Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 14
The Impact of Nonsurgical Periodontal Therapy on Serum Levels of Dickkopf-Related Protein-1 in Smokers and Nonsmokers with Periodontitis: A Prospective Comparative Study
Authors Azab E , Attia A, Yaghmoor W, Aldahlawi S , Youssef AR
Received 16 February 2022
Accepted for publication 16 June 2022
Published 28 June 2022 Volume 2022:14 Pages 191—198
DOI https://doi.org/10.2147/CCIDE.S362801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Ehab Azab,1 Alaa Attia,1,2 Wael Yaghmoor,1 Salwa Aldahlawi,1 Abdel-Rahman Youssef1,3
1Department of Basic and Clinical Oral Sciences, Faculty of Dentistry, Umm Al-Qura University, Makkah, Saudi Arabia; 2Department of Oral Medicine and Periodontology, Faculty of Dentistry, Al-Azhar University, Assiut, Egypt; 3Department of Microbiology and Immunology, Faculty of Medicine, Suez Canal University, Ismailia, Egypt
Correspondence: Ehab Azab, Department of Basic and Clinical Oral Sciences, Faculty of Dentistry, Umm Al-Qura University, Prince Sultan Road, Makkah, 21421, Saudi Arabia, Tel +966 12 527 0000, Email [email protected]
Purpose: This study aimed to evaluate and compare Dickkopf-related protein-1 (DKK1) serum levels and periodontal clinical parameters of smokers and nonsmokers with periodontitis at baseline and after nonsurgical periodontal treatment.
Patients and Methods: A prospective comparative study was conducted among 24 patients with periodontitis who were divided according to the smoking habits into two groups: nonsmokers (G1) and smokers (G2). All the participants were assessed clinically by recording the probing depth (PD), clinical attachment loss (CAL), plaque index (PI), and bleeding index (BI), and immunologically by measuring the DKK1 serum levels at baseline and six weeks after nonsurgical periodontal therapy.
Results: The two groups showed a significant decrease in PI, BI, and CAL after periodontal therapy (p < 0.05), while PD was significantly reduced in G1 (p = 0.005). The PI mean value was significantly higher at the baseline in G2 versus G1 (p = 0.050), while PD, BI, and CAL values were not significantly different between the groups (p = 0.056, p = 0.241, and p = 0.381, respectively). For DKK1 serum levels, there was a statistically significant decrease after treatment compared to the baseline for both groups (G1: p < 0.001; G2: p < 0.001) but no significant difference before (p = 0.131) and six weeks after treatment (p = 0.334) between the two groups.
Conclusion: Although nonsurgical periodontal treatment effectively improved periodontal clinical parameters and reduced DKK1 serum levels, there were no significant differences in the DKK1 serum levels among the smokers and nonsmokers with periodontitis.
Keywords: periodontitis, dickkopf-related protein-1, periodontal therapy, Wnt signaling, bone cells
Introduction
Periodontitis is a form of inflammatory disease that affects tooth surrounding structures as a result of interactions between the host’s immune cells and periodontal pathogenic bacteria.1 These interactions involve releasing many substances that provoke inflammation, connective tissue degradation, and alveolar bone destruction, which may result in tooth loss.2,3 The host susceptibility to periodontal pathogens has been reported to be primarily responsible for the disease initiation and progression.4 Smoking and other conditions, including hereditary diseases, immunological diseases, and systemic diseases, have been reported to induce the risk of periodontitis in patients.5
Smoking is a well-recognized environmental risk factor for periodontal disease initiation and progression.6 Studies have found that smoker patients are more susceptible to periodontitis than nonsmoker controls, and show deeper probing depth (PD) and greater attachment and alveolar bone loss.7 Smoking modifies the periodontal microbial colonies and the host immune response.8,9 These modifications lead to changes in vascular permeability, reduction in antibody production, impairment in neutrophil chemotaxis and phagocytosis, increase in reactive oxidative stress, and disturbance in the other immune cells’ activities, causing an increase in proinflammatory mediators’ formation.9,10 Moreover, smoking affects periodontal healing capacity and posttreatment tissue response.11
