunji Takahashi has received medical writing support from Eisai Co., Ltd.; grants or contracts from MSD K.K., Chugai Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., Bristol-Myers Squibb K.K., Ono Pharmaceutical Co., Ltd., Eisai Co., Ltd., Novartis Pharma K.K., Taiho Pharmaceutical Co., Ltd., and Daiichi Sankyo, Co., Ltd.; and payments or honoraria from Eisai Co., Ltd., Novartis Pharma K.K., Chugai Pharmaceutical Co., Ltd., AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., MSD K.K., Taiho Pharmaceutical Co., Ltd., Bayer Ltd., and Bristol-Myers Squibb K.K. Koji Matsumoto has received medical writing support from Eisai Co., Ltd.; payments or honoraria from Eisai Co., Ltd., Kyowa Kirin Co., Ltd., MSD K.K., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Bristol-Myers Squibb K.K., ACTmed Co., Ltd., Bayer Yakuhin Ltd., Ono Pharmaceutical Co., Ltd., and Kaken Pharmaceutical Co., Ltd.; and participated on a data safety monitoring board or advisory board for Eisai Co., Ltd, AstraZeneca K.K., MSD K.K., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., and Genmab K.K. Kojiro Ohba, Yasuhiro Nakano, Yasushi Miyazawa, and Takumi Kawaguchi have received payment or honoraria from Eisai Co., Ltd., and medical writing support for the present manuscript. Yasushi Miyazawa has received consulting fees from AIM Services Co., Ltd., Forico Foods Co., Ltd., and TOP Corporation; payment or honoraria from Nestlé Health Science Co., Ltd. and Otsuka Pharmaceutical Factory, Inc.; and has a leadership or fiduciary role in The Japan Association for Nutritional Management Practice. Takumi Kawaguchi has received consulting fees from ASKA Pharmaceutical Co., Ltd., Kowa Co., Ltd., and Taisho Pharmaceutical Co., Ltd.; received payment or honoraria from ASKA Pharmaceutical Co., Ltd., AstraZeneca K.K., AbbVie GK., Chugai Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., Gilead Sciences, Inc., Janssen Pharmaceutical K.K., KM Biologics, Kowa Company, Ltd., Merck & Co., Inc., Miyarisan Pharmaceutical Co., Ltd., Nobelpharma Co., Ltd., Novartis AG., Otsuka Pharmaceutical Factory, Inc., Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., SMC Laboratories, Inc., and Taisho Pharmaceutical Co., Ltd.; and has received medical writing support from Eisai Co., Ltd. The authors report no other conflicts of interest in this work.
References
1. Hopkinson JB, Wright DN, McDonald JW, Corner JL. The prevalence of concern about weight loss and change in eating habits in people with advanced cancer. J Pain Symptom Manage. 2006;32:322–331. doi:10.1016/j.jpainsymman.2006.05.012
2. Poole K, Froggatt K. Loss of weight and loss of appetite in advanced cancer: a problem for the patient, the carer, or the health professional? Palliat Med. 2002;16:499–506. doi:10.1191/0269216302pm593oa
3. Nishikawa H, Goto M, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Cancer cachexia: its mechanism and clinical significance. Int J Mol Sci. 2021;22:8491. doi:10.3390/ijms22168491
4. Peixoto da Silva S, Santos JMO, Costa E, Silva MP, da Costa RM G, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11:619–635. doi:10.1002/jcsm.12528
5. Hariyanto TI, Kurniawan A. Appetite problem in cancer patients: pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun. 2021;27:100336. doi:10.1016/j.ctarc.2021.100336
6. Laviano A, Koverech A, Seelaender M. Assessing pathophysiology of cancer anorexia. Curr Opin Clin Nutr Metab Care. 2017;20:340–345. doi:10.1097/MCO.0000000000000394
7. Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–6248. doi:10.1200/JCO.2005.06.866
8. Herremans KM, Riner AN, Cameron ME, Trevino JG. The microbiota and cancer cachexia. Int J Mol Sci. 2019;20:6267. doi:10.3390/ijms20246267
9. Barajas Galindo DE, Vidal-Casariego A, Calleja-Fernández A, et al. Appetite disorders in cancer patients: impact on nutritional status and quality of life. Appetite. 2017;114:23–27. doi:10.1016/j.appet.2017.03.020
10. Cooper C, Burden ST, Cheng H, Molassiotis A. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99–111. doi:10.1002/jcsm.12010
11. Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865–871. doi:10.1016/S1470-2045(09)70200-1
12. Ezeoke CC, Morley JE. Pathophysiology of anorexia in the cancer cachexia syndrome. J Cachexia Sarcopenia Muscle. 2015;6:287–302. doi:10.1002/jcsm.12059
13. Del Fabbro E. Current and future care of patients with the cancer anorexia-cachexia syndrome. Am Soc Clin Oncol Educ Book. 2015;35:e229–37. doi:10.14694/EdBook_AM.2015.35.e229
14. Arends J, Strasser F, Gonella S, et al. Cancer cachexia in adult patients: ESMO clinical practice guidelines. ESMO Open. 2021;6:100092. doi:10.1016/j.esmoop.2021.100092
15. Li Y, Jin H, Chen Y, Huang T, Mi Y, Zou Z. Cancer cachexia: molecular mechanism and pharmacological management. Biochem J. 2021;478:1663–1688. doi:10.1042/BCJ20201009
16. Mondello P, Mian M, Aloisi C, Famà F, Mondello S, Pitini V. Cancer cachexia syndrome: pathogenesis, diagnosis, and new therapeutic options. Nutr Cancer. 2015;67:12–26. doi:10.1080/01635581.2015.976318
17. Laviano A, Inui A, Marks DL, et al. Neural control of the anorexia-cachexia syndrome. Am J Physiol Endocrinol Metab. 2008;295:E1000–1008. doi:10.1152/ajpendo.90252.2008
18. Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncol Nurs Forum. 2008;35:802–807. doi:10.1188/08.ONF.802-807
19. Asakawa A, Fujimiya M, Niijima A, et al. Parathyroid hormone-related protein has an anorexigenic activity via activation of hypothalamic urocortins 2 and 3. Psychoneuroendocrinology. 2010;35:1178–1186. doi:10.1016/j.psyneuen.2010.02.003
20. Cha SH, Lane MD. Central lactate metabolism suppresses food intake via the hypothalamic AMP kinase/malonyl-CoA signaling pathway. Biochem Biophys Res Commun. 2009;386:212–216. doi:10.1016/j.bbrc.2009.06.017
21. Dwarkasing JT, Marks DL, Witkamp RF, van Norren K. Hypothalamic inflammation and food intake regulation during chronic illness. Peptides. 2016;77:60–66. doi:10.1016/j.peptides.2015.06.011
22. Khatib MN, Gaidhane A, Gaidhane S, Quazi ZS. Ghrelin as a promising therapeutic option for cancer cachexia. Cell Physiol Biochem. 2018;48:2172–2188. doi:10.1159/000492559
23. Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol. 2018;15:9–20. doi:10.1038/s41574-018-0123-0
24. Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia--pathophysiology and management. J Gastroenterol. 2013;48:574–594. doi:10.1007/s00535-013-0787-0
25. Mondello P, Lacquaniti A, Mondello S, et al. Emerging markers of cachexia predict survival in cancer patients. BMC Cancer. 2014;14:828. doi:10.1186/1471-2407-14-828
26. Garcia JM, Garcia-Touza M, Hijazi RA, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–2926. doi:10.1210/jc.2004-1788
27. Legakis I, Stathopoulos J, Matzouridis T, Stathopoulos GP. Decreased plasma ghrelin levels in patients with advanced cancer and weight loss in comparison to healthy individuals. Anticancer Res. 2009;29:3949–3952.
