Back to Journals » Infection and Drug Resistance » Volume 12
The impact of lifestyle upon the probability of late bacterial infection after soft-tissue filler augmentation
Authors Marusza W, Olszanski R, Sierdzinski J , Szyller K, Ostrowski T, Gruber-Miazga J, Netsvyetayeva I
Received 4 January 2019
Accepted for publication 28 March 2019
Published 23 April 2019 Volume 2019:12 Pages 855—863
DOI https://doi.org/10.2147/IDR.S200357
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Wojciech Marusza,1 Romuald Olszanski,2 Janusz Sierdzinski,3 Kamila Szyller,1 Tomasz Ostrowski,4 Joanna Gruber-Miazga,1 Irina Netsvyetayeva5
1Academy of Face Sculpting, Warsaw, Poland; 2Military Institute of Health Services, Warsaw, Poland; 3Department of Medical Informatics and Telemedicine, Medical University of Warsaw, Warsaw, Poland; 4Department of General and Endocrine Surgery, Medical University of Warsaw, Warsaw, Poland; 5Department of Microbiology, Medical University of Warsaw, Warsaw, Poland
Purpose: Little is known about the influence of lifestyle-related factors upon the risk of late bacterial infection (LBI) emerging at the site of soft-tissue filler augmentation. The aim of this study was to analyze the impact of some such factors on the risk of LBI by comparing their respective prevalence between two groups of previously healthy women: a group in which infection occurred at a site of cross-linked hyaluronic acid (HA) augmentation and a second group which did not have such an infection.
Patients and methods: The infection group featured 25 women who developed LBI at a site of cross-linked HA augmentation; the control group featured 92 women who did not experience complications during a 24-month period of observation after the same procedure. Data was analyzed statistically using Chi-square tests and logistic regression.
Results: The two groups did not differ significantly in terms of age. However, the frequency of antibiotic therapy, household pet ownership, occupation, hormone replacement therapy or contraception use, and attendance at a swimming pool, sauna, or gym attendance were found to vary with statistical significance, P<0.05.
Conclusions: Women in the control group practiced a more active lifestyle. Antibiotic therapy in the year preceding cross-linked HA augmentation was a factor which rendered a patient predisposed towards the development of LBI. Pet ownership was more prominent among women who did not suffer LBI than within the group in which soft tissue filler-related complications had occurred.
Keywords: late bacterial infection, bacterial biofilm, lifestyle, hyaluronic acid, soft tissue fillers
Introduction
Cosmetic procedures involving soft-tissue filler augmentation have become rather common. For example, in the United States, 2,676,970 soft-tissue filler augmentations were conducted in 2018 alone. Cross-linked hyaluronic acid (HA) augmentation procedures, characterized by minimal invasiveness, are among the most often undertaken and their number has been steadily increasing. The number of such procedures undertaken in the United States alone was 2,091,476 in 2017, and by 2018 had increased to 2,128,923; these procedures involved a range of HA gels, including Juvederm Ultra, Juvederm Ultra Plus, Perlane, Restylane and Belotero.1 Late bacterial infections (LBI) at the sites of soft-tissue filler augmentation, such as cross-linked hyaluronic acid (HA), are relatively rare but are difficult to treat and often cause permanent disfigurement to the faces of patients.2 Such complications occur in 0.01–0.1% of the total number of procedures carried out.3,4 These aesthetic procedures should thus be performed in compliance with guidelines that minimize the risks of complications.5 Meticulous evaluation of whether a patient qualifies for a procedure is therefore essential for patient safety. This entails assessing both general and dermatological health so that appropriate adjustments can be made.6 It is of particular importance to assess for patient-dependent risk factors via a detailed medical interview. When such risk factors are detected, it may be necessary to reduce the scope of the procedure, or in some cases, avoid the procedure altogether, in order to ensure patient safety.7 Relative contraindications for soft-tissue filler procedures include active skin infections, active localized infections (eg, ear, nose, dental abscess), and active generalized infections (eg, gastroenteritis, urinary bladder infection, active collagenosis, immune compromise, autoimmune conditions, catabolic status, tuberculosis). Absolute contraindications to soft-tissue filler procedures include skin inflammation (eg, atopic, allergic dermatitis, sensitive skin syndrome, seborrheic dermatitis, active acne rosacea), non-infectious gastrointestinal conditions, transplant patients, thyroid dysfunction, and cutaneous collagenosis.7,8 Aseptic and antiseptic application is fundamental in mitigating the risk of infection in the setting of procedures compromising skin integrity. Skin disinfection, the use of sterile tools and materials, contamination avoidance and infection prevention are thus key measures.6,9 Early infection occurring within the first week after the procedure is usually directly related to a lack of compliance with such measures5 and are most commonly caused by Staphylococcus aureus or Streptococcus pyogenes.10 Late infection occurring within several weeks to months after the procedure is more likely to be related to the formation of bacterial biofilm, for which culture tests are usually negative.10 Late infection cannot be completely prevented merely by the application of aseptic and antiseptic measures.2,5 In addition, several factors that are known to increase the risk of LBI have not yet been fully elucidated. In order to mitigate the risk of such infection, patients are usually advised to abstain from the application of makeup throughout the month preceding the procedure and the administration of broad-spectrum antibiotics as perioperative prophylactics has been encouraged.2 In recent years, associations between particular lifestyle factors and the risk of developing a variety of illnesses including infections, have been reported.11–13 Lifestyle-related factors that are likely to affect the risk of LBI occurrence after invasive soft-tissue filler augmentation, however, have not been investigated thus far.