Periodontal pathogenic bacteria have many virulence factors that trigger the host immune cells to release numerous proinflammatory cytokines, inducing connective tissue degradation and causing an imbalance in bone metabolism that favors bone resorption.3,12,13 Bone homeostasis is maintained by balanced activity between osteoblastic bone-forming cells and osteoclastic bone-resorbing cells. Bone metabolism is a complex process that utilizes many transduction pathways.14,15 Understating the different mechanisms of bone metabolism could help in the management of periodontal disease.16
Osteocytes have been shown as a central regulator cell that organizes both osteoblast and osteoclast activities either directly by cell connection or indirectly by factors secretion.17,18 Osteocytes produce Dickkopf-related protein-1 (DKK1), which blocks the Wnt/ß-catenin signaling pathway required for osteoblast differentiation and bone formation.19–23 DKK1 interferes with the Wnt signaling pathway by binding to the osteoblast’s extracellular region of lipoprotein receptor-related protein 5/6 (LRP5/6), inducing ß-catenin degradation by preventing its translocation to the nucleus. This leads to the inhibition of the Wnt/β-catenin signaling pathway and the suppression of bone formation.24,25
Several studies have demonstrated that DKK1 is implicated in periodontal disease. A significant increase in the expression of DKK1 mRNA was found in the gingival tissue of patients with chronic periodontitis compared to the gingival tissue of healthy individuals, while DKK1 serum levels did not change significantly in patients with chronic periodontitis compared to healthy individuals.26 In addition, it has been found that smokers with chronic periodontitis had higher DKK1 serum levels compared to nonsmokers with chronic periodontitis.27 Moreover, systemic administration of the DKK1 antibody enhanced alveolar bone regeneration in the experimental molar extraction model in rats.28
Early detection of periodontitis plays a crucial role in its prevention and progression.2,29 Clinical and radiographical examinations are still the best available tools for the diagnosis of periodontal disease; however, they do not carefully measure the disease activity or evaluate the patient’s treatment response or predict future disease progression.30 Hence, the search is ongoing to discover a reliable diagnostic tool such as biomarkers for early detection and monitoring of periodontal disease.
There are scarce data regarding the impact of nonsurgical periodontal therapy on DKK1 serum levels in smoker and nonsmoker patients with periodontitis. Therefore, we hypothesized that there is no difference in the DKK1 serum levels in smoker and nonsmoker patients with periodontitis after nonsurgical periodontal therapy.
Materials and Methods
Study Design and Participants
This study was approved by the Umm Al-Qura University Faculty of Dentistry Institutional Review Board Committee (IRB: 157–19). Twenty-four patients with periodontitis were recruited from the Dental Teaching Hospital, Umm Al-Qura University, Makkah, Saudi Arabia, after obtaining their informed consent. Participants who were 25–55 years old, systemically healthy, had ≥ 15 remaining teeth (excluding third molars) and had periodontitis (interproximal clinical attachment loss (CAL) ≥ 3 mm in at least two nonadjacent teeth, probing depth (PD) ≥ 5 mm, and radiographic bone loss ≥ 20%31) were included in this study. In addition, smokers were defined as regular users of any smoking habit, including cigarettes, electronic cigarettes, cigars, water pipes, and smokeless tobacco.
Participants who received periodontal treatment in the last six months; were pregnant or lactating women; were diagnosed with osteoporosis; used antibiotics in the previous six months; needed antibiotic premedication; used glucocorticoids, bisphosphonates, or denosumab; were regularly using nonsteroidal anti-inflammatory drugs; or were unable to sign the consent form were excluded from this study.
Study Procedures
Periodontal clinical parameters, including PD, plaque index (PI), bleeding index (BI), and CAL, and the smoking habits of the participants were assessed and recorded. Peripheral blood samples were collected in serum separator tubes, kept for clotting for up to 30 minutes at room temperature, and then centrifuged at 3000 rpm for 10 minutes. Serum was collected and stored at −80°C. The participants were asked to visit the clinic one week later to receive nonsurgical periodontal treatment, including oral hygiene instructions and scaling and root planing. Six weeks after nonsurgical periodontal treatment, the participants’ periodontal clinical parameters were assessed, and serum samples were obtained as described above.
