Back to Journals » Infection and Drug Resistance » Volume 16
The Impact of the COVID-19 Pandemic on Respiratory Syncytial Virus Infection: A Narrative Review
Authors Chuang YC , Lin KP, Wang LA, Yeh TK , Liu PY
Received 6 November 2022
Accepted for publication 12 January 2023
Published 30 January 2023 Volume 2023:16 Pages 661—675
DOI https://doi.org/10.2147/IDR.S396434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yu-Chuan Chuang,1 Kuan-Pei Lin,1 Li-An Wang,1 Ting-Kuang Yeh,2,3 Po-Yu Liu2– 6
1Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 2Division of Infectious Disease, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; 3Genomic Center for Infectious Diseases, Taichung Veterans General Hospital, Taichung, Taiwan; 4Ph.D. in Translational Medicine, National Chung Hsing University, Taichung, Taiwan; 5Rong Hsing Research Center for Translational Medicine, National Chung Hsing University, Taichung, Taiwan; 6Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
Correspondence: Po-Yu Liu, Division of Infectious Disease, Department of Internal Medicine, Taichung Veterans General Hospital, No. 1650, Sec. 4, Taiwan Blvd., Xitun Dist, Taichung City, 407219, Taiwan, Tel +886 4 2359 2525, Email [email protected]
Abstract: Respiratory syncytial virus (RSV) is one of the most common respiratory viruses. It not only affects young children but also the elderly and immunocompromised patients. After the emergence of SARS-CoV-2 and the corona virus disease 2019 (COVID-19) era, a dramatic reduction in RSV activity was found, which coincided with the implementation of public health and social measures (PHSMs). However, the correlation is more complicated than we initially thought. After PHSMs were gradually lifted, a seasonality shift and a delayed RSV outbreak with greater number of infected patients were found in numerous countries, such as Israel, Australia, South Africa, New Zealand, France, United States, and Japan. Several hypotheses and possible reasons explaining the interaction between SARS-CoV-2 and RSV were mentioned. Since RSV vaccinations are still under investigation, administration of palivizumab should be considered in high-risk patients. In the post-COVID-19 era, greater attention should be paid to a further resurgence of RSV. In this narrative review, we conducted a thorough review of the current knowledge on the epidemiology of RSV during the COVID-19 era, the out-of-season outbreak of RSV, and the data on co-infection with RSV and SARS-CoV-2.
Keywords: COVID-19 pandemic, SARS-CoV-2, respiratory syncytial virus, influenza virus, respiratory tract infection
Introduction
RSV belongs to the Pneumoviridae family, which is a single-stranded and negative-sense RNA virus. RSV can be grouped according to two main antigens, known as A and B subtypes.1 Respiratory syncytial virus (RSV) is one of the most common respiratory viruses. It not only affects young children but also the elderly and immunocompromised patients. The elderly, patients with particular comorbidities, such as chronic lung disease, those who have undergone transplantation, and immunocompromised patient are more vulnerable to severe RSV infection.2 In temperate countries, RSV is predominant in winter. In comparison, outbreaks of RSV may be observed during cooler months or wet months in tropical and sub-tropical countries.3,4 RSV infection has become a major public healthcare issue, which has caused significant hospitalization and mortality rates, partially due to the lack of vaccination against RSV.5 During the corona virus disease 2019 (COVID-19) era, clinicians and researchers have noted similarities and differences between RSV and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The COVID-19 era has seen the widespread implementation of public health and social measures (PHSMs), which include personal protective measures, such as mask-wearing, hand hygiene, and social distancing, environmental measures, and surveillance and response measures.6 PHSMs play an indispensable role against SARS-CoV-2 transmission. The implemented PHSMs have simultaneously had an enormous impact on other respiratory viral diseases. Many studies have shown a remarkable decrease in the incidence of RSV and other common respiratory pathogens during the COVID-19 era.7 However, while it appears that there is a simple direct correlation, the true nature of the relationship may be more complicated. When PHSMs were gradually lifted, a seasonality shift and a delayed RSV outbreak with greater number of infected patients were found in a number of countries, such as Israel, Australia, South Africa, New Zealand, France, United States, and Japan.8,9 Delayed outbreaks also caused a higher hospitalization rate, even though the clinical symptoms seemed to be milder.10 Therefore, it is important to pay attention to and prepare well for the possibility of further out-of-season RSV peaks.9 The symptoms caused by SARS-CoV-2 may be difficult to differentiate from those of other common respiratory viruses. Since the detection of these viruses might provide potentially life-saving treatments in certain circumstances, commercial multiplex PCR assays may aid the physician in reaching a diagnosis. Research aimed at developing RSV vaccinations are currently underway, although there are still a great challenges to overcome.11 As such, administration of palivizumab should be considered in patients with higher risk of developing severe clinical symptoms and fatality.12 During the post-COVID-19 era, it is vital to recognize the clinical importance of RSV infection as well as other common upper respiratory viral infections. In this narrative review, we conducted a thorough review of the current knowledge on the epidemiology of RSV during the COVID-19 era, out-of-season outbreaks of RSV, and data on co-infections of RSV and SARS-CoV-2.
Materials and Methods
We organized published articles and current evidence on the epidemiology of RSV during the COVID-19 pandemic in comparison to the pre-COVID-19 era. This is a narrative review by performing a thorough literature search using PubMed, Embase, and Google Scholar. For the bibliographic search, MeSH terminology was used with the following search strategy: [((SARS-CoV-2[MeSH Terms]) OR (COVID-19[MeSH Terms])) AND (Respiratory Syncytial Virus [MeSH Terms])]. The searches were not restricted by date. All retrieved records (including original articles, letters to the editor, editorials, and case reports) in English and records with English translation were downloaded and evaluated. Subsequently, the abstracts were evaluated for eligibility. Articles resulting from these searches and relevant references cited in those articles were reviewed. All duplicates were eliminated. Finally, all of the articles were analyzed. The searches were not restricted by date; however, most of the studies on RSV during the COVID-19 era are still ongoing, time constraint is the major limitation to our study. The figure in this study was created with MapChart (available at mapchart.net).13
Results and Discussion
Epidemiology
Before the emergence of SARS-CoV-2, Tin Tin Htar et al had conducted a systematic review and meta-analysis showing that the incidence rates of hospitalization due to RSV-related respiratory infection was 7 to 13 cases in every 10,000 people in Asia and Africa and 190 to 254 in every 10,000 people in the USA (United States of America). Furthermore, people older than 50 years old had much higher incidence rates, from 195 up to 1790 cases in every 10,000 people in the USA.2 However, a considerable decrease in RSV incidence and hospitalization rate were found during the COVID-19 era. During the COVID-19 pandemic, while strict PHSMs were implemented and RSV was found to have decreased in prevalence worldwide. Moreover, it had almost disappeared in several countries and regions. The overall RSV activity after the emergence of SARS-CoV-2 is presented in Figure 1.