This prospective study aimed to compare the prevalence of selected lifestyle-related factors among patients managed at various aesthetic clinics for LBI that emerged after cross-linked HA augmentation with that of patients who underwent similar procedures with comparable doses of cross-linked HA and, in the following 24-month post-procedure observation period, were not found to suffer any complications at the injection site. In carrying out this study, we aimed to elucidate whether lifestyle affects the occurrence of LBI in the setting of cross-linked HA augmentation.
Materials and methods
The study was conducted at Akademia Rzeźbienia Twarzy (Academy of Face Sculpting – Warsaw, Poland) between 2012 and 2018.
Participants
We recruited healthy female patients, for control group, from across Poland who underwent aesthetic procedures involving cross-linked HA augmentation at the Akademia Rzeźbienia Twarzy.
Infection group was recruited from women who sought treatment for LBI at the Akademia Rzeźbienia Twarzy after HA augmentation at other private clinics across the country
Criteria for study inclusion
Patients were recruited if they were not suffering from extant skin inflammation (except for LBI at a site of HA augmentation) or other localized inflammation (eg, periodontal lesions, sepsis, hormonal dysfunctions requiring treatment other than hormone replacement therapy or contraception, autoimmunological diseases, immunosuppression). All patients underwent subcutaneous skin augmentation with at least 1 ml of filler containing 20–26 ng/ml of stabilized cross-linked HA gel. Following the acquisition of written consent, all patients were interviewed in order to obtain data pertaining to lifestyle.
Exclusion criteria
Allergic reaction to administered cross-linked HA gel.
Early bacterial infection in the site of HA augmentation.
Incompletely or erroneously filled questionnaire
Study group
In total, 30 women were referred for inclusion in the study. Of these, five were found to be non-eligible: 3 were diagnosed to have an allergic reaction to HA gel, while the other two developed an early bacterial infection which occurred within 7 days of HA augmentation. Hence, the infection group consisted of 25 Polish women who sought treatment for LBI at the Akademia Rzeźbienia Twarzy after HA augmentation at other private clinics across the country. This study group is shown in Table 1. All patients from the infection group successfully completed the study and all presented with redness, swelling, pain, induration, and the discharge of pus at the site of cross-linked HA augmentation within 1–18 months after the procedure.
 | Table 1 Description of the study group |
Control group
The control group consisted of 92 Polish female regular patients of the Akademia Rzeźbienia Twarzy who had undergone at least two cosmetic facial procedures involving at least 1 ml of subcutaneous filler containing 20–26 ng/ml of stabilized cross-linked HA gel in each procedure and suffered no complications over the course of a 24-month observation period that started from the first procedure and lasted until study inclusion.
Lifestyle-related factors
• Age;
• Occupation;
• Antibiotic therapy during the 12 months preceding the study;
• Hospitalizations during the 12 months preceding the study;
• Household pet ownership (eg, dog, cat, small rodent);
• Reproductive state (menses or menopause);
• Hormone replacement therapy or the use of contraceptives;
• Gym attendance at least once a week;
• Swimming pool attendance at least once a week;
• Sauna attendance at least once a month;
• Solarium attendance at least once a month.
Statistical analysis
SAS 9.4 software suite (SAS Institute Inc., Cary, NC,USA) was used for all data analysis and P-values <0.05 were considered to be statistically significant. Basic statistics were used to describe groups and characterize variables. In order to demonstrate the significance of differences between the infection and control groups, we applied Mann–Whitney U and Chi-square (Chisq) tests. Logistic regression was used to investigate independent factors capable of influencing the development of LBI development.