28. Uysal P, Afsar CU, Sozer V, et al. Evaluation of the relationship between serum ghrelin levels and cancer cachexia in patients with locally advanced non-small-cell lung cancer treated with chemoradiotherapy. J Cancer Res Ther. 2020;16:855–859. doi:10.4103/jcrt.JCRT_10_19
29. Imai H, Soeda H, Komine K, Otsuka K, Shibata H. Preliminary estimation of the prevalence of chemotherapy-induced dysgeusia in Japanese patients with cancer. BMC Palliat Care. 2013;12:38. doi:10.1186/1472-684X-12-38
30. Childs DS, Jatoi A. A hunger for hunger: a review of palliative therapies for cancer-associated anorexia. Ann Palliat Med. 2019;8:50–58. doi:10.21037/apm.2018.05.08
31. Pottier C, Fresnais M, Gilon M, Jérusalem G, Longuespée R, Sounni NE. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers. 2020;12:731. doi:10.3390/cancers12030731
32. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. 2019;12:27. doi:10.1186/s13045-019-0718-5
33. Li J, Gu J. Risk of gastrointestinal events with newly approved (after 2011) vascular endothelial growth factor receptor tyrosine kinase inhibitors in cancer patients: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2017;73:1209–1217. doi:10.1007/s00228-017-2299-y
34. Santoni M, Conti A, De Giorgi U, et al. Risk of gastrointestinal events with sorafenib, sunitinib and pazopanib in patients with solid tumors: a systematic review and meta-analysis of clinical trials. Int J Cancer. 2014;135:763–773. doi:10.1002/ijc.28544
35. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi:10.1016/j.clnu.2008.06.013
36. Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36:1187–1196. doi:10.1016/j.clnu.2017.06.017
37. Arena C, Troiano G, De Lillo A, Testa NF, Lo Muzio L. Stomatitis and VEGFR-tyrosine kinase inhibitors (VR-TKIs): a review of current literature in 4369 patients. Biomed Res Int. 2018;2018:5035217. doi:10.1155/2018/5035217
38. Lasheen W, Walsh D. The cancer anorexia-cachexia syndrome: myth or reality? Support Care Cancer. 2010;18:265–272. doi:10.1007/s00520-009-0772-6
39. Yavuzsen T, Walsh D, Davis MP, et al. Components of the anorexia-cachexia syndrome: gastrointestinal symptom correlates of cancer anorexia. Support Care Cancer. 2009;17:1531–1541. doi:10.1007/s00520-009-0623-5
40. Thomas S, Walsh D, Aktas A. Systematic bias in cancer patient-reported outcomes: symptom ‘orphans’ and ‘champions’. BMJ Support Palliat Care. 2019;9:67–74. doi:10.1136/bmjspcare-2014-000835
41. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi:10.1056/NEJMoa1712126
42. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi:10.1200/JCO.2008.20.1293
43. Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi:10.1056/NEJMoa1003825
44. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi:10.1016/S0140-6736(06)69446-4
45. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi:10.1056/NEJMoa060655
46. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi:10.1056/NEJMoa0708857
47. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, Phase 3 trial. Lancet. 2014;384:319–328. doi:10.1016/S0140-6736(14)60421-9
48. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi:10.1056/NEJMoa1303989
49. Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 2013;14:1287–1294. doi:10.1016/S1470-2045(13)70465-0
50. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi:10.1016/S0140-6736(12)61857-1
51. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi:10.1016/S0140-6736(12)61900-X
52. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi:10.1016/S0140-6736(16)32453-9
53. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–1823. doi:10.1056/NEJMoa1510016
54. Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. doi:10.1200/JCO.2012.48.4659
55. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi:10.1056/NEJMoa1717002
56. Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi:10.1056/NEJMoa1406470
57. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi:10.1016/S0140-6736(18)30207-1
58. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, Phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482. doi:10.1016/S1470-2045(15)00290-9
59. Ni J, Zhang L. Cancer cachexia: definition, staging, and emerging treatments. Cancer Manag Res. 2020;12:5597–5605. doi:10.2147/CMAR.S261585
60. Fonseca GWPD, Farkas J, Dora E, von Haehling S, Lainscak M. Cancer cachexia and related metabolic dysfunction. Int J Mol Sci. 2020;21:2321. doi:10.3390/ijms21072321