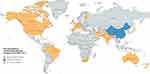 |
Figure 1 Regions with a decreased RSV infection rate during the COVID-19 pandemic. Notes: Created with mapchart.net.13 |
In the USA, before the COVID-19 pandemic, RSV accounted for 5.4% in all detected positive specimens of respiratory pathogens during December 2019 to March 2020. However, after PHSMs were implemented, the RSV positive rate dramatically dropped to 0.03% during December 2020 to March 2021. Nevertheless, a delayed surge of RSV was found from April 2021 to October 2021.14 In New York City, before the COVID-19 era, RSV was the third most commonly detected respiratory virus. During the outbreak of COVID-19, Yuan et al observed a significant 86% reduction in the cumulative incidence of RSV.15 Another study conducted in New York City also revealed a similar result. During the 2020–2021 RSV season, which was from November to April, the total cases of RSV positive rate in a pediatric emergency department had dramatically decreased from 1162 cases in the 2019–2020 season to 143 cases.16 In rural Alaska, there was a dramatic decrease in RSV-related hospitalizations, which coincided with the timing of the implementation of PHSMs due to the COVID-19 pandemic.17 Groves et al analyzed data from the Canadian Respiratory Virus Detection Surveillance System. They found a dramatic reduction in the average weekly RSV positive rate. It was 5.96% before the COVID-19 pandemic; however, it dropped to 0.047% during the 2020–2021 season.18 Doroshenko et al also revealed a similar reduction in RSV infections beginning in July 2020, following the implementation of PHSMs in Canada.19 In South America, in a Brazilian cohort investigated by Varela et al, no RSV was detected from May to August 2020 via RT-PCR (real-time polymerase-chain reaction) test. Meanwhile, SARS-CoV-2 was detected in 32.7% of the patients.20 In Puerto Rico, the RSV test-positivity rate was 4% during 2012 to 2018. However, during the COVID-19 pandemic and the period when PHSMs were implemented, no cases of RSV were detected.21
In Europe, several countries, such as Austria, found a significant reduction of RSV cases beginning in March 2020, the start of COVID-19 pandemic.22 In the United Kingdom (UK), Bardsley et al extracted national data on RSV-associated disease from 2014 to March 2022. The analysis showed a dramatic reduction of RSV activity in the 2020–2021 winter season, with an estimated relative reduction of 99.6% in RSV test positivity. The observed RSV test positivity rate was only 0.1%, as well with 7 cases testing positive among 7094 tests. Since winter was typically the peak season for RSV during the pre-COVID-19 period, the 2020–2021 winter seemed to be unusual.23 An enormous reduction in RSV cases of up to 99% was observed in Belgium in 2020 during the RSV season.24 In Italy, the RSV detection rate was 99% lower during 2020 to 2021, compared to the previous two seasons, and the rate of RSV in hospitalized children decreased from 38.1% to 4.7% in 2020–2021.25,26 In Spain, there was a reduction of up to 86% in RSV-related disease during the RSV peak season in 2020–2021.27 Moreover, a reduction of RSV infection number was found in a pediatric emergency department. The numbers of cases of RSV infection in patients younger than 2 years old totalled around 6700 to 8600 for the period 2016 to 2019. During the COVID-19 pandemic, RSV cases dropped to 2489 cases in 2020.28 Another study in a pediatric emergency department in Spain also showed a dramatic reduction in RSV infections with only 21 cases detected in the 2020 winter.29 As for France, although the RSV peak season showed a delay, the number of RSV cases was generally than in the 2020–2021 season.30 Moreover, no patients admitted during April to November 2020 had an RSV-related infection in a tertiary hospital in Paris, France.10 In Germany, similarly, the RSV positivity rate dropped from 10–21% to 0% in the 2020/21 season until the end of March 2021.31,32 In Nordic countries, RSV has a biennial epidemiological pattern. In Finland, the number of cases of RSV infection declined rapidly in the beginning of the COVID-19 pandemic in March 2020, which was expected to be a high-incidence year.33 Moreover, RSV occurrence was significantly lower compared to the occurrence in low-incidence years during the COVID-19 pandemic between August 2020 and January 2021. This was possibly owing to implementation of PHSMs.34
In the southern hemisphere, a similar pattern was observed. The detection rate of RSV infection was decreased by up to 98% in different regions in Australia as well in winter 2020, compared to 2015 to 2019.35–37 The low RSV detection in 2020 included both subtype A and B.38 In South Africa, RSV was detected in 4.1% of outpatients and 10.5% of inpatients in 2020. Compared to 2013–2019, there was an infection rate of 6% in outpatients and 15.4% in in patients.39 A children’s hospital in New Zealand revealed that RSV-positive PCR cases reduced from 200 to 300 cases annually to 2 cases in the 2022 season.40 Another study using multiple national surveillance systems found a 98% reduction of detected RSV in 2020 compared to 2015–2019.41
In Asia, investigators in Japan found an association between implementation of PHSMs and the decrease in RSV cases. There was not only a decrease in pediatric hospitalized patients, but also a significant drop in RSV. The number of cases admitted due to RSV had dropped from 4 cases per month to 0 cases per month in Hokkaido, Japan.42 Moreover, Wagatsuma performed a surveillance study of data using a national database, which revealed an approximately 85% reduction in monthly RSV average cases in comparison to the preceding 6 years. This might also be evidence that PHSMs potentially curb the transmission of RSV.43 Similarly, in South Korea, the RSV weekly positive rate had 81% and 91% reduction in the outpatients and inpatients, respectively, during 2020 to 2021 winter.44 Other studies in South Korea showed a fall in RSV monthly incidence rates of up to 99% in 2020.45 During the COVID-19 era, Taiwan found a significantly lower number of RSV cases as well in the RSV peak reason.46 Chiu et al compared the incidence rates of RSV in children during February 2020 to January 2021 with January 2016 to 2020 in Hong Kong. They found a 98% relative reduction in RSV.47 In western region of India, the positivity rate during January 2017, to March 2020, was 7.6%, which significantly reduced to 0.6% between March 2020 and December 2020.48 In Pakistan, the impact due to COVID-19 and the implemented PHSMs reduced RSV cases by up to 93%.49 In Iran, Letafati et al showed no detected RSV infection in children under 5 years old between March and May of 2021, which are typically cold months during the year. Compared to the pre-COVID-19 RSV rate of 24% in acute respiratory hospitalized patient, RSV disappeared during the period of COVID-19 pandemic.50 Table 1 summarizes the aforementioned major findings from articles focusing on the RSV activity during the COVID-19 era in different countries and region.
 |
Table 1 Summary of Major Findings from Articles Focusing on RSV Activity During the COVID-19 Era in Different Countries and Regions |
Although most countries and regions revealed a significant reduction of RSV infection after the outbreak of COVID-19 pandemic, China showed conflicting data. In Zhejiang, China, RSV positive cases in children during COVID-19 outbreak from February to April in 2020 showed a decrease compared to 2018 and 2019.51 However, in Hangzhou, China, Du et al evaluated the RSV positivity rate before and during the COVID-19 pandemic, ie, October 2017 to February 2021. Compared with the season before 2020, no obvious reduction was found. The RSV positivity rate remained high, although the number of samples was lower than in the previous season.52 In another study conducted by Li et al there was even a prominent increase in the RSV infection rate from 6.6% to 20.1%, during September to December in 2019 and 2020, respectively. There were 2298 cases in 2019 and 3398 in 2020.53 Ye et al also demonstrated an increase in the positive rate of RSV in 2020, which was 9.35%, compared to 6.31% in 2019.54 Nevertheless, in Shanghai, China, there was an obvious drop in RSV infections during the COVID-19 pandemic and school closures, during the period from February to May in 2020.55 Overall, the conflicting data may have been partly due to China’s large geographic size. As the world’s third largest country, China spans five geographical time zones, and therefore, there might have been regional differences and relatively wide range of RSV activity. Another possible reason is that proper wearing of masks is more difficult in children under 2 years old and there is greater susceptibility to RSV infection in children younger than 2 years old.52,53
Overall, the most important reason for such a significant reduction of RSV during the COVID-19 pandemic is likely the implementation of PHSMs. Since the beginning of the SARS-CoV-2 outbreak, governments have paid considerable attention to these measures, which include personal protective measures, such as facial coverings, mask-wearing, hand hygiene, social distancing, environmental measures, and surveillance and response measures.6 Moreover, border controls of many countries as well as mandatory quarantine after arrival all limited the viral transmission among countries.40 It seems highly probable that these measures played an essential role protecting against SARS-CoV-2 transmission. The implemented PHSMs simultaneously appeared to have an enormous impact on the transmission of RSV.14,38 Another reason for this finding is the hypothesized competition among respiratory viruses.20 In the initial outbreak of the COVID-19 pandemic, Leuzinger et al observed the fact that SARS-CoV-2 had quickly and nearly completely replaced other common seasonal respiratory viruses.56 The phenomenon of competition and viral interference between SARS-CoV-2 and RSV has been described and further supported by an Italian multicenter study. Nenna et al observed a resurgence of RSV in the fall with a major peak in November 2021; meanwhile, the SARS-CoV-2 activity was relatively low. In contrast, when the outbreak of the Omicron variant of SARS-CoV-2 occurred, RSV saw a sharp reduction immediately.57 However, some might question this hypothesis because several studies showed a substantial rate of co-infection with SARS-CoV-2 and other respiratory viruses.58 Some experts argued that the reduction of RSV cases was in part because of the decrease in the sample number and hospital visits during the COVID-19 pandemic.59 Nevertheless, another study disproved this possibility because the number being tested in 2020 was as twice that observed than in previous years.38 Bardsley et al has also demonstrated the reduction of laboratory testing could not explain the decrease in RSV since the RSV positivity rate was also reduced by a similar order of magnitude.23 In sum, the implementation of PHSMs appears to be the most commonly mentioned factor associated with the reduction of RSV activity during the beginning of the COVID-19 era, but other factors also seemed to play an important role.