Results
The mean age of the infection group (n=25 women) was 46.80 years, while the mean age of the control group (n=92 women) was 45.26 years (Table 2).
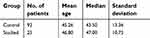 | Table 2 Patient age comparison between the two groups of subjects |
In the infection group, 13 patients (52%) had non-medical white-collar professions (bank clerk, public sector officer, sale manager, businesswoman, accountant, teacher, attorney, lawyer, information technology specialist, judge, architect, economist, academic teacher, journalist, secretary, engineer), 4 (16%) had medical or paramedical occupations (cosmetician, nurse, medical doctor, veterinarian, dentist), and 8 (32%) were housewives (housewife, pensioner).
In the control group, 66 patients (71.74%) had non-medical white-collar professions, 17 (18.48%) had medical or paramedical occupations, and 9 (9.78%) were housewives (Table 3). Data comparing hospitalization, antibiotic therapy, hormone replacement therapy or the use of contraceptives, household pet ownership, as well as attendance at the swimming pool, gym, sauna, or solarium, between the two groups are presented in Table 4.
 | Table 3 The occupations of our study cohort |
 | Table 4 Comparison of the prevalence of selected lifestyle factors between the two groups |
Antibiotics were used within the 12 months prior to study inclusion by 20 women (80%) from the infection group and 26 women (28.26%) from the control group. During the 12 months prior to study inclusion, 20 women from the infection group underwent 37 antibiotic treatments, while 26 women from the control group underwent 30 antibiotic treatments. The mean frequencies of antibiotic use among patients from respective groups are presented in Table 5. Statistical analyses revealed significant differences (P<0.05) in the total number of antibiotic therapies used, hormone replacement therapy or the use of contraceptives, household pet ownership, as well as attendance at the swimming pool, sauna, and gym when comparing patients from the infection group to those from the control group.
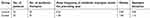 | Table 5 Comparison of the mean frequencies of antibiotic use in the two groups over the 12 months preceding the study |
Multifactorial logistic regression revealed that use of antibiotic therapy, use of hormone replacement therapy or contraception, household pet ownership, and sauna attendance all had significant impact upon the probability of LBI development. Logistic regression data are presented in Table 6. This statistical test analyzed many concomitant independent variables in order to assess their relative impact upon the presence of the analyzed factor (the presence of biofilm). The results generated are of great scientific value because they identify factors which can impact upon the presence of biofilm using independent datasets.
 | Table 6 Multifactorial logistic regression analysis (Chi-square model =22.70 for P=0.0001) |
Discussion
A number of previous studies have confirmed that lifestyle can influence the risk of infection.11,13 In order to determine which lifestyle-related factors affect the prevalence of LBI after cross-linked HA augmentation, we evaluated two groups of female patients undergoing aesthetic medicinal treatment. All patients underwent augmentation with at least 1 ml of filler containing 20–26 ng/ml of stabilized cross-linked HA gel, produced in sterile conditions, approved by relevant national institutions across Western Europe and certified for use in aesthetic procedures in Poland. The gel was subcutaneously administered into face tissues with sterile needles (27 G, 13 mm; 30 G, 13 mm) or via cannula (25 G, 40–50 mm); these were all produced in sterile conditions, approved by relevant national institutions across Western Europe and certified for use in aesthetic procedures in Poland. Consequently, our patients didn’t undergo the procedures with the use of the HA gel, needles and cannulas produced in non-sterile conditions. Therefore we didn’t consider abovementioned factors as reasons for the occurrence of LBI.
