Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The Impact of Inhaled Corticosteroids on the Prognosis of Chronic Obstructive Pulmonary Disease
Authors Park JW, Hong Y , Rhee CK , Choi HS , Kim K, Yoo KH , Jung KS, Park JH
Received 11 September 2022
Accepted for publication 6 April 2023
Published 2 May 2023 Volume 2023:18 Pages 733—743
DOI https://doi.org/10.2147/COPD.S388367
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Ji Won Park,1,* Yoonki Hong,2,* Chin Kook Rhee,3 Hye Sook Choi,4 Kyungjoo Kim,3 Kwang Ha Yoo,5 Ki-Suck Jung,6 Joo Hun Park1
1Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Suwon, South Korea; 2Department of Internal Medicine, Kangwon National University, Chuncheon, South Korea; 3Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, South Korea; 4Department of Internal Medicine, Kyung Hee University Medical Center, Seoul, South Korea; 5Department of Internal Medicine, Konkuk University School of Medicine, Seoul, South Korea; 6Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, South Korea
*These authors contributed equally to this work
Correspondence: Joo Hun Park, Department of Pulmonary and Critical Care Medicine, Ajou University School of Medicine, Worldcup road 164, Suwon, Gyeonggi-do, 16499, South Korea, Tel +82-31-219-5116 ; +82-10-8379-8299, Fax +82-31-219-5124, Email [email protected]; [email protected]
Background: A comprehensive analysis of the effects of inhaled corticosteroids (ICS) on COPD in a real-world setting is required due to safety concerns regarding ICS in COPD. This study aimed to explore the impact of ICS on the prognosis of Asian COPD patients in the real-life world.
Methods: We examined 978 COPD patients registered in the Korean National Health and Nutrition Examination Survey (KNHANES) database and with their data linked to Health Insurance and Review Assessment (HIRA) data. The outcome measures were ascertained by HIRA from January 1, 2009, to December 31, 2012. This study enrolled two arms; ICS users (N = 85, mean age = 66.7 ± 8.9 years) and non-ICS users (N = 893, mean age = 63.7 ± 9.7 years).
Results: Compared to the non-ICS users, the ICS users had a higher rate of pneumonia, tuberculosis, and acute exacerbations (P< 0.05). Hospitalization due to respiratory causes was also higher among ICS users (P< 0.05). Multivariate analysis showed that acute exacerbation was independently associated with the development of pneumonia (P< 0.05), whereas ICS therapy had a tendency to be associated with pneumonia. Another multivariate analysis demonstrated that old age, FEV1, ICS therapy, and pneumonia were independently associated with the occurrence of acute exacerbation (P< 0.05). The concomitant pneumonia (HR = 3.353, P = 0.004) was independently associated with higher mortality (P< 0.05).
Conclusion: Our data demonstrated that the ICS users had a higher rate of pneumonia and tuberculosis and the concomitant pneumonia was independently associated with higher mortality, highlighting the importance of cautious and targeted administration of ICS in COPD.
Keywords: COPD, inhaled corticosteroids, pneumonia, mortality
Introduction
Although chronic obstructive pulmonary disease (COPD) has been a major public health concern and was expected to rank third among all the causes of death by 2030, there is still no therapeutic intervention that modifies the natural course of COPD.1,2 The pharmacological treatment of COPD comprises long-acting muscarinic antagonist (LAMA), long-acting beta-agonists (LABA), the combination of LAMA and LABA, the combination of LABA and inhaled corticosteroids (ICS), and triple therapy including LABA, LAMA, and ICS. The benefits of ICS in COPD reported by various studies include the reduction of frequency of COPD exacerbations, a lower rate of hospitalization, and a reduction of all-cause mortality in some COPD subgroups.3–6 However, the use of ICS in COPD is much debated because of the associated safety concerns;7–10 there is a concern about the attendant side effects of ICS on COPD including pneumonia and tuberculosis.11,12 Consequently, the withdrawal of ICS in COPD patients is common due to ICS-related adverse events or treatment-related risks outweighing the expected benefits.13,14 In addition, ICS treatment is associated with several challenges; observational studies suggested that overprescription of ICS is common in patients with COPD and smoking can result in steroid resistance by decreasing the efficacy of ICS in COPD.13,15,16
However, so far, there are few comprehensive analyses of the effects of ICS on COPD in a real-world setting. Hence, this study was conducted to investigate the impact of ICS on the prognosis of Asian COPD patients in the real world setting by analyzing the Korean National Health and Nutrition Examination Survey (KNHANES) merged with Health Insurance and Review Assessment (HIRA) data.