Out-of-Season Epidemic and Delayed Surge of RSV
During the COVID-19 era, the interaction between RSV and SARS-CoV-2 has been widely discussed. In 2009 influenza pandemic, RSV showed a seasonal delay of up to 2.5 months, compared with the previous RSV season. A viral interference effect and the implementation of non-pharmaceutical interventions have been postulated to explain these findings.60 Recent data have identified a seasonal change in RSV circulation in both northern and southern hemispheres.3 The resurgence of RSV after the lightning of PHSMs is a major concern that has been raised by many experts. Lina et al developed a model simulating the RSV season after the end of PHSMs in 2022–2023 in Tokyo, Japan. The result showed a possible severe outbreak partly because of the increased size of populations that are susceptible to RSV during the period of PHSM implementation for COVID-19.61 Similarly, Baker et al in the US also reported this concern using an epidemic model.62 In fact, the resurgence of RSV infection had already been found in Australia during January to March 2021.63 Moreover, since the start of 2021 summer in the northern hemisphere, an unusual circulation of RSV was observed in the USA by the New Vaccine Surveillance Network (NVSN).64 A study in Western Australia found an unusual RSV resurgence in late 2020. In patients admitted due to respiratory tract admission, the RSV positive rate was relatively low during May to September 2020. However, during November and December 2020, which is summer in the southern hemisphere, Foley et al found RSV positive rate of 81.7%.65 In addition, a similar interseasonal increase in RSV cases was reported in other studies, in regions such as in New South Wales, Australia, and New York, USA.66,67
In Europe, an unexpected resurgence of RSV was detected in summer 2021 in the UK. Moreover, the number of reported cases was even greater than in the pre-COVID-19 era.23 An interseasonal surge of RSV infection was found during the first half of 2021 in Switzerland. Hammerstein et al reported a shift in the usual RSV season in both infants and children, which coincided with the lifting of PHSMs and a local decline in COVID-19 cases.68 A change in RSV seasonality was also found in Germany. A dramatic decrease of RSV cases was observed in winter during 2020 to 2021. However, once the requirements for PHSMs were officially lifted, a delayed RSV epidemic occurred.69 Southern Italy also suffered a seasonal shift of RSV epidemic beginning in August 2021. Moreover, a delayed peak with increasing RSV cases was also noted in November 2021.70 An early RSV season was detected in Finland after the lifting of PHSMs in September 2021.71 A seasonality shift was observed in Portugal as well. The RSV peak season used to occur during December and January, however, a significant delay in the RSV peak season to July and August was documented in 2021.72 In France, a late RSV outbreak was found in the 2020–2021 season. The RSV season used to begin in the fall, around October. However, it was not until December 2020 that the RSV epidemic began, peaking between February and March 2021.10 In Italy, no RSV specimens were detected in 2020. However, there was a surge in RSV in the fall of 2021. In those positive nasopharyngeal specimens, RSV account for 14.5%, which was only less than SARS-CoV-2 of 25.6%.73
In eastern Asia, an unusual outbreak of RSV infection with a greater magnitude than in the pre-COVID-19 season occurred in Tokyo, Japan in the spring of 2021. This outbreak was associated with the return to school.74 In Shanghai, China, the RSV positivity rate in the post-COVID-19 period, from June 2020 to January 2021 increased. The resurgence was also accompanied by a seasonal shift, with RSV being detected during the whole post-COVID-19 period and peaking in the summer.55 As in Taiwan, the usual peak RSV season is in spring and fall. However, in the 2020–2021 season, Lee et al noted a delay in the RSV season, which occurred in the winter of 2020. The delay was mainly related to the COVID-19 mitigation measures.46 In Iran, the first RSV outbreak in 2022 among children less than 5 years old occurred after the emergence of SARS-CoV-2. A local children’s hospital in southwest Iran reported that as many as 53% of children admitted due to acute respiratory infection suffered from RSV.75
Several factors have been reported described to be responsible for the seasonality change in RSV and its resurgence. First, PHSMs were not as strictly enforced as at the beginning of COVID-19 pandemic. As the COVID-19 pandemic wore on, requirements for PHSMs were loosened; children could return to schools and day care centers, and restrictions on social gatherings were lifted.10 Among all of the PHSMs, a thorough analysis of RSV surveillance data from 11 countries revealed a strong association of school reopenings and lifting of stay-at-home requirements with increased RSV activity.76 Moreover, the role of adults in RSV transmission seems to be more important than previously thought. During the COVID-19 era, PHSMs were mainly aimed at older children and adults, partly because of the difficulty of strictly implementing these measures in infants, toddlers, and younger children. The resurgence of RSV was associated with the lifting of PHSMs and the reopening of borders rather than the opening of childcare facilities. This result has given rise to speculation that it was the change in daily habits in adult is actually affecting RSV activity.77 Second, decreasing immunity in older children and adults to RSV is another prominent factor. Due to the COVID-19 era, there was a RSV‐naïve infant cohort that possessed increased susceptibility to RSV.30,78 One of the reasons for this concern was a phenomenon termed “immunity debt”. Before the COVID-19 pandemic, most children less than 2 years old would become infected with RSV sooner or later and then develop a certain level of immunity against severe disease.79 It has become apparent that because of the significant decrease of RSV activity since the emergence of SARS-CoV-2, there is now a cohort of children who are more susceptible to severe RSV infection due to their immunologically naïve status.80 In fact, entire populations, especially those who are younger than 2 years old, lack immune stimulation because of the reduction of RSV circulation.81 This might result in very young children having greater susceptibility to RSV as well as a decrease in adult immunity.3 The hypothesis of waning immunity has been further supported by Reicherz et al. Serum prefusion RSV F protein IgG level and RSV neutralization titers were examined in women of reproductive age and in infants before and during the COVID-19 pandemic. The results showed a significant reduction of RSV antibody levels after the emergence of SARS-CoV-2.82 Furthermore, Foley et al also found the median patient age during the RSV resurgence was 18.4 months, which was significantly higher than in the previous season, which ranged from 7.3 to 12.5 months.66 The “immunity debt” might raise the risk to the whole population of a serious outbreak and potentially more severe RSV infection.83 Moreover, household transmission was shown, to some degree, to be partially responsible for the RSV surge.84 Other possible factors include the reopening of travel between countries and climatic factors.30,78 Importantly, the RSV resurgence was not associated with a novel RSV strain. A study conducted in Greece in 2021–2022 reported a sudden RSV outbreak, which was caused by both RSV subtype A and B. An analysis revealed that the genotypes were the same as in the pre-COVID-19 season.85 This suggests the possibility of a novel strain-related outbreak is low.