Enzyme-Linked Immunosorbent Assay
DKK1 serum levels at baseline and after periodontal therapy were measured using an enzyme-linked immunosorbent assay (ELISA) (Human DKK1 Quantikine ELISA Kit; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol. The microplate was precoated with human DKK1 monoclonal antibody. Standard or diluted serums (8-fold dilution) were added per well and incubated for 2 hours. After washing, human DKK1 conjugate was added to each well, incubated for 2 hours, and then washed. Substrate was added for 30 minutes, and then the reaction was stopped. The optical density of each well was determined at 450 nm using an ELISA spectrophotometric reader (SPECTROstar; BMG LABTECH, Offenburg, Germany). The total amounts of DKK1 were displayed as picogram/milliliter (pg/mL).
Statistical Analysis
Statistical Package for the Social Sciences (version 26) and GraphPad Prism 7 (GraphPad Software, San Diego, CA) were utilized for analytical statistics and data presentation. The data were represented using mean and standard error (SE). Paired t-test was utilized to analyze the effect of periodontal treatment in the same group, while unpaired t-test was used to compare the means of the smoker and the nonsmoker groups. The difference was considered statistically significant at p ≤ 0.05.
Results
Twenty-four patients with periodontitis were selected for this study and divided based on their smoking history into group 1 (G1: 12 nonsmokers) and group 2 (G2: 12 smokers).
Evaluation of Clinical Parameters
The descriptive analysis of the periodontal clinical parameters for both the nonsmokers (G1) and the smokers (G2) was expressed in mean, standard error, and t- and p-values as shown in Tables 1 and 2.
 |
Table 1 Comparisons of Clinical Parameters Between Nonsmoker and Smoker Patients with Periodontitis at the Baseline and Six Weeks After Nonsurgical Periodontal Therapy |
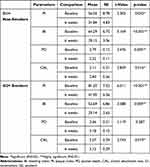 |
Table 2 Comparisons of Clinical Parameters Within Nonsmoker and Smoker Patients with Periodontitis Patients at Baseline and Six Weeks After Nonsurgical Periodontal Therapy |
At baseline, G2 (smokers) showed a worse clinical finding as a higher mean PD and CAL and lower BI; however, this difference was not statistically significant. The PI was significantly higher in G2 compared to G1 (p < 0.05) (Table 1).
After nonsurgical periodontal treatment, both groups showed significant improvements in all clinical parameters (PI, BI, and CAL) (Table 2). Although the reduction in PD was significant in G1 (p = 0.005), the change in PD in G2 was not statistically significant (p = 0.287) (Table 2). Comparing the clinical findings of both groups six weeks after nonsurgical therapy revealed that both groups had a similar presentation, with the only difference being a deeper residual PD in G2 (p < 0.001) (Table 1).
Serum DKK1 Levels
Descriptive statistical findings of serum DKK1 levels in G1 and G2 are presented in Table 3. Despite the increase in the mean values of DKK1 levels in the smokers versus the nonsmokers, there was no statistically significant difference in the DKK1 serum levels at the baseline (p = 0.131) and six weeks following the nonsurgical periodontal treatment (p = 0.334) (Table 3). However, the intragroup comparison showed that the DKK1 serum level was significantly reduced following nonsurgical treatment compared to the baseline for both groups (p < 0.001) (Figure 1).