61. Oakvik J, Ready D. Updates in cancer-related symptom management of anorexia and cachexia syndrome. Semin Oncol Nurs. 2022;38:151254. doi:10.1016/j.soncn.2022.151254
62. Roeland EJ, Bohlke K, Baracos VE, et al. Management of cancer cachexia: ASCO guideline. J Clin Oncol. 2020;38:2438–2453. doi:10.1200/JCO.20.00611
63. Chambers MS, Rugo HS, Litton JK, Meiller TF. Stomatitis associated with mammalian target of rapamycin inhibition: a review of pathogenesis, prevention, treatment, and clinical implications for oral practice in metastatic breast cancer. J Am Dent Assoc. 2018;149:291–298. doi:10.1016/j.adaj.2017.10.024
64. Shono H, Tsutsumi R, Beppu K, et al. Dietary supplementation with monosodium glutamate suppresses chemotherapy-induced downregulation of the T1R3 taste receptor subunit in head and neck cancer patients. Nutrients. 2021;13:2921. doi:10.3390/nu13092921
65. Pulito C, Cristaudo A, Porta C, et al. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res. 2020;39:210. doi:10.1186/s13046-020-01715-7
66. Tang G, Huang W, Zhang L, Wei Z. Role of glutamine in the management of oral mucositis in patients with cancer: a meta-analysis of randomized controlled trials. Nutr Cancer. 2022;74:482–495. doi:10.1080/01635581.2021.1889623
67. Peng TR, Lin HH, Yang LJ, Wu TW. Effectiveness of glutamine in the management of oral mucositis in cancer patients: a meta-analysis of randomized controlled trials. Support Care Cancer. 2021;29:4885–4892. doi:10.1007/s00520-021-06060-9
68. Shuai T, Tian X, Xu LL, et al. Oral glutamine may have no clinical benefits to prevent radiation-induced oral mucositis in adult patients with head and neck cancer: a meta-analysis of randomized controlled trials. Front Nutr. 2020;7:49. doi:10.3389/fnut.2020.00049
69. Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Marti S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;2013(3):CD004310. doi:10.1002/14651858.CD004310.pub3
70. Sandhya L, Devi Sreenivasan N, Goenka L, et al. Randomized double-blind placebo-controlled study of olanzapine for chemotherapy-related anorexia in patients with locally advanced or metastatic gastric, hepatopancreaticobiliary, and lung Cancer. J Clin Oncol. 2023;41:2617–2627.
71. Celichowska M, Miedziaszczyk M, Lacka K. Pharmacotherapy in cachexia: a review of endocrine abnormalities and steroid pharmacotherapy. J Pain Palliat Care Pharmacother. 2022;36:117–131. doi:10.1080/15360288.2022.2063469
72. Mantovani G, Macciò A, Massa E, Madeddu C. Managing cancer-related anorexia/cachexia. Drugs. 2001;61:499–514. doi:10.2165/00003495-200161040-00004
73. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39:2216–2229. doi:10.1016/j.clinthera.2017.09.011
74. Wang Y, Yang M, Tao M, et al. Corticosteroid administration for cancer-related indications is an unfavorable prognostic factor in solid cancer patients receiving immune checkpoint inhibitor treatment. Int Immunopharmacol. 2021;99:108031. doi:10.1016/j.intimp.2021.108031
75. Drakaki A, Dhillon PK, Wakelee H, et al. Association of baseline systemic corticosteroid use with overall survival and time to next treatment in patients receiving immune checkpoint inhibitor therapy in real-world US oncology practice for advanced non-small cell lung cancer, melanoma, or urothelial carcinoma. Oncoimmunology. 2020;9:1824645. doi:10.1080/2162402X.2020.1824645
76. Skribek M, Rounis K, Afshar S, et al. Effect of corticosteroids on the outcome of patients with advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Eur J Cancer. 2021;145:245–254. doi:10.1016/j.ejca.2020.12.012
77. Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12:14–16. doi:10.1002/jcsm.12675
78. Bai Y, Hu Y, Zhao Y, et al. Anamorelin for cancer anorexia-cachexia syndrome: a systematic review and meta-analysis. Support Care Cancer. 2017;25:1651–1659. doi:10.1007/s00520-016-3560-0
79. Nishie K, Yamamoto S, Nagata C, Koizumi T, Hanaoka M. Anamorelin for advanced non-small-cell lung cancer with cachexia: systematic review and meta-analysis. Lung Cancer. 2017;112:25–34. doi:10.1016/j.lungcan.2017.07.023
80. Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519–531. doi:10.1016/S1470-2045(15)00558-6
81. Currow D, Temel JS, Abernethy A, Milanowski J, Friend J, Fearon KC. ROMANA 3: a phase 3 safety extension study of anamorelin in advanced non-small-cell lung cancer (NSCLC) patients with cachexia. Ann Oncol. 2017;28:1949–1956. doi:10.1093/annonc/mdx192
82. Katakami N, Uchino J, Yokoyama T, et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer. 2018;124:606–616. doi:10.1002/cncr.31128