Although the implementation of PHSMs disrupted the transmission of RSV, it is unlikely that they completely eliminated infections. An interseasonal shift of the RSV peak season has already been found. Therefore, we should pay more attention to a resurgence of RSV in the near future with the possibility of a more severe outbreak and impact.86 Moreover, an out-of-season RSV outbreak might make preventive RSV screening on the ward a potential measure for physicians to detect positive cases earlier. Once a case of RSV has been noted, quarantine could stop transmission between high-risk patients, such as those with hematological disease. Considering the potentially serious outcomes in such patients, a further strategy on RSV prevention should be reviewed and investigated.87 Clinicians should be aware of the possibility of future outbreaks of a more serious RSV epidemic following the easing of strict PHSMs. Surveillance of certain common respiratory infections, especially RSV, will be important as we enter the post-COVID-19 era.79
SARS-CoV-2 Co-Infection with Respiratory Syncytial Virus
Physicians have raised concerns about co-infection with SARS-CoV-2 and RSV. Although a resurgence of RSV was noted in the post-COVID-19 era, the rate of co-infection with SARS-CoV-2 and RSV was substantially lower than expected. During the COVID-19 pandemic in the USA, Uhteg et al found only two patients had co-infection. One had SARS-CoV-2 and enterovirus/rhinovirus and the other was co-infected with enterovirus/rhinovirus and adenovirus. In sum, the co-infection rate of SARS-CoV-2 was only 0.8% between December 2019 and October 2021 in the study.14 A retrospective review in the USA showed only 1.4% of cases were infected with SARS-CoV-2 and RSV simultaneously in 2021.88 In Italy, the co-infection rate of SARS-CoV-2 and other respiratory viruses was around 7.6% in all positive samples. None of these cases showed co-infection with SARS-CoV-2 and RSV.73 In another study conducted in Warsaw, Poland, no cases of co-infection with SARS-CoV-2 and RSV were found.89 In South Africa, no co-infection was found for either SARS-CoV-2 or RSV during March to October 2020.39 In the UK, the co-infection rate among SARS-CoV-2-positive patients with RSV between February 2020 and December 2021 was around 3.2%. Meanwhile, the co-infection rates of SARS-CoV-2 with influenza viruses and adenoviruses was 3.3% and 2.0%, respectively. Nevertheless, only co-infection of SARS-CoV-2 with influenza virus was associated with increased odds of mechanical ventilation use and in-hospital mortality.90 Cong et al conducted a systematic review on the impact of SARS-CoV-2 and RSV co-infection on mortality, which included 12 published studies. The review showed no obvious effect on the overall mortality; however, due to the lack of high-quality studies, the results revealed insufficient evidence. Therefore, further investigation on this topic is necessary.91
However, a high co-infection rate was found in several regions. In Brazil, Alvares et al conducted an observational retrospective study that included 32 children younger than 24 months of age who suffered from COVID-19 during March to September 2020. Among these patients, an RSV co-infection rate of 18.7% was observed. Although patients with co-infection did not have a high risk of intensive care unit admission, use of mechanical ventilation, or death, length of stay was longer.92 Hashemi et al observed an RSV co-infection rate of 9.7% in children who died from SARS-CoV-2 infection in Iran. However, the co-infection rate was as high as 22.3% in the cases with influenza virus.93
In contrast to patients infected with SARS-CoV-2, those with RSV were more likely to have bacterial co-infection.94 Clinicians should be aware of the substantial rate of co-infection with RSV and other respiratory viruses, especially SARS-CoV-2. Early recognition might alter the further treatment plan for such patients. In practice, it might be necessary to carefully detect all suspected respiratory pathogens, not only for accurate clinical diagnosis and management but also for further investigation of these respiratory viruses.
Diagnostic Approach for RSV in COVID-19 Era
Laboratory confirmation of RSV could assist in the clinical diagnosis of RSV infection. Evidence of an RSV infection should alert the physician to the possibility that further testing may be needed, especially for patients at high risk of developing severe illness or hospitalization, such as preterm birth, comorbidity of underlying lung disease, Down syndrome, immunocompromised patients, as well as the elderly with chronic pulmonary disease or functional disability.95–97 Nucleic acid amplification test is the preferred method, rather than antigen detection or viral culture. The specimen can be collected via nasopharyngeal swab of bronchoalveolar lavage fluid.98,99 When PHSMs were lifted, increasing cases of RSV as well as influenza viruses were detected, which led to increased testing demand to examine these viruses.14 The World Health Organization (WHO) has suggested testing other common respiratory viruses, which include influenza viruses and SARS-CoV-2, once RSV infection is suspected.100
Table 2 lists some of the currently available multiplex PCR assay that can detect SARS-CoV-2, RSV, and influenza virus simultaneously. Most of the listed PCR assays received emergency use authorization (EUA) by the United States Food and Drug Administration (FDA). The first EUA-approved multiplex PCR assays for detecting these viruses was the Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV Test, which has showed high accuracy and a good correlation with to standard-of-care tests.101–103 Both the NeuMoDx™ Flu A-B/RSV/SARSCoV-2 Vantage Assay and the Alinity m Resp-4-Plex have also provided high sensitivity and accuracy as well, which may aid physicians in performing diagnosis and differential diagnosis.104 The Allplex™ SARS-CoV-2/FluA/FluB/RSV Assay and the PowerChek™ SARS-CoV-2, Influenza A&B Multiplex Real-time PCR Kit are both commercially available tools for detecting SARS-CoV-2, RSV, and influenza virus simultaneously in Korea.105 In Australia, AusDiagnostics SARS-CoV-2, Influenza and the RSV 8-well assay have also shown efficacy in detecting these viruses at an intermediate concentration, which makes sample pooling a possible issue.106 Recently, the first non-prescription Labcorp’s Seasonal Respiratory Virus RT-PCR DTC test has received EUA approval. Patients can now use a home collection kit to collect nasal swab specimens themselves.107 The high sensitivity and accuracy of these PCR assays aid in the differentiation and detection of viruses in patients with a viral infection.108
 |
Table 2 Summary of the Currently Available Commercial Multiplex PCR Assay for Detecting SARS-CoV-2, Influenza Viruses, and RSV |
Treatments for Respiratory Syncytial Virus in COVID-19 Era
In general, respiratory syncytial virus infections are mainly treated with best supportive care. Special consideration should be made in immunocompromised patients, which included hematopoietic cell transplant recipients, lung transplant recipients, other solid organ transplant recipients, and patients with hematologic malignancy.109 There are three currently available pharmacotherapeutic agents benefit these patients, which includes palivizumab, immune globulin, and ribavirin.110 Ribavirin is the only approved antiviral drug for treating RSV infection by the United States FDA, albeit its effectiveness and expensiveness are major concern. Several small trials showed reduction in days of hospitalization and days of ventilation in infants and young children.111 Besides, a retrospective analysis included 23 haematopoietic stem cell transplant recipients with RSV-related respiratory tract infection has demonstrated that oral ribavirin use is associated with reduced morbidity and mortality.112 Similarly, a review on treating RSV infected adult HSCT recipients with ribavirin also revealed a lower mortality rate.113 However, ribavirin is contraindicated to pregnant females and during the 6 months prior to pregnancy.114 On the other hand, administration of intravenous immunoglobulin (IVIG) might be effective in immunocompromised patients suffered RSV infection.115 Prophylaxis of severe RSV illness with palivizumab, a humanized monoclonal antibody against RSV, is recommended to high-risk patients under 2 years of age.116 The use of palivizumab among adult, especially the elderly and immunocompromised patient, is lack of clinical evidence currently.117 Although the efficacy is uncertain, palivizumab is a well-tolerated and safe option among immunocompromised patients. It might be reasonable to treat immunocompromised patient with palivizumab.118,119 Since RSV seasonality has an obvious change after the emergence of SARS-CoV-2, the American Academy of Pediatrics (AAP) supported the administration of palivizumab in those eligible patients. More than 5 consecutive doses of palivizumab should be considered because of the shift in seasonality and the interseasonal spread of RSV.120 Individualized consideration should be made especially during the atypical interseason. Besides, the physician should pay attention on RSV epidemiology in the following days.121
Conclusion
In this narrative review, we collected the most current studies on the epidemiology of RSV in the COVID-19 era as well as data on the RSV resurgence. The importance of RSV infection in those at risk of developing severe disease has attracted attention among experts in this field. It is apparent that RSV remains one of the most common respiratory viruses even after the emergence of SARS-CoV-2. However, to date, there is still no available RSV vaccine.122 The development of an RSV reproducible human experimental model could aid further investigations and research on anti-viral medication and vaccine.123 Besides quarantine and lockdown, hand washing, mask wearing, and social distancing are all effective at decreasing RSV transmission. Maintaining use of PHSMs could provide a benefit against future outbreaks of those common respiratory viruses. Passive immunization with monoclonal antibody palivizumab should also be considered and administrated in high-risk patients during the RSV season. In addition to future investigations and the development of passive and active immunization, thorough surveillance of RSV variations is also of critical importance as we move into the post-COVID-19 era.