 |
Table 3 Comparisons of DDK1 Levels (Pg/Ml) Before and After Nonsurgical Periodontal Treatment for Both Groups |
Discussion
Periodontal pathogens and smoking induce the production of several mediators that can be detected in the saliva, serum, and gingival crevicular fluid (GCF) of patients with periodontitis, which could be used as biomarkers to diagnose and monitor periodontal tissue degradation and bone resorption.32 Biomarkers are widely used nowadays in the medical field. With advances in genomic and proteomic analysis, biomarkers are likely to succeed in disease diagnosis and treatment. Several proinflammatory and bone homeostasis molecules have been studied and evaluated for use as biomarkers for periodontitis, such as interleukin-1β (IL-1β), tumor necrosis factor-alpha, IL-6, IL-8, IL-17, C-reactive protein, matrix metalloproteinases, prostaglandins, osteocalcin, receptor activator of nuclear factor κB ligand (RANKL), and osteoprotegerin (OPG).33,34 Although using these mediators might improve the diagnosis and prognosis of periodontal disease, none has been confirmed until now as a definitive biomarker.35
Bone homeostasis is maintained by balanced activity between bone-forming cells and bone-resorbing cells. Two major pathways are involved in this process. The first pathway is mediated via the balance between RANKL and OPG. Osteoclast differentiation and activation are stimulated by the interaction of RANK with its ligand, RANKL. Periodontal inflammation causes an imbalance in the osteoblast—osteoclast axis that favors osteoclastic bone resorption.36 The second pathway is the Wnt/ß-catenin signaling, which is essential for bone formation, and its related inhibitors produced by osteocytes such as DKK1 have been involved in several inflammatory and bone disorders.37 The DKK1 serum levels reflect the suppression of bone formation.38 In addition, high DKK1 expression reduces alkaline phosphatase activity and endogenous β-catenin, while silencing Wnt receptor mRNAs inhibited alkaline phosphatase activity, which is essential for osteoblast differentiation.39 However, the role of Wnt/ß-catenin signaling and its regulators in periodontal disease needs to be further elucidated.
The present study explored the effect of nonsurgical periodontal therapy on DKK1 levels in smoker and nonsmoker patients with periodontitis. Periodontal treatment positively improved the clinical parameters and significantly reduced the DKK1 serum levels in both groups when compared to the baseline. The smokers in the present study had worse periodontal clinical parameters than the nonsmokers, which is consistent with the findings of several studies.40,41 In the current study, there was an improvement in periodontal outcomes after periodontal therapy in both the smokers and the nonsmokers, but the response was higher in the nonsmokers than the smokers. When comparing periodontal parameters six weeks after nonsurgical periodontal therapy in the nonsmokers versus the smokers, there was a considerable reduction in PI and PD in the nonsmokers compared to the smokers. These results are in line with a previous report, reflecting the significant impact of smoking on periodontal tissues and the response to periodontal therapy.41
We hypothesized that there would be no significant difference in the DKK1 serum levels in smokers and nonsmokers with periodontitis after nonsurgical periodontal therapy. However, there was a significant decrease in DKK1 serum levels after nonsurgical periodontal therapy at six weeks in both the smokers and the nonsmokers, but there was no significant difference between the two groups. Despite the lack of statistically significant differences between the groups, the DKK1 levels were still greater in the smokers compared to the nonsmokers. These results demonstrate the beneficial effects of nonsurgical periodontal therapy on DKK1 reduction in patients with periodontitis.
Previous studies have shown that the mRNA expression and protein levels of DKK1 are significantly increased in the gingival specimen as well as the serum of patients with chronic periodontitis compared to those without periodontitis.26,28 Similarly, DKK1 has been reported to be upregulated in patients with chronic periodontitis presenting with type II diabetes and/or smoking.27 Moreover, GCF-DKK1 levels have been found to be associated with periodontal bone loss, periodontitis, and its severity in patients with early rheumatoid arthritis.42 Both DKK1 and smoking were found to impact bone homeostasis negatively, DKK1 could block the expression of Wnt signaling, consequently decreasing OPG expression and resulting in bone loss.43 Additionally, long lifetime smoking exposure could negatively impact local OPG production, increasing RANKL/OPG ratio and resulting in bone resorption.44
This study has some limitations including the small sample size and only assessed the effect of nonsurgical periodontal therapy on systemic levels of DKK1. Further studies are needed to evaluate the effect of nonsurgical periodontal therapy on levels of DKK1 in gingival crevicular fluid and saliva. Despite these limitations, the main strength of this study is that it provides insight into the effect of nonsurgical treatment on DKK1 levels in patients with periodontitis.