Methods
Study Population
This study analyzed the KNHANES data that was linked to HIRA data, from Jan 2007 to Dec 2012. KNHANES is a nationally representative cross-sectional complex survey that includes approximately 10,000 individuals each year, collecting information on socioeconomic status, health-related behaviors, quality of life, health-care utilization, anthropometric measures, biochemical and clinical profiles for non-communicable diseases, and dietary intakes with three component surveys: health interview, health examination and nutrition survey.17 HIRA contains comprehensive and rich information on health-care services such as treatments, pharmaceuticals, procedures, and diagnoses for almost 50 million South Korean beneficiaries.18
Data on baseline comorbidities were obtained through HIRA and analyzed further for future events. Spirometry data of 17,472 subjects whose aged 40 years or older were obtained out of the 43,864 subjects of KNHANES. Among them, 2,450 patients with COPD who met the inclusion criteria were enrolled in our study. Patients with missing smoking data (N = 34), smoking amount less than 10 pack years (N = 1,277), and detection of cancer (N = 161) during the screening period from Jan 2007 to Dec 2008 were excluded (Figure 1). Finally, 978 patients linked with NHIS were analyzed for the primary and secondary outcomes of this study.
 |
Figure 1 Flow diagram of this study. |
Main Outcomes
The occurrences of events including primary and secondary outcomes were investigated during the observation period, from Jan 2009 to Dec 2012 (Figure 2). Primary outcomes included pneumonia, acute exacerbation, and mortality. Secondary outcomes comprised co-morbidities including tuberculosis, ischemic heart disease, cerebrovascular stroke, lung cancer, osteoporosis, diabetes mellitus, hypertension, arrhythmia, and heart failure. The outcome measures were ascertained by HIRA data from January 1, 2009, to December 31, 2012. The first incident event was only used in the analyses for participants with more than one event. International Classification of Disease, Tenth Revision (ICD-10) codes were used to identify outcomes as follows: lung cancer (C33, C34), osteoporosis (M80, M81, M82), diabetes (E10-E14), ischemic heart disease (I20-I25), cerebrovascular disease (I60-I69), arrhythmia (I44-I49), and heart failure (I50).
 |
Figure 2 Study design. |
Definition and Covariates
The inclusion criteria for COPD patients were as follows: (1) age ≥40 years; (2) current or former smoker with a smoking history of ≥10 pack-years; (3) pre-bronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity <0.7; (4) no history of any cancer during the screening period.
Tuberculosis was defined if ICD-10 diagnoses of TB (A15–A19, U88.0–U88.1) were met and two or more of following anti-tuberculosis medications were prescribed in 90 days: simultaneously prescribed isoniazid and rifampicin (considered as one anti-tuberculosis medicine), ethambutol, pyrazinamide, prothionamide, para-aminosalicylate, and cycloserine.19 Pneumonia was defined via the presence of ICD codes ranging from J12.x to J18.x, and prescription of antibiotics.20
An eligible 85 ICS users (13 patients on ICS, 42 patients with ICS/LABA, and 30 patients with ICS/LABA/LAMA) were identified from all the patients treated by ICS in the cohort who had a prescription for an inhaled respiratory medication for 120 days or longer during the observation period (Supplement Table 1). The ICS users consisted of all individuals who were dispensed at least one of the following inhaled respiratory medications: beclomethasone, budesonide, triamcinolone, ciclesonide, fluticasone, or flunisolide.19 A detailed history of smoking (smoking amount, duration, and non-smoking period) was evaluated by self-administered questionnaires at baseline. Former smokers were defined as smokers whose smoking cessation period was one year or more at enrollment.21 Data on body mass index (body weight in kilograms divided by height in meters squared; kg/m2), systolic and diastolic blood pressure, fasting serum glucose, and fasting total cholesterol level measured at baseline were obtained.