Acknowledgments
The authors did not receive any financial support for conducting this review or publication. PYL was supported in part by the NSTC with grant numbers MOST 110-2314-B-075A-011 and by the Taichung Veterans General Hospital with grant numbers TCVGH-1123901C and TCVGH-1123901D.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ. 2019;366:l5021. doi:10.1136/bmj.l5021
2. Tin Tin Htar M, Yerramalla MS, Moisi JC, Swerdlow DL. The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e48. doi:10.1017/S0950268820000400
3. Gomez GB, Mahe C, Chaves SS. Uncertain effects of the pandemic on respiratory viruses. Science. 2021;372(6546):1043–1044. doi:10.1126/science.abh3986
4. Haynes AK, Manangan AP, Iwane MK, et al. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis. 2013;208(Suppl 3):S246–54. doi:10.1093/infdis/jit515
5. Coutinho Baldoto Gava Chakr V. Which should we fear more in preschoolers and infants: SARS-CoV-2 or respiratory syncytial virus? Postgrad Med J. 2022;98(1161):e7. doi:10.1136/postgradmedj-2021-141012
6. World Health Organization. Considerations for Implementing and Adjusting Public Health and Social Measures in the Context of COVID-19: Interim Guidance, 14 June 2021. 2021. Available from: https://apps.who.int/iris/handle/10665/341811.
7. Achangwa C, Park H, Ryu S, Lee MS. Collateral Impact of Public Health and Social Measures on Respiratory Virus Activity during the COVID-19 Pandemic 2020-2021. Viruses. 2022;14(5):May. doi:10.3390/v14051071
8. Weinberger Opek M, Yeshayahu Y, Glatman-Freedman A, Kaufman Z, Sorek N, Brosh-Nissimov T. Delayed respiratory syncytial virus epidemic in children after relaxation of COVID-19 physical distancing measures, Ashdod, Israel, 2021. Euro Surveill. 2021;26(29). doi:10.2807/1560-7917.ES.2021.26.29.2100706
9. Williams TC, Sinha I, Barr IG, Zambon M. Transmission of paediatric respiratory syncytial virus and influenza in the wake of the COVID-19 pandemic. Euro Surveill. 2021;26(29). doi:10.2807/1560-7917.ES.2021.26.29.2100186
10. Fourgeaud J, Toubiana J, Chappuy H, et al. Impact of public health measures on the post-COVID-19 respiratory syncytial virus epidemics in France. Eur J Clin Microbiol Infect Dis. 2021;40(11):2389–2395. doi:10.1007/s10096-021-04323-1
11. Kim S, Williams TC, Viboud C, Campbell H, Chen J, Spiro DJ. RSV genomic diversity and the development of a globally effective RSV intervention. Vaccine. 2021;39(21):2811–2820. doi:10.1016/j.vaccine.2021.03.096
12. Mosscrop LG, Williams TC, Tregoning JS. Respiratory syncytial virus after the SARS-CoV-2 pandemic - what next? Nat Rev Immunol. 2022;22(10):589–590. doi:10.1038/s41577-022-00764-7
13. Giannekas M. MapChart. Available from: https://www.mapchart.net/.
14. Uhteg K, Amadi A, Forman M, Mostafa HH. Circulation of Non-SARS-CoV-2 Respiratory Pathogens and Coinfection with SARS-CoV-2 Amid the COVID-19 Pandemic. Open Forum Infect Dis. 2022;9(3):ofab618. doi:10.1093/ofid/ofab618
15. Yuan H, Yeung A, Yang W. Interactions among common non-SARS-CoV-2 respiratory viruses and influence of the COVID-19 pandemic on their circulation in New York City. Influenza Other Respir Viruses. 2022;16(4):653–661. doi:10.1111/irv.12976
16. Halabi KC, Saiman L, Zachariah P. The Epidemiology of Respiratory Syncytial Virus in New York City during the Coronavirus Disease-2019 Pandemic Compared with Previous Years. J Pediatr. 2022;242:242–244 e1. doi:10.1016/j.jpeds.2021.10.057
17. Nolen LD, Seeman S, Bruden D, et al. Impact of Social Distancing and Travel Restrictions on Non–Coronavirus Disease 2019 (Non–COVID-19) Respiratory Hospital Admissions in Young Children in Rural Alaska. Clin Infect Dis. 2020;72(12):2196–2198. doi:10.1093/cid/ciaa1328
18. Groves HE, Piche-Renaud PP, Peci A, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: a population-based study. Lancet Reg Health Am. 2021;1:100015. doi:10.1016/j.lana.2021.100015
19. Doroshenko A, Lee N, MacDonald C, Zelyas N, Asadi L, Kanji JN. Decline of Influenza and Respiratory Viruses With COVID-19 Public Health Measures: Alberta, Canada. Mayo Clin Proc. 2021;96(12):3042–3052. doi:10.1016/j.mayocp.2021.09.004
20. Varela FH, Scotta MC, Polese-Bonatto M, et al. Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: likely role of lower transmission in the community. J Glob Health. 2021;11:05007. doi:10.7189/jogh.11.05007
21. Quandelacy TM, Adams LE, Munoz J, et al. Reduced spread of influenza and other respiratory viral infections during the COVID-19 pandemic in southern Puerto Rico. PLoS One. 2022;17(4):e0266095. doi:10.1371/journal.pone.0266095
22. Redlberger-Fritz M, Kundi M, Aberle SW, Puchhammer-Stockl E. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol. 2021;137:104795. doi:10.1016/j.jcv.2021.104795
23. Bardsley M, Morbey RA, Hughes HE, et al. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: a retrospective observational study. Lancet Infect Dis. 2014;1:548. doi:10.1016/S1473-3099(22)00525-4
24. Van Brusselen D, De Troeyer K, Ter Haar E, et al. Bronchiolitis in COVID-19 times: a nearly absent disease? Eur J Pediatr. 2021;180(6):1969–1973. doi:10.1007/s00431-021-03968-6
25. Vittucci AC, Piccioni L, Coltella L, et al. The Disappearance of Respiratory Viruses in Children during the COVID-19 Pandemic. Int J Environ Res Public Health. 2021;18(18):9550. doi:10.3390/ijerph18189550
26. Nenna R, Matera L, Pierangeli A, et al. First COVID-19 lockdown resulted in most respiratory viruses disappearing among hospitalised children, with the exception of rhinoviruses. Acta Paediatr. 2022;111(7):1399–1403. doi:10.1111/apa.16326
27. Coma E, Vila J, Mendez-Boo L, et al. Respiratory Syncytial Virus Infections in Young Children Presenting to Primary Care in Catalonia During the COVID-19 Pandemic. J Pediatric Infect Dis Soc. 2022;11(2):69–72. doi:10.1093/jpids/piab121
28. Reyes Dominguez AI, Pavlovic Nesic S, Urquia Marti L, Reyes Suarez D, Garcia-Munoz Rodrigo F. Effects of public health measures during the SARS-CoV-2 pandemic on the winter respiratory syncytial virus epidemic: an interrupted time series analysis. Paediatr Perinat Epidemiol. 2022;36(3):329–336. doi:10.1111/ppe.12829
29. Torres-Fernandez D, Casellas A, Mellado MJ, Calvo C, Bassat Q. Acute bronchiolitis and respiratory syncytial virus seasonal transmission during the COVID-19 pandemic in Spain: a national perspective from the pediatric Spanish Society (AEP). J Clin Virol. 2021;145:105027. doi:10.1016/j.jcv.2021.105027
30. Delestrain C, Danis K, Hau I, et al. Impact of COVID-19 social distancing on viral infection in France: a delayed outbreak of RSV. Pediatr Pulmonol. 2021;56(12):3669–3673. doi:10.1002/ppul.25644
31. Stamm P, Sagoschen I, Weise K, et al. Influenza and RSV incidence during COVID-19 pandemic-an observational study from in-hospital point-of-care testing. Med Microbiol Immunol. 2021;210(5–6):277–282. doi:10.1007/s00430-021-00720-7
32. Engels G, Sack J, Weissbrich B, et al. Very Low Incidence of SARS-CoV-2, Influenza and RSV but High Incidence of Rhino-, Adeno- and Endemic Coronaviruses in Children With Acute Respiratory Infection in Primary Care Pediatric Practices During the Second and Third Wave of the SARS-CoV-2 Pandemic. Pediatr Infect Dis J. 2022;41(4):e146–e148. doi:10.1097/INF.0000000000003460