Our infection group featured 25 patients with symptoms which met the criteria for LBI diagnosis after cross-linked HA augmentation. All of these patients were successfully treated, mostly in accordance with the M&N Scheme14 (scheme of biofilm comprehensive treatment, proposed and published by Marusza and Netsvyetayeva). The control group consisted of 92 patients who had undergone the procedure at least twice yet developed no undesirable effects within a 24-month post-procedure period of observation. Only healthy patients, who exhibited neither relative nor absolute contraindications to foreign substance implantation, were included in our study.6–8 Previous studies have reported a number of lifestyle factors which could significantly influence the risk of LBI development, including antibiotic therapy, hospitalization,15–18 household pet ownership (eg, dog, cat, small rodent),19 the use of hormone replacement therapy, menopause,20 swimming pool or solarium attendance,21–23 and gym attendance.24 In the present study, we did not identify any statistically significant differences between the infection and control groups in terms of participant age. A greater proportion of patients from the infection group were unemployed and lived as housewives. In addition, a greater proportion of patients from the infection group had entered menopause whereas most of the patients in the control group used hormone replacement therapy or contraceptives. Our present findings are consistent with those from previously published studies describing a general reduction in immune function as a result of lower levels of sex hormones with aging, as well as the benefits of hormone replacement therapy. In addition, hormone replacement therapy and the use of contraceptives have been associated with more active lifestyles.20 We found that the patients in our control group attended a gym or swimming facilities more frequently than those in the infection group. To some extent, these findings contradict those arising from existing publications.21–24 Existing literature reports an increased risk of infection (particularly in terms of dermatological and pulmonary infection) in individuals who use shared gym equipment24 and frequent public swimming pools.21 Control group patients reported more frequent sauna attendance compared to those in the infection group. While sauna attendance is often associated with beneficial health effects and is considered to reduce the risk of respiratory diseases, very little evidence exists to support these claims.25 We determined that the highest predictive value for an increased risk of LBI subsequent to cross-linked HA augmentation correlated with the frequency of antibiotic use. This finding is likely to be related to antibiotics adversely affecting physiological microflora and ultimately distorting its composition.26 In turn, alterations in physiological microbial flora result in increased vulnerability to opportunistic, nosocomial infections subsequent to interruptions in the integrity of the skin or mucous membranes.15,27 Interestingly, household pet ownership in the control group patients was higher than that in the infection group. Humans have been reported to share physiological flora with their dogs,28 thereby increasing their exposure to microbial diversity.29 Nevertheless, further research is still required in terms of the human bacterial flora originating from animals. In addition, microbiota in the dust of pet-owning households is significantly more diverse than in non-pet-owning households.19,29 Numerous studies have reported that a high level of diversity in physiological microflora can precondition humans to good general health and contributes significantly to the prevention of disease.30 Such reported biodiversity is consistent with the well-known “Old Friends” hypotheses.13 In this context, direct social milieu bears significance.28 For instance, contact sport team members are known to share similar microbiota.31 While social interactions are a crucial consequence of environmental access, they also increase the microbial diversity to which individuals are exposed to.13 In the present study, we established that control group patients led more active lifestyles, in that they frequented the gym, pool, or sauna facilities more often. Furthermore, fewer patients in the control group were unemployed. Their more frequent use of hormone replacement therapy or contraception also correlated to more active lifestyles. Finally, we found that members of the control group reported more prevalent ownership of household pets and far less frequent use of antibiotics when compared to patients in the infection group. Collectively, these factors are highly likely to influence the diversity of physiological bacterial flora and thus protect against the development of LBI after soft-tissue filler augmentation procedures.
Conclusions
Our analysis showed that while patients in the infection group suffered LBI subsequent to soft-tissue filler augmentation, there were no such complications in patients in the control group who led more active lifestyles. The control group reported a higher incidence of exercising at a gym or swimming pool, attending a sauna, pursuing professional careers or using appropriately prescribed hormone replacement therapy or contraception. Use of antibiotic therapy within the 12 months preceding soft-tissue filler augmentation was shown to be a factor that most likely predisposes patients to the occurrence of LBI. We also found that pet ownership correlated with a lower likelihood of LBI following soft-tissue filler augmentation.
Abbreviation List
LBI, Late bacterial infection; HA, Hyaluronic acid gel; M&N Scheme, scheme of biofilm comprehensive treatment proposed and published by Marusza and Netsvyetayeva.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Department of Microbiology, Warsaw Medical University and was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Consent for publication
The authors hereby certify that all work contained in this article is original. All authors contributed to this research, claim full responsibility for the contents of this article, and have read and approved the final manuscript.
Availability of data and material
All available data are presented in the tables herein.
Acknowledgments
This study was not supported by any grants. The funding for the research and manuscript preparation was sourced from the following authors: Marusza W, Olszanski R, Szyller K, Ostrowski T, and Gruber-Miazga J.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1.
2. Alhede M, Bjarnsholt T. Are biofilms responsible for the adverse effects experienced following soft-tissue fillers? Future Microbiol. 2014;9(8):931–933. doi:10.2217/fmb.14.57
3. Sathianathan M, Hegde A, Vickery K, Deva AK. The role of biofilm in hyaluronic acid filler: an in vitro study. Plast Reconstr Surg. 2013;132(4S–1):100–101. doi:10.1097/01.prs.0000435976.32791.50
4. Grippaudo FR, Pacilio M, Di Girolamo M, Dierckx RA, Signore A. Radiolabelled white blood cell scintigraphy in the work-up of dermal filler complications. Eur J Nucl Med Mol Imaging. 2013;40(3):418–425. doi:10.1007/s00259-012-2334-2
5. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. doi:10.2147/CCID.S50546
6. Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Preventing the complications associated with the use of dermal fillers in facial aesthetic procedures: an expert group consensus report. Aesthetic Plast Surg. 2017;41(3):667–677. doi:10.1007/s00266-017-0798-y
7. De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;15(8):205–214. doi:10.2147/CCID.S80446
8. Christensen L. Normal and pathologic tissue reactions of soft tissue gel fillers. Dermatol Surg. 2007;33(Suppl 2):168–175.
9. Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31(1):110–121. doi:10.1177/1090820X11423764
10. Urdiales-Gálvez F, Delgado N, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498–510. doi:10.1007/s00266-018-1194-y
11. Ehlers S, Kaufmann SHE. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends Immunol. 2010;31(5):184–190. doi:10.1016/j.it.2010.02.003
12. Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: A systematic review. PLoS Negl Trop Dis. 2018;12(6):e0006577. doi:10.1371/journal.pntd.0006577
13. Flandroy L, Poutahidis T, Berg G, et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci Total Environ. 2018;15(627):1018–1038. doi:10.1016/j.scitotenv.2018.01.288
14. Marusza W, Olszanski R, Sierdzinski J, et al. Treatment of late bacterial infections resulting from soft-tissue filler injections. Infect Drug Resist. 2019;12:469–480. doi:10.2147/IDR.S186996
15. Madan JC, Salari RC, Saxena D, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):456–462. doi:10.1136/fetalneonatal-2011-301373
16. Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216–3223. doi:10.1099/mic.0.040618-0
17. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;29(352):539–544. doi:10.1126/science.aad9378
18. Van Puyvelde S, Deborggraeve S, Jacobs J. Why the antibiotic resistance crisis requires a one health approach. Lancet Infect Dis. 2018;18(2):132–134. doi:10.1016/S1473-3099(17)30704-1
19. Fujimura KE, Johnson CC, Ownby DR, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126(2):410–412. doi:10.1016/j.jaci.2010.05.042
20. Ghosh M, Rodriguez-Garcia M, Wira CR. The immune system in menopause: pros and cons of hormone therapy. J Steroid Biochem Mol Biol. 2014;142:171–175. doi:10.1016/j.jsbmb.2013.09.003
21. Barna Z, Kádár M. The risk of contracting infectious diseases in public swimming pools: a review. Ann Ist Super Sanita. 2012;48(4):374–386. doi:10.4415/ANN_12_04_05
22. Smith A, Reacher M, Smerdon W, Adak GK, Nichols G, Chalmers RM. Outbreaks of waterborne infectious intestinal disease in England and Wales, 1992-2003. Epidemiol Infect. 2006;134(6):1141–1149. doi:10.1017/S0950268806006406
23. Roser DJ, Van Den Akker B, Boase S, Haas CN, Ashbolt NJ, Rice SA. Pseudomonas aeruginosa dose response and bathing water infection. Epidemiol Infect. 2014;142(3):449–462. doi:10.1017/S0950268813002690
24. Grosset-Janin A, Nicolas X, Saraux A. Sport and infectious risk: a systematic review of the literature over 20 years. Med Mal Infect. 2012;42(11):533–544. doi:10.1016/j.medmal.2012.10.002
25. Kunutsor SK, Laukkanen T, Laukkanen JA. Sauna bathing reduces the risk of respiratory diseases: a long-term prospective cohort study. Eur J Epidemiol. 2017;32(12):1107–1111. doi:10.1007/s10654-017-0311-6
26. Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2015;6:1543.
27. Netsvyetayeva I, Marusza W, Olszanski R, et al. Skin bacterial flora as a potential risk factor predisposing to late bacterial infection after cross-linked hyaluronic acid gel augmentation. Infect Drug Resist. 2018;11:213–222. doi:10.2147/IDR.S154328
28. Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi:10.7554/eLife.00458
29. Dunn RR, Fierer N, Henley JB, Leff JW, Menninger HL. Home life: factors structuring the bacterial diversity found within and between homes. PLoS One. 2013;8(5):e64133. doi:10.1371/journal.pone.0064133
30. Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci U S A. 2013;110(46):18360–18367. doi:10.1073/pnas.1313731110
31. Meadow JF, Bateman AC, Herkert KM, O’Connor TK, Green JL. Significant changes in the skin microbiome mediated by the sport of roller derby. Peer J. 2013;1:e53. doi:10.7717/peerj.190
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