Measurements
According to the guidelines of the American Thoracic Society and European Respiratory Society, spirometry (Elite-DX or CPFS; Medgraphics, St Paul, MN, USA) was performed, and the acceptability and repeatability criteria of the spirometry were established.22 Normal predictive values of spirometry in the Korean population were obtained based on the second KNHANES data.23
HRQL Assessed Using the EQ-5D Instrument
The EuroQol five-dimensions five-level (EQ-5D) includes a descriptive module (two pages) and the EuroQol Visual Analogue Scale (three pages). The descriptive module explores mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has a five-level response corresponding to no problem, slight problems, moderate problems, severe problems, and extreme problems.24
Statistical Analyses
All values were expressed as mean ± standard deviation for continuous data and number (percent) for categorical data. Student’s t-test, one-way ANOVA test, or Kruskal–Wallis test was performed for continuous variables. χ2 test or Fisher’s exact test was used for categorical data. A P< 0.05 was considered to be significant. A Cox proportional hazard regression analysis was performed to find the significant variables associated with the development of pneumonia, the occurrence of acute exacerbation, and mortality. A P-value of less than 0.05 was deemed significant. All statistical analyses were performed using SAS version 9.2 software (SAS Institute Inc., Cary, NC, USA).
Ethics Statement
The current study was approved by the institutional review board of Konkuk University Medical Center (Approval Number = 177 KHH1010338). The data accessed complied with relevant data protection and privacy regulations. The requirement for informed consent was waived by the ethics review board due to the retrospective nature of the study.
Results
Baseline Characteristics of the Patients with COPD
Baseline characteristics (n = 978) are presented in Table 1; the mean age was 64.0 ± 9.6 years, mean FEV1 was 76.0 ± 15.6% (pre-bronchodilator), 52.6% were current smokers, and 95.9% were males. Co-morbidities at the time of enrollment were diabetes mellitus (12.2%), hypertension (33.2%), coronary artery disease (3.8%), myocardial infarction (1.6%), and cerebrovascular stroke (0.8%). At the time of enrollment, the ICS users had a higher smoking amount, lower EQ-5D score, lower FEV1, and FEV1/FVC than non-ICS users (P<0.05) (Supplement Table 2).
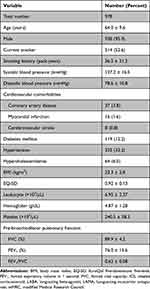 |
Table 1 Baseline Characteristics of the Patients with Chronic Obstructive Pulmonary Disease |
Adverse Events Based on ICS Usage and the Severity of COPD
Compared to non-ICS users, the ICS users had a higher rate of pneumonia, tuberculosis, acute exacerbation, and lung cancer. Hospitalization due to respiratory causes was higher among the ICS users (P<0.05) (Table 2). The incidence of lung cancer was higher among ICS users in moderate COPD subgroup, although the number of cases was small (P < 0.05) (Table 3). Hospital admissions due to respiratory causes were higher among the ICS users in mild and moderate COPD subgroups (Table 3) (P < 0.05). However, there was no difference in the mortality rate between ICS users and non-ICS users in all the COPD subgroups (Table 3). The rate of pneumonia infection was higher among ICS users in all the mild, moderate, and severe COPD subgroups, whereas the rate of pulmonary tuberculosis was higher among ICS users in moderate COPD subgroup (Table 3, Supplement Table 3) (P < 0.05).
 |
Table 2 Adverse Events Based on ICS Usage from 2009 to 2012 |
 |
Table 3 Adverse Events According to the Severity of COPD |
Risk factors associated with primary outcomes include the development of pneumonia, acute exacerbation, and mortality.