33. Kuitunen I, Artama M, Makela L, Backman K, Heiskanen-Kosma T, Renko M. Effect of Social Distancing Due to the COVID-19 Pandemic on the Incidence of Viral Respiratory Tract Infections in Children in Finland During Early 2020. Pediatr Infect Dis J. 2020;39(12):e423–e427. doi:10.1097/INF.0000000000002845
34. Kuitunen I, Renko M. Lessons to learn from the current pandemic for future non-pharmaceutical interventions against the respiratory syncytial virus - nationwide register-study in Finland. Infect Dis. 2021;53(6):476–478. doi:10.1080/23744235.2021.1894351
35. Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of Coronavirus Disease 2019 Public Health Measures on Detections of Influenza and Respiratory Syncytial Virus in Children During the 2020 Australian Winter. Clin Infect Dis. 2021;72(12):2199–2202. doi:10.1093/cid/ciaa1475
36. El-Heneidy A, Ware RS, Robson JM, Cherian SG, Lambert SB, Grimwood K. Respiratory virus detection during the COVID-19 pandemic in Queensland, Australia. Aust N Z J Public Health. 2022;46(1):10–15. doi:10.1111/1753-6405.13168
37. Abo YN, Clifford V, Lee LY, et al. COVID-19 public health measures and respiratory viruses in children in Melbourne. J Paediatr Child Health. 2021;57(12):1886–1892. doi:10.1111/jpc.15601
38. Britton PN, Hu N, Saravanos G, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health. 2020;4(11):e42–e43. doi:10.1016/S2352-4642(20)30307-2
39. Tempia S, Walaza S, Bhiman JN, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Euro Surveill. 2021;26(29). doi:10.2807/1560-7917.ES.2021.26.29.2001600
40. Trenholme A, Webb R, Lawrence S, et al. COVID-19 and Infant Hospitalizations for Seasonal Respiratory Virus Infections, New Zealand, 2020. Emerg Infect Dis. 2021;27(2):641–643. doi:10.3201/eid2702.204041
41. Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 non pharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12(1):1001. doi:10.1038/s41467-021-21157-9
42. Maruo Y, Ishikawa S, Oura K, et al. The impact of the coronavirus disease 2019 pandemic on pediatric hospitalization in Kitami, Japan. Pediatr Int. 2022;64(1):e14937. doi:10.1111/ped.14937
43. Wagatsuma K, Koolhof IS, Shobugawa Y, Saito R. Decreased human respiratory syncytial virus activity during the COVID-19 pandemic in Japan: an ecological time-series analysis. BMC Infect Dis. 2021;21(1):734. doi:10.1186/s12879-021-06461-5
44. Kim JH, Roh YH, Ahn JG, et al. Respiratory syncytial virus and influenza epidemics disappearance in Korea during the 2020-2021 season of COVID-19. Int J Infect Dis. 2021;110:29–35. doi:10.1016/j.ijid.2021.07.005
45. Park S, Michelow IC, Choe YJ. Shifting Patterns of Respiratory Virus Activity Following Social Distancing Measures for Coronavirus Disease 2019 in South Korea. J Infect Dis. 2021;224(11):1900–1906. doi:10.1093/infdis/jiab231
46. Lee CY, Wu TH, Fang YP, et al. Delayed respiratory syncytial virus outbreak in 2020 in Taiwan was correlated with two novel RSV-A genotype ON1 variants. Influenza Other Respi Viruses. 2022;16(3):511–520. doi:10.1111/irv.12951
47. Chiu SS, Cowling BJ, Peiris JSM, Chan ELY, Wong WHS, Lee KP. Effects of Non pharmaceutical COVID-19 Interventions on Pediatric Hospitalizations for Other Respiratory Virus Infections, Hong Kong. Emerg Infect Dis. 2022;28(1):62–68. doi:10.3201/eid2801.211099
48. Bhardwaj S, Choudhary ML, Jadhav S, et al. A retrospective analysis of respiratory virus transmission before and during the COVID-19 pandemic in Pune the western region of India. Front Public Health. 2022;10:936634. doi:10.3389/fpubh.2022.936634
49. Rana MS, Usman M, Alam MM, et al. Impact of COVID-19 preventive measures on other infectious and non-infectious respiratory diseases in Pakistan. J Infect. 2021;82(5):e31–e32. doi:10.1016/j.jinf.2021.01.018
50. Letafati A, Aghamirmohammadali FS, Rahimi-Foroushani A, Hasani SA, Mokhtari-Azad T, Yavarian J. No human respiratory syncytial virus but SARS-CoV-2 found in children under 5 years old referred to Children Medical Center in 2021, Tehran, Iran. J Med Virol. 2022;94(7):3096–3100. doi:10.1002/jmv.27685
51. Zhu Y, Li W, Yang B, et al. Epidemiological and virological characteristics of respiratory tract infections in children during COVID-19 outbreak. BMC Pediatr. 2021;21(1):195. doi:10.1186/s12887-021-02654-8
52. Du X, Wu G, Zhu Y, Zhang S. Exploring the epidemiological changes of common respiratory viruses since the COVID-19 pandemic: a hospital study in Hangzhou, China. Arch Virol. 2021;166(11):3085–3092. doi:10.1007/s00705-021-05214-8
53. Li L, Wang H, Liu A, et al. Comparison of 11 respiratory pathogens among hospitalized children before and during the COVID-19 epidemic in Shenzhen, China. Virol J. 2021;18(1):202. doi:10.1186/s12985-021-01669-y
54. Ye Q, Liu H. Impact of non-pharmaceutical interventions during the COVID-19 pandemic on common childhood respiratory viruses - An epidemiological study based on hospital data. Microbes Infect. 2022;24(1):104911. doi:10.1016/j.micinf.2021.104911
55. Liu P, Xu M, Lu L, et al. The changing pattern of common respiratory and enteric viruses among outpatient children in Shanghai, China: two years of the COVID-19 pandemic. J Med Virol. 2022;94(10):4696–4703. doi:10.1002/jmv.27896
56. Leuzinger K, Roloff T, Gosert R, et al. Epidemiology of SARS-CoV-2 Emergence Amidst Community-Acquired Respiratory Viruses. medRxiv. 2020. doi:10.1101/2020.07.07.20148163
57. Nenna R, Matera L, Licari A, et al. An Italian Multicenter Study on the Epidemiology of Respiratory Syncytial Virus During SARS-CoV-2 Pandemic in Hospitalized Children. Front Pediatr. 2022;10:930281. doi:10.3389/fped.2022.930281
58. Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA. 2020;323(20):2085–2086. doi:10.1001/jama.2020.6266
59. Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 non pharmaceutical interventions on the future dynamics of endemic infections. Proce National Acad Sci. 2020;117(48):30547–30553. doi:10.1073/pnas.2013182117
60. Li Y, Wang X, Msosa T, de Wit F, Murdock J, Nair H. The impact of the 2009 influenza pandemic on the seasonality of human respiratory syncytial virus: a systematic analysis. Influenza Other Respir Viruses. 2021;15(6):804–812. doi:10.1111/irv.12884
61. Madaniyazi L, Seposo X, Ng CFS, et al. Respiratory Syncytial Virus Outbreaks Are Predicted after the COVID-19 Pandemic in Tokyo, Japan. Jpn J Infect Dis. 2022;75(2):209–211. doi:10.7883/yoken.JJID.2021.312
62. Baker RE, Park SW, Yang W, Vecchi GA, Metcalf CJE, Grenfell BT. The impact of COVID-19 non pharmaceutical interventions on the future dynamics of endemic infections. Proc Natl Acad Sci U S A. 2020;117(48):30547–30553. doi:10.1073/pnas.2013182117
63. Cooney HC, Fleming C, Scheffer IE. Respiratory syncytial virus epidemic during the COVID-19 pandemic. J Paediatr Child Health. 2022;58(1):215–216. doi:10.1111/jpc.15847
64. Perez A, Lively JY, Curns A, et al. Respiratory Virus Surveillance Among Children with Acute Respiratory Illnesses - New Vaccine Surveillance Network, United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2022;71(40):1253–1259. doi:10.15585/mmwr.mm7140a1
65. Foley DA, Phuong LK, Peplinski J, et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch Dis Child. 2022;107(3):e7. doi:10.1136/archdischild-2021-322507
66. Foley DA, Yeoh DK, Minney-Smith CA, et al. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019-Related Public Health Measures. Clin Infect Dis. 2021;73(9):e2829–e2830. doi:10.1093/cid/ciaa1906