Conclusion
Although nonsurgical periodontal treatment effectively improved the periodontal clinical parameters and reduced the DKK1 serum levels, there were no significant differences in the DKK1 serum levels between the smokers and nonsmokers with periodontitis.
Abbreviations
DKK1, Dickkopf-Related Protein-1; PD, probing depth; CAL, clinical attachment loss; PI, plaque index; BI, bleeding index; LRP5/6, Lipoprotein receptor-related protein 5/6; ELISA, enzyme-linked immunosorbent assay (ELISA); SE, standard error; GCF, Gingival crevicular fluid; IL-1β, Interleukin-1β; TNF-α, tumor necrosis factor -α; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor κB ligand.
Ethics Approval and Consent to Participate
This study was approved by Umm Al-Qura University Faculty of Dentistry Institutional Review Board Committee (IRB: 157-19) and followed the guidelines of Helsinki declaration. The signed consent form was acquired from all participants before enrollment in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lovegrove JM. Dental plaque revisited: bacteria associated with periodontal disease. J N Z Soc Periodontol. 2004;87(87):7–21.
2. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi:10.1016/S0140-6736(05)67728-8
3. Bascones-Martinez A, Munoz-Corcuera M, Noronha S, Mota P, Bascones-Ilundain C, Campo-Trapero J. Host defence mechanisms against bacterial aggression in periodontal disease: basic mechanisms. Med Oral Patol Oral Cir Bucal. 2009;14(12):e680–685. doi:10.4317/medoral.14.e680
4. Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82(2):82–90. doi:10.1177/154405910308200202
5. Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7(1):3–7.
6. Bergstrom J, Preber H. Tobacco use as a risk factor. J Periodontol. 1994;65(5 Suppl):545–550. doi:10.1902/jop.1994.65.5s.545
7. Bergstrom J, Eliasson S, Preber H. Cigarette smoking and periodontal bone loss. J Periodontol. 1991;62(4):242–246. doi:10.1902/jop.1991.62.4.242
8. Haffajee AD, Socransky SS. Relationship of cigarette smoking to the subgingival microbiota. J Clin Periodontol. 2001;28(5):377–388. doi:10.1034/j.1600-051x.2001.028005377.x
9. Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors–tobacco smoking. J Clin Periodontol. 2005;32(6):180–195. doi:10.1111/j.1600-051X.2005.00786.x
10. Matthews JB, Chen FM, Milward MR, et al. Effect of nicotine, cotinine and cigarette smoke extract on the neutrophil respiratory burst. J Clin Periodontol. 2011;38(3):208–218. doi:10.1111/j.1600-051X.2010.01676.x
11. Johnson GK, Slach NA. Impact of tobacco use on periodontal status. J Dent Educ. 2001;65(4):313–321. doi:10.1002/j.0022-0337.2001.65.4.tb03401.x
12. Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33–53. doi:10.1111/j.1600-0757.1997.tb00191.x
13. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–759. doi:10.1038/s41579-018-0089-x
14. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi:10.1210/endo.142.12.8536
15. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79(5–6):243–253. doi:10.1007/s001090100226
16. Chatzopoulos GS, Mansky KC, Lunos S, Costalonga M, Wolff LF. Sclerostin and WNT-5a gingival protein levels in chronic periodontitis and health. J Periodontal Res. 2019;54(5):555–565. doi:10.1111/jre.12659
17. Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … and more. Endocr Rev. 2013;34(5):658–690. doi:10.1210/er.2012-1026
18. Heino TJ, Kurata K, Higaki H, Vaananen HK. Evidence for the role of osteocytes in the initiation of targeted remodeling. Technol Health Care. 2009;17(1):49–56. doi:10.3233/THC-2009-0534
19. Uda Y, Azab E, Sun N, Shi C, Pajevic PD. Osteocyte mechanobiology. Curr Osteoporos Rep. 2017;15(4):318–325. doi:10.1007/s11914-017-0373-0
20. Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19(13):1842–1844. doi:10.1096/fj.05-4221fje
21. Silverman SL. Sclerostin. J Osteoporos. 2010;2010:941419. doi:10.4061/2010/941419