Multi-variable Cox regression analyses were performed in two models: Model 1 (adjusted for age, sex, smoking, FEV1 and significant factors in the univariate analysis), Model 2 (including all factors). Variables included in the primary outcomes by univariate Cox regression analysis are presented in Table 4–6. The multivariable Cox regression analysis revealed that the development of pneumonia was significantly associated with acute exacerbation (model 1, Hazard ratio (HR) = 17.016, P < 0.001; model 2, HR = 16.860, P < 0.001) and had a tendency to be associated with ICS therapy (model 1, HR = 1.554, P = 0.074; model 2, HR = 1.574, P = 0.067) (Table 4). Another multivariable Cox regression analysis (model 2) showed that old age (HR = 1.043, P < 0.001), FEV1 (HR = 0.972, P < 0.001), ICS therapy (HR = 4.040, P < 0.001), and pneumonia (HR = 6.610, P < 0.001) were independently associated with the occurrence of acute exacerbation (Table 5). The concomitant pneumonia (model 1, HR = 3.113, P = 0.001; model 2, HR = 3.353, P = 0.004) was independently associated with higher mortality (Table 6).
 |
Table 4 Risk Factors Associated with the Development of Pneumonia |
 |
Table 5 Risk Factors Associated with the Development of Acute Exacerbation (None Vs ≥1) |
 |
Table 6 Risk Factors Associated with Mortality |
Discussion
Our study aimed to explore the impact of ICS on the prognosis of Asian COPD patients in the real-world setting. Our study also provides a comprehensive analysis of the clinical course of COPD in the light of the development of comorbidities according to ICS intervention.
Our Study Presents Some Interesting Findings
First, our data demonstrated that ICS therapy was associated with the development of pneumonia in univariate analysis along with a tendency to have an association with pneumonia through multivariate analysis. Also, ICS users in COPD of this study had a higher rate of tuberculosis and were accompanied by more frequent hospitalization due to respiratory diseases. However, the number of subjects enrolled in our study is not big enough to conclude the harmful side effects of ICS in subgroups of COPD. The study was also limited by its nature of not being a randomized controlled trial and lower baseline pulmonary function in the ICS group. Therefore, the subgroup analysis based on COPD severity was performed considering the lower baseline FEV1 in ICS users. This subgroup analysis also showed that ICS users had a higher rate of pneumonia in all COPD subgroups and had a higher admission rate due to respiratory causes in mild and moderate COPD subgroups. These data are supported by a recent study in a real-world setting where the incidence of severe pneumonia requiring hospitalization was higher with the use of a triple combination of LAMA, LABA, and ICS compared to a combination of LAMA and LABA.9 A meta-analysis in COPD treatment showed that the incidence of adverse events including pneumonia was lower in LABA/LAMA versus LABA/ICS, and LABA/LAMA presented a lower risk for withdrawals due to lack of efficacy versus LAMA and adverse events versus LABA/ICS.10
Recent randomized controlled trials provided some bodies of evidence on the benefits of ICS in the COPD subtype with high blood eosinophil count and frequent exacerbation.3,4 However, other observational studies reported that overprescription of ICS in COPD is widespread and clinicians should consider the potential harm even when appropriately prescribed in COPD.13,25 Previous studies supporting withdrawal of ICS in a group of COPD patients for whom the risk of ICS-related adverse effects outweigh the expected benefits or the clinical benefits are unproven.25,26 Potential negative health consequences related to inappropriate prescribing of ICS include a high incidence of respiratory infection, hoarseness, skin bruising, decreased bone density, fracture, increased risk of diabetes, and cataract.7,26 ICS therapy can increase the risk of respiratory infections including not only pneumonia but also oropharyngeal candidiasis, tuberculosis, and nontuberculous mycobacterial pulmonary infection through the impairment of bactericidal activity, interleukin-1, tumor necrosis factor α production, and T-cell activation.11,12,19,27–30 In addition, Suissa et al reported that weaning of ICS in COPD has decreased the rate of serious pneumonia by 20% in the first month and to 50% by the fourth month after discontinuation.8 Therefore, targeted ICS therapy is required in COPD where the clinical benefits can outweigh the harmful risks.