67. Agha R, Avner JR. Delayed Seasonal RSV Surge Observed During the COVID-19 Pandemic. Pediatrics. 2021;148(3). doi:10.1542/peds.2021-052089
68. von Hammerstein AL, Aebi C, Barbey F, et al. Interseasonal RSV infections in Switzerland - rapid establishment of a clinician-led national reporting system (RSV EpiCH). Swiss Med Wkly. 2021;151:w30057. doi:10.4414/SMW.2021.w30057
69. Cai W, Durrwald R, Biere B, et al. Determination of respiratory syncytial virus epidemic seasons by using 95% confidence interval of positivity rates, 2011-2021, Germany. Influenza Other Respir Viruses. 2022;16(5):854–857. doi:10.1111/irv.12996
70. Loconsole D, Centrone F, Rizzo C, et al. Out-of-Season Epidemic of Respiratory Syncytial Virus during the COVID-19 Pandemic: the High Burden of Child Hospitalization in an Academic Hospital in Southern Italy in 2021. Children. 2022;9(6):Jun. doi:10.3390/children9060848
71. Kuitunen I, Artama M, Haapanen M, Renko M. Respiratory virus circulation in children after relaxation of COVID-19 restrictions in fall 2021-A nationwide register study in Finland. J Med Virol. 2022;94(9):4528–4532. doi:10.1002/jmv.27857
72. Moro G, Mamo C. 2021 EIP abstract book. Cogent Med. 2021;8(1):2002558. doi:10.1080/2331205X.2021.2002558
73. Sberna G, Lalle E, Valli MB, Bordi L, Garbuglia AR, Amendola A. Changes in the Circulation of Common Respiratory Pathogens among Hospitalized Patients with Influenza-like Illnesses in the Lazio Region (Italy) during Fall Season of the Past Three Years. Int J Environ Res Public Health. 2022;19(10):5962. doi:10.3390/ijerph19105962
74. Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N. Resurgence of Respiratory Syncytial Virus I nfections during COVID-19 Pandemic, Tokyo, Japan. Emerg Infect Dis. 2021;27(11):2969–2970. doi:10.3201/eid2711.211565
75. Mohebi L, Karami H, Mirsalehi N, et al. A delayed resurgence of respiratory syncytial virus (RSV) during the COVID-19 pandemic: an unpredictable outbreak in a small proportion of children in the Southwest of Iran, April 2022. J Med Virol. 2022;94(12):5802–5807. doi:10.1002/jmv.28065
76. Billard M-N, van de Ven PM, Baraldi B, Kragten-Tabatabaie L, Bont LJ, Wildenbeest JG. International changes in respiratory syncytial virus (RSV) epidemiology during the COVID-19 pandemic: association with school closures. Influenza Other Respi Viruses. 2022;16(5):926–936. doi:10.1111/irv.12998
77. Binns E, Koenraads M, Hristeva L, et al. Influenza and respiratory syncytial virus during the COVID-19 pandemic: time for a new paradigm? Pediatr Pulmonol. 2022;57(1):38–42. doi:10.1002/ppul.25719
78. Casalegno J, Javouhey E, Ploin D, et al. Delayed Start of the Respiratory Syncytial Virus Epidemic at the End of the 20/21 Northern Hemisphere Winter Season, Lyon, France. medRxiv. 2021. doi:10.1101/2021.03.12.21253446
79. van Summeren J, Meijer A, Aspelund G, et al. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill. 2021;26(29). doi:10.2807/1560-7917.ES.2021.26.29.2100639
80. Di Mattia G, Nenna R, Mancino E, et al. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol. 2021;56(10):3106–3109. doi:10.1002/ppul.25582
81. Cohen R, Ashman M, Taha MK, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51(5):418–423. doi:10.1016/j.idnow.2021.05.004
82. Reicherz F, Xu RY, Abu-Raya B, et al. Waning immunity against respiratory syncytial virus during the COVID-19 pandemic. J Infect Dis. 2022;226(12):2064–2068. doi:10.1093/infdis/jiac192
83. Hatter L, Eathorne A, Hills T, Bruce P, Beasley R. Respiratory syncytial virus: paying the immunity debt with interest. Lancet Child Adolesc Health. 2021;5(12):e44–e45. doi:10.1016/S2352-4642(21)00333-3
84. Emanuels A, Heimonen J, O’Hanlon J, et al. Remote household observation for noninfluenza respiratory viral illness. Clin Infect Dis. 2021;73(11):e4411–e4418. doi:10.1093/cid/ciaa1719
85. Pappa S, Haidopoulou K, Zarras C, et al. Early initiation of the respiratory syncytial virus season in 2021-2022, Greece. J Med Virol. 2022;94(7):3453–3456. doi:10.1002/jmv.27671
86. Eden JS, Sikazwe C, Xie R, et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun. 2022;13(1):2884. doi:10.1038/s41467-022-30485-3
87. Baier C, Huang J, Reumann K, et al. Target capture sequencing reveals a monoclonal outbreak of respiratory syncytial virus B infections among adult hematologic patients. Antimicrob Resist Infect Control. 2022;11(1):88. doi:10.1186/s13756-022-01120-z
88. Kahanowitch R, Gaviria S, Aguilar H, et al. How did respiratory syncytial virus and other pediatric respiratory viruses change during the COVID-19 pandemic? Pediatr Pulmonol. 2022;57(10):2542–2545. doi:10.1002/ppul.26053
89. Kuchar E, Zaleski A, Wronowski M, et al. Children were less frequently infected with SARS-CoV-2 than adults during 2020 COVID-19 pandemic in Warsaw, Poland. Eur J Clin Microbiol Infect Dis. 2021;40(3):541–547. doi:10.1007/s10096-020-04038-9
90. Swets MC, Russell CD, Harrison EM, et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022;399(10334):1463–1464. doi:10.1016/S0140-6736(22)00383-X
91. Cong B, Deng S, Wang X, Li Y. The role of respiratory co-infection with influenza or respiratory syncytial virus in the clinical severity of COVID-19 patients: a systematic review and meta-analysis. J Glob Health. 2022;12:05040. doi:10.7189/jogh.12.05040
92. Alvares PA. SARS-CoV-2 and Respiratory Syncytial Virus Coinfection in Hospitalized Pediatric Patients. Pediatr Infect Dis J. 2021;40(4):e164–e166. doi:10.1097/INF.0000000000003057
93. Hashemi SA, Safamanesh S, Ghasemzadeh-Moghaddam H, Ghafouri M, Azimian A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J Med Virol. 2021;93(2):1008–1012. doi:10.1002/jmv.26364
94. Hedberg P, Johansson N, Ternhag A, Abdel-Halim L, Hedlund J, Naucler P. Bacterial co-infections in community-acquired pneumonia caused by SARS-CoV-2, influenza virus and respiratory syncytial virus. BMC Infect Dis. 2022;22(1):108. doi:10.1186/s12879-022-07089-9
95. Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus--a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–379. doi:10.1007/s12016-013-8368-9
96. Walsh EE, Peterson DR, Falsey AR. Risk factors for severe respiratory syncytial virus infection in elderly persons. J Infect Dis. 2004;189(2):233–238. doi:10.1086/380907
97. Manzoni P, Figueras-Aloy J, Simoes EAF, et al. Defining the Incidence and Associated Morbidity and Mortality of Severe Respiratory Syncytial Virus Infection Among Children with Chronic Diseases. Infect Dis Ther. 2017;6(3):383–411. doi:10.1007/s40121-017-0160-3
98. Miller JM, Binnicker MJ, Campbell S, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67(6):e1–e94. doi:10.1093/cid/ciy381
99. Kim SH, AlMutawa F. Tracheal Aspirate and Bronchoalveolar Lavage as Potential Specimen Types for COVID-19 Testing Using the Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV. Microbiol Spectr. 2022;10(3):e0039922. doi:10.1128/spectrum.00399-22
100. Teirlinck AC, Broberg EK, Stuwitz Berg A, et al. Recommendations for respiratory syncytial virus surveillance at the national level. Eur Respir J. 2021;58(3):2003766. doi:10.1183/13993003.03766-2020
101. Mostafa HH, Carroll KC, Hicken R, et al. Multicenter Evaluation of the Cepheid Xpert Xpress SARS-CoV-2/Flu/RSV Test. J Clin Microbiol. 2021;59(3):e02955–20. doi:10.1128/JCM.02955-20