22. van Bezooijen RL, Roelen BA, Visser A, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–814. doi:10.1084/jem.20031454
23. Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev. 2012;33(5):747–783. doi:10.1210/er.2011-1060
24. Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–19887. doi:10.1074/jbc.M413274200
25. Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775. doi:10.1074/jbc.M504308200
26. Napimoga MH, Nametala C, da Silva FL, et al. Involvement of the Wnt-beta-catenin signalling antagonists, sclerostin and dickkopf-related protein 1, in chronic periodontitis. J Clin Periodontol. 2014;41(6):550–557. doi:10.1111/jcpe.12245
27. Miranda TS, Napimoga MH, Feres M, et al. Antagonists of Wnt/beta-catenin signalling in the periodontitis associated with type 2 diabetes and smoking. J Clin Periodontol. 2018;45(3):293–302. doi:10.1111/jcpe.12854
28. Liu M, Kurimoto P, Zhang J, et al. Sclerostin and DKK1 inhibition preserves and augments alveolar bone volume and architecture in rats with alveolar bone loss. J Dent Res. 2018;97(9):1031–1038. doi:10.1177/0022034518766874
29. Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–251. doi:10.1196/annals.1384.028
30. Barros SP, Williams R, Offenbacher S, Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 2000. 2016;70(1):53–64. doi:10.1111/prd.12107
31. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018;89(12):1475. Erratum for: J Periodontol. 2018;89 Suppl 1:S159-S172. doi:10.1002/JPER.18-0006
32. Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011;38(11):85–105. doi:10.1111/j.1600-051X.2010.01670.x
33. Kinney JS, Morelli T, Oh M, et al. Crevicular fluid biomarkers and periodontal disease progression. J Clin Periodontol. 2014;41(2):113–120. doi:10.1111/jcpe.12194
34. Ghallab NA. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: review of the current evidence. Arch Oral Biol. 2018;87:115–124. doi:10.1016/j.archoralbio.2017.12.022
35. Periodontitis O. American academy of periodontology task force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86(7):835–838. doi:10.1902/jop.2015.157001
36. Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. J Clin Periodontol. 2012;39(3):239–248. doi:10.1111/j.1600-051X.2011.01810.x
37. Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–192. doi:10.1038/nm.3074
38. Anastasilakis AD, Polyzos SA, Gkiomisi A, Bisbinas I, Gerou S, Makras P. Comparative effect of zoledronic acid versus denosumab on serum sclerostin and dickkopf-1 levels of naive postmenopausal women with low bone mass: a randomized, head-to-head clinical trial. J Clin Endocrinol Metab. 2013;98(8):3206–3212. doi:10.1210/jc.2013-1402
39. Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD
40. Sreedevi M, Ramesh A, Dwarakanath C. Periodontal status in smokers and nonsmokers: a clinical, microbiological, and histopathological study. Int J Dent. 2012;2012:571590. doi:10.1155/2012/571590
41. Chang J, Meng HW, Lalla E, Lee CT. The impact of smoking on non-surgical periodontal therapy: a systematic review and meta-analysis. J Clin Periodontol. 2021;48(1):60–75. doi:10.1111/jcpe.13384
42. Romero-Sanchez C, Giraldo S, Heredia PA, et al. Association of serum and crevicular fluid dickkopf-1 levels with disease activity and periodontitis in patients with early rheumatoid arthritis. Curr Rheumatol Rev. 2021. doi:10.2174/1573397117666211116105118
43. Fujita K, Janz S. Attenuation of WNT signaling by DKK-1 and −2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. doi:10.1186/1476-4598-6-71
44. Tang TH, Fitzsimmons TR, Bartold PM. Effect of smoking on concentrations of receptor activator of nuclear factor kappa B ligand and osteoprotegerin in human gingival crevicular fluid. J Clin Periodontol. 2009;36(9):713–718. doi:10.1111/j.1600-051X.2009.01444.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