Second, the current study revealed that the concomitant pneumonia was independently associated with a higher rate of exacerbation and higher mortality. Therefore, this study suggests that the development of pneumonia in COPD functioning as a predictor of acute exacerbation and mortality should be deemed an important clinical outcome. Although COPD patients were at increased risk for pneumonia, it remained controversial whether a concomitant pneumonia in COPD is associated with higher mortality.31–33 It was reported that a pneumonia during hospitalization due to acute exacerbations of COPD increased in-hospital morbidity comprising increased length of hospital stay and more frequent ICU admissions.33–36 Some studies showed that community acquired pneumonia in COPD was associated with higher mortality, in addition to other worse outcomes including longer hospital stay and more ICU admissions.33,36 On the other hand, a few studies reported otherwise that the mortality during hospitalization is not affected by pneumonia in COPD, although pneumonia can increase in-hospital morbidity in COPD.34,35 Hence, this topic merits further verification.
Third, the occurrence of acute exacerbation in this cohort was independently associated with pneumonia and ICS therapy, along with older age and lower FEV1. Our finding that ICS therapy was associated with exacerbations should be interpreted with caution considering the observational study design. Since ICS therapy is indicated in COPD patients with frequent exacerbations, the frequent exacerbations associated with ICS therapy may simply reflect a clinical practice of prescribing ICS therapy in COPD exacerbators in South Korea and cannot explain the causal link without a randomized control design study. While big randomized controlled trials including Wisdom and FLAME supported a safe withdrawal of ICS in COPD, a recent IMPACT trial reported that triple therapy including ICS, LAMA, and LABA resulted in a lower rate of COPD exacerbations and hospitalization and even improved the survival rate.3,37,38 Therefore, further validation seems to be required in this area.
We Acknowledge Several Limitations of This Study
First, analysis on blood eosinophil was not performed due to a lack of data in the KNAHES. Second, post-bronchodilator values were not obtained due to the lack of information in the KNHANES data, and the definition of COPD by less than 70% of prebronchodilator FEV1/FVC can harbor the potential risk of overdiagnosis. Third, the small number of ICS users (85 out of 978, 8.7%) is a limitation in the interpretation of our analysis, therefore this study cannot determine the causal link between ICS therapy and adverse events. Fourth, we cannot rule out the influence of unmeasured confounders despite subgroup analysis of our data. Fifth, COPD patients of this cohort were not treated by current therapeutic strategies because observation period is not up-to-date. Sixth, a higher rate of lung cancer in ICS users cannot be generalized because of lower FEV1 and higher smoking amount in ICS users.
In conclusion, our data demonstrated that the ICS users had a higher rate of pneumonia and tuberculosis and the concomitant pneumonia was independently associated with higher mortality, highlighting the importance of cautious and targeted administration of ICS in COPD.
Acknowledgment
We thank Kyungjoo Kim for the statistical analysis. Ji Won Park and Yoonki Hong are co-first authors of this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the research program funded from Korea National Institute of Health (Funding code 2016ER670100, 2016ER670101, 2016ER670102, 2018ER67100, 2018ER67101, 2018ER67102, and 2021ER120500) and by National Research Foundation of Korea funded from Korean Government (NRF-2021R1I1A3056129).
Disclosure
All authors report no potential conflicts of interest in this work.