102. Wolters F, Grunberg M, Huber M, et al. European multicenter evaluation of Xpert(R) Xpress SARS-CoV-2/Flu/RSV test. J Med Virol. 2021;93(10):5798–5804. doi:10.1002/jmv.27111
103. Leung EC, Chow VC, Lee MK, Tang KP, Li DK, Lai RW. Evaluation of the Xpert Xpress SARS-CoV-2/Flu/RSV Assay for Simultaneous Detection of SARS-CoV-2, Influenza A and B Viruses, and Respiratory Syncytial Virus in Nasopharyngeal Specimens. J Clin Microbiol. 2021;59(4). doi:10.1128/jcm.02965-20
104. Quinton M, Geahr M, Gluck L, Jarrett J, Mostafa HH. Evaluation of the respiratory NeuMoDx Flu A-B/RSV/SARS-CoV-2 Vantage and Alinity m Resp-4-Plex assays. J Clin Virol. 2022;150-151:105164. doi:10.1016/j.jcv.2022.105164
105. Yun J, Park JH, Kim N, et al. Evaluation of Three Multiplex Real-time Reverse Transcription PCR Assays for Simultaneous Detection of SARS-CoV-2, Influenza A/B, and Respiratory Syncytial Virus in Nasopharyngeal Swabs. J Korean Med Sci. 2021;36(48):e328. doi:10.3346/jkms.2021.36.e328
106. Phan T, Tran NYK, Gottlieb T, Siarakas S, McKew G. Evaluation of the influenza and respiratory syncytial virus (RSV) targets in the AusDiagnostics SARS-CoV-2, Influenza and RSV 8-well assay: sample pooling increases testing throughput. Pathology. 2022;54(4):466–471. doi:10.1016/j.pathol.2022.02.002
107. Larkin HD. First Nonprescription COVID-19 Test That Also Detects Flu and RSV. JAMA. 2022;328(1):11. doi:10.1001/jama.2022.11031
108. Kim TY, Kim JY, Shim HJ, et al. Comparison of the PowerChek SARS-CoV-2, Influenza A&B, RSV Multiplex Real-time PCR Kit and BioFire Respiratory Panel 2.1 for simultaneous detection of SARS-CoV-2, influenza A and B, and respiratory syncytial virus. J Virol Methods. 2021;298:114304. doi:10.1016/j.jviromet.2021.114304
109. Beaird OE, Freifeld A, Ison MG, et al. Current practices for treatment of respiratory syncytial virus and other non-influenza respiratory viruses in high-risk patient populations: a survey of institutions in the Midwestern Respiratory Virus Collaborative. Transpl Infect Dis. 2016;18(2):210–215. doi:10.1111/tid.12510
110. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56(2):258–266. doi:10.1093/cid/cis844
111. Ventre K, Randolph A. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract in infants and young children. Cochrane Database Syst Rev. 2004;(4):CD000181. doi:10.1002/14651858.CD000181.pub2
112. Gorcea CM, Tholouli E, Turner A, et al. Effective use of oral ribavirin for respiratory syncytial viral infections in allogeneic haematopoietic stem cell transplant recipients. J Hosp Infect. 2017;95(2):214–217. doi:10.1016/j.jhin.2016.11.012
113. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117(10):2755–2763. doi:10.1182/blood-2010-08-263400
114. Sinclair SM, Jones JK, Miller RK, Greene MF, Kwo PY, Maddrey WC. The Ribavirin Pregnancy Registry: an Interim Analysis of Potential Teratogenicity at the Mid-Point of Enrollment. Drug Saf. 2017;40(12):1205–1218. doi:10.1007/s40264-017-0566-6
115. Ottolini MG, Porter DD, Hemming VG, Zimmerman MN, Schwab NM, Prince GA. Effectiveness of RSVIG prophylaxis and therapy of respiratory syncytial virus in an immunosuppressed animal model. Bone Marrow Transplant. 1999;24(1):41–45. doi:10.1038/sj.bmt.1701813
116. American Academy of Pediatrics Committee on Infectious D, American Academy of Pediatrics Bronchiolitis Guidelines C. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):e620–38. doi:10.1542/peds.2014-1666
117. Villanueva DH, Arcega V, Rao M. Review of respiratory syncytial virus infection among older adults and transplant recipients. Ther Adv Infect Dis. 2022;9:20499361221091413. doi:10.1177/20499361221091413
118. Boeckh M, Berrey MM, Bowden RA, Crawford SW, Balsley J, Phase CL. 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184(3):350–354. doi:10.1086/322043
119. Permpalung N, Mahoney MV, McCoy C, et al. Clinical characteristics and treatment outcomes among respiratory syncytial virus (RSV)-infected hematologic malignancy and hematopoietic stem cell transplant recipients receiving palivizumab. Leuk Lymphoma. 2019;60(1):85–91. doi:10.1080/10428194.2018.1468896
120. American Academy of Pediatrics. Updated Guidance: use of Palivizumab Prophylaxis to Prevent Hospitalization From Severe Respiratory Syncytial Virus Infection During the 2022-2023 RSV Season. Available from: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/interim-guidance-for-use-of-palivizumab-prophylaxis-to-prevent-hospitalization/.
121. Taylor RS. Respiratory syncytial virus and palivizumab prophylaxis in the COVID-19 era. CMAJ. 2021;193(15):E523. doi:10.1503/cmaj.78240
122. Tripp RA, Stambas J. Intervention Strategies for Seasonal and Emerging Respiratory Viruses with Drugs and Vaccines Targeting Viral Surface Glycoproteins. Viruses. 2021;13(4):Apr. doi:10.3390/v13040625
123. Lambkin-Williams R, DeVincenzo JP. A COVID-19 human viral challenge model. Learning from experience. Influenza Other Respir Viruses. 2020;14(6):747–756. doi:10.1111/irv.12797
124. Zhen W, Manji R, Smith E, Wuitschick J, Lucic D, Berry GJ. Evaluation of the Alinity m Resp-4-Plex Assay for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2, Influenza A Virus, Influenza B Virus, and Respiratory Syncytial Virus. Microbiol Spectr. 2022;10(1):e0109021. doi:10.1128/spectrum.01090-21
125. Cheng A, Riedel S, Arnaout R, Kirby JE. Verification of the Abbott Alinity m Resp-4-Plex assay for detection of SARS-CoV-2, influenza A/B, and respiratory syncytial virus. Diagn Microbiol Infect Dis. 2022;102(2):115575. doi:10.1016/j.diagmicrobio.2021.115575
126. Sahajpal NS, Mondal AK, Ananth S, et al. Clinical validation of a multiplex PCR-based detection assay using saliva or nasopharyngeal samples for SARS-Cov-2, influenza A and B. Sci Rep. 2022;12(1):3480. doi:10.1038/s41598-022-07152-0
127. Prevention CfDCa. Table 4. Multiplex Assays Authorized for Simultaneous Detection of Influenza Viruses and SARS-CoV-2 by FDA.
128. Administration USFaD. In vitro diagnostics EUAs - Molecular Diagnostic Tests for SARS-CoV-2. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2#individual-molecular.
129. Lim HJ, Park JE, Park MY, et al. Assay System for Simultaneous Detection of SARS-CoV-2 and Other Respiratory Viruses. Diagnostics. 2021;11(6):548. doi:10.3390/diagnostics11061084
130. Neopane P, Nypaver J, Shrestha R, Beqaj S. Performance Evaluation of TaqMan SARS-CoV-2, Flu A/B, RSV RT-PCR Multiplex Assay for the Detection of Respiratory Viruses. Infect Drug Resist. 2022;15:5411–5423. doi:10.2147/IDR.S373748
131. Mboumba Bouassa RS, Tonen-Wolyec S, Veyer D, Pere H, Belec L. Analytical performances of the AMPLIQUICK(R) Respiratory Triplex assay for simultaneous detection and differentiation of SARS-CoV-2, influenza A/B and respiratory syncytial viruses in respiratory specimens. PLoS One. 2022;17(1):e0262258. doi:10.1371/journal.pone.0262258
132. Chung HY, Jian MJ, Chang CK, et al. Multicenter study evaluating one multiplex RT-PCR assay to detect SARS-CoV-2, influenza A/B, and respiratory syncytial virus using the LabTurbo AIO open platform: epidemiological features, automated sample-to-result, and high-throughput testing. Aging. 2021;13(23):24931–24942. doi:10.18632/aging.203761
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