83. Hamauchi S, Furuse J, Takano T, et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer. 2019;125:4294–4302. doi:10.1002/cncr.32406
84. Yennurajalingam S, Basen-Engquist K, Reuben JM, et al. Anamorelin combined with physical activity, and nutritional counseling for cancer-related fatigue: a preliminary study. Support Care Cancer. 2022;30:497–509. doi:10.1007/s00520-021-06463-8
85. Ligibel JA, Bohlke K, May AM, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40:2491–2507. doi:10.1200/JCO.22.00687
86. Grande AJ, Silva V, Sawaris Neto L, Teixeira Basmage JP, Peccin MS, Maddocks M. Exercise for cancer cachexia in adults. Cochrane Database Syst Rev. 2021;3:CD010804. doi:10.1002/14651858.CD010804.pub3
87. Hall CC, Cook J, Maddocks M, Skipworth RJE, Fallon M, Laird BJ. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: a systematic review. Support Care Cancer. 2019;27:2371–2384. doi:10.1007/s00520-019-04749-6
88. Uster A, Ruehlin M, Mey S, et al. Effects of nutrition and physical exercise intervention in palliative cancer patients: a randomized controlled trial. Clin Nutr. 2018;37:1202–1209. doi:10.1016/j.clnu.2017.05.027
89. Solheim TS, Laird BJA, Balstad TR, et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:778–788. doi:10.1002/jcsm.12201
90. Solheim TS, Laird BJA, Balstad TR, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care. 2018;8:258–265. doi:10.1136/bmjspcare-2017-001440
91. Tobberup R, Carus A, Rasmussen HH, et al. Feasibility of a multimodal intervention on malnutrition in patients with lung cancer during primary anti-neoplastic treatment. Clin Nutr. 2021;40:525–533. doi:10.1016/j.clnu.2020.05.050
92. Miura S, Naito T, Mitsunaga S, et al. A randomized phase II study of nutritional and exercise treatment for elderly patients with advanced non-small cell lung or pancreatic cancer: the NEXTAC-TWO study protocol. BMC Cancer. 2019;19:528. doi:10.1186/s12885-019-5762-6
93. Vaughan VC, Martin P. Multidisciplinary approaches to cancer cachexia: current service models and future perspectives. Expert Rev Anticancer Ther. 2022;22:737–749. doi:10.1080/14737140.2022.2088516
94. Parmar MP, Vanderbyl BL, Kanbalian M, Windholz TY, Tran AT, Jagoe RT. A multidisciplinary rehabilitation programme for cancer cachexia improves quality of life. BMJ Support Palliat Care. 2017;7:441–449. doi:10.1136/bmjspcare-2017-001382
95. Bland KA, Harrison M, Zopf EM, et al. Quality of life and symptom burden improve in patients attending a multidisciplinary clinical service for cancer cachexia: a retrospective observational review. J Pain Symptom Manage. 2021;62:e164–e176. doi:10.1016/j.jpainsymman.2021.02.034
96. Vaughan VC, Farrell H, Lewandowski PA, McCoombe SG, Martin P. Defining a new model of interdisciplinary cancer cachexia care in regional Victoria, Australia. Support Care Cancer. 2020;28:3041–3049. doi:10.1007/s00520-019-05072-w
97. Lu Z, Fang Y, Liu C, et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39:748–756. doi:10.1200/JCO.20.01254
98. Ray H, Beaumont A, Loeliger J, et al. Implementation of a multidisciplinary allied health optimisation clinic for cancer patients with complex needs. J Clin Med. 2020;9:2431. doi:10.3390/jcm9082431
99. Zhao Y, Pang D, Lu Y. The role of nurse in the multidisciplinary management of cancer cachexia. Asia Pac J Oncol Nurs. 2021;8:487–497. doi:10.4103/apjon.apjon-2123
100. Sato R, Hayashi N, Nakayama N, Okimura A. Factors affecting the assessment of cancer cachexia by nurses caring for patients with advanced cancer undergoing chemotherapy: a cross-sectional survey. Asia Pac J Oncol Nurs. 2022;9:100075. doi:10.1016/j.apjon.2022.100075
101. Sato R, Naito T, Hayashi N. Barriers in nursing practice in cancer cachexia: a scoping review. Asia Pac J Oncol Nurs. 2021;8:498–507. doi:10.4103/apjon.apjon-2152
102. Tanaka K, Nakamura S, Narimatsu H. Nutritional approach to cancer cachexia: a proposal for dietitians. Nutrients. 2022;14:345. doi:10.3390/nu14020345
103. van der Meij BS, Teleni L, McCarthy AL, Isenring EA. Cancer cachexia: an overview of diagnostic criteria and therapeutic approaches for the accredited practicing dietitian. J Hum Nutr Diet. 2021;34:243–254. doi:10.1111/jhn.12811
104. Johnson SB, Park HS, Gross CP, Yu JB. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4:1375–1381. doi:10.1001/jamaoncol.2018.2487
105. Johnson SB, Park HS, Gross CP, Yu JB. Use of alternative medicine for cancer and Its impact on survival. J Natl Cancer Inst. 2018;110:121–124.