References
1. Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi:10.1164/rccm.200703-456SO
2. Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):502–506. doi:10.1513/pats.200701-001FM
3. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
4. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
5. Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–338. doi:10.1164/rccm.200712-1869OC
6. Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(12):1508–1516. doi:10.1164/rccm.201911-2207OC
7. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012(7):Cd002991. doi:10.1002/14651858.CD002991.pub3
8. Suissa S, Coulombe J, Ernst P. Discontinuation of inhaled corticosteroids in COPD and the risk reduction of pneumonia. Chest. 2015;148(5):1177–1183. doi:10.1378/chest.15-0627
9. Suissa S, Dell’Aniello S, Ernst P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: cohort Study in Real-World Clinical Practice. Chest. 2020;157(4):846–855. doi:10.1016/j.chest.2019.11.007
10. Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi:10.2147/COPD.S130482
11. Kim JH, Park JS, Kim KH, Jeong HC, Kim EK, Lee JH. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest. 2013;143(4):1018–1024. doi:10.1378/chest.12-1225
12. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
13. Avdeev S, Aisanov Z, Arkhipov V, et al. Withdrawal of inhaled corticosteroids in COPD patients: rationale and algorithms. Int J Chron Obstruct Pulmon Dis. 2019;14:1267–1280. doi:10.2147/COPD.S207775
14. Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;6(11):855–862. doi:10.1016/S2213-2600(18)30368-0
15. Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745–756. doi:10.1016/S2213-2600(19)30190-0
16. Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J. 2005;25(3):552–563. doi:10.1183/09031936.05.00117504
17. Kweon S, Kim Y, Jang MJ, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014;43(1):69–77. doi:10.1093/ije/dyt228
18. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32(5):718–728. doi:10.3346/jkms.2017.32.5.718
19. Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68(12):1105–1113. doi:10.1136/thoraxjnl-2012-203175
20. Kim MH, Rhee CK, Shim JS, et al. Inhaled corticosteroids in asthma and the risk of pneumonia. Allergy Asthma Immunol Res. 2019;11(6):795–805. doi:10.4168/aair.2019.11.6.795
21. Sheen S, Sun JS, Park JH, et al. Unique features of non-obstructive emphysema and pure airway obstruction. Int J Tuberc Lung Dis. 2014;18(1):109–116. doi:10.5588/ijtld.13.0258
22. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
23. Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58(3):230–242. doi:10.4046/trd.2005.58.3.230
24. Choi HS, Yang DW, Rhee CK, et al. The health-related quality-of-life of chronic obstructive pulmonary disease patients and disease-related indirect burdens. Korean J Intern Med. 2020;35(5):1136–1144. doi:10.3904/kjim.2018.398
25. Cataldo D, Derom E, Liistro G, et al. Overuse of inhaled corticosteroids in COPD: five questions for withdrawal in daily practice. Int J Chron Obstruct Pulmon Dis. 2018;13:2089–2099. doi:10.2147/COPD.S164259
26. White P, Thornton H, Pinnock H, Georgopoulou S, Booth HP. Overtreatment of COPD with inhaled corticosteroids--implications for safety and costs: cross-sectional observational study. PLoS One. 2013;8(10):e75221. doi:10.1371/journal.pone.0075221
27. Suissa S, McGhan R, Niewoehner D, Make B. Inhaled corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4(7):535–542. doi:10.1513/pats.200701-024FM
28. Dekhuijzen PNR, Batsiou M, Bjermer L, et al. Incidence of oral thrush in patients with COPD prescribed inhaled corticosteroids: effect of drug, dose, and device. Respir Med. 2016;120:54–63. doi:10.1016/j.rmed.2016.09.015
29. Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J. 2017;50:3. doi:10.1183/13993003.00037-2017
30. Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006;27(2):413–426. doi:10.1183/09031936.06.00125404
31. Loke YK, Kwok CS, Wong JM, Sankaran P, Myint PK. Chronic obstructive pulmonary disease and mortality from pneumonia: meta-analysis. Int J Clin Pract. 2013;67(5):477–487. doi:10.1111/ijcp.12120
32. Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. doi:10.1378/chest.128.4.2099
33. Myint PK, Lowe D, Stone RA, Buckingham RJ, Roberts CM. U.K. National COPD Resources and Outcomes Project 2008: patients with chronic obstructive pulmonary disease exacerbations who present with radiological pneumonia have worse outcome compared to those with non-pneumonic chronic obstructive pulmonary disease exacerbations. Respiration. 2011;82(4):320–327. doi:10.1159/000327203
34. Huerta A, Crisafulli E, Menéndez R, et al. Pneumonic and nonpneumonic exacerbations of COPD: inflammatory response and clinical characteristics. Chest. 2013;144(4):1134–1142. doi:10.1378/chest.13-0488
35. Andreassen SL, Liaaen ED, Stenfors N, Henriksen AH. Impact of pneumonia on hospitalizations due to acute exacerbations of COPD. Clin Respir J. 2014;8(1):93–99. doi:10.1111/crj.12043
36. Søgaard M, Madsen M, Løkke A, Hilberg O, Sørensen HT, Thomsen RW. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:455–465. doi:10.2147/COPD.S96179
37. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234. doi:10.1056/NEJMoa1516385
38. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