106. Greenlee H, Neugut AI, Falci L, et al. Association between complementary and alternative medicine use and breast cancer chemotherapy initiation: the Breast Cancer Quality of Care (BQUAL) study. JAMA Oncol. 2016;2:1170–1176. doi:10.1001/jamaoncol.2016.0685
107. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi:10.1016/j.clnu.2016.07.015
108. Wei L, Wu Z, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids-an update. Cancer Lett. 2022;526:193–204. doi:10.1016/j.canlet.2021.11.023
109. de van der Schueren MAE, Laviano A, Blanchard H, Jourdan M, Arends J, Baracos VE. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol. 2018;29:1141–1153. doi:10.1093/annonc/mdy114
110. Braha A, Albai A, Timar B, et al. Nutritional interventions to improve cachexia outcomes in cancer-a systematic review. Medicina. 2022;58:966. doi:10.3390/medicina58070966
111. de Castro GS, Andrade MF, Pinto FCS, Faiad JZ, Seelaender M. Omega-3 fatty acid supplementation and its impact on systemic inflammation and body weight in patients with cancer cachexia-a systematic review and meta-analysis. Front Nutr. 2022;8:797513. doi:10.3389/fnut.2021.797513
112. Ukovic B, Porter J. Nutrition interventions to improve the appetite of adults undergoing cancer treatment: a systematic review. Support Care Cancer. 2020;28:4575–4583. doi:10.1007/s00520-020-05475-0
113. Salama S, Shou Q, Abd El-Wahed AA, et al. Royal jelly: beneficial properties and synergistic effects with chemotherapeutic drugs with particular emphasis in anticancer strategies. Nutrients. 2022;14:4166. doi:10.3390/nu14194166
114. Miyata Y, Sakai H. Anti-cancer and protective effects of royal jelly for therapy-induced toxicities in malignancies. Int J Mol Sci. 2018;19:3270. doi:10.3390/ijms19103270
115. Nainu F, Masyita A, Bahar MA, et al. Pharmaceutical prospects of bee products: special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics. 2021;10:822. doi:10.3390/antibiotics10070822
116. Yuno T, Miyata Y, Mukae Y, et al. Mechanisms underlying the inhibition of tyrosine kinase inhibitor-induced anorexia and fatigue by royal jelly in renal cell carcinoma patients and the correlation between macrophage colony stimulating factor and inflammatory mediators. Med Sci. 2020;8:43.
117. El-Seedi HR, Eid N, Abd El-Wahed AA, et al. Honey bee products: preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front Nutr. 2022;8:761267. doi:10.3389/fnut.2021.761267
118. Miyata Y, Araki K, Ohba K, et al. Oral intake of royal jelly improves anti-cancer effects and suppresses adverse events of molecular targeted therapy by regulating TNF-α and TGF-β in renal cell carcinoma: a preliminary study based on a randomized double-blind clinical trial. Mol Clin Oncol. 2020;13:29. doi:10.3892/mco.2020.2148
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.