Back to Journals » Cancer Management and Research » Volume 15
The Impact of Endoscopic Endonasal Surgery on Quality of Life in Patients with Malignant Tumors of the Anterior Skull Base: A Prospective Study
Authors Xu H, Li W, Zhang H, Wang H, Hu L, Sun X , Wang D
Received 17 February 2023
Accepted for publication 10 June 2023
Published 16 June 2023 Volume 2023:15 Pages 523—535
DOI https://doi.org/10.2147/CMAR.S409091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Haoyuan Xu,* Wanpeng Li,* Huankang Zhang, Huan Wang, Li Hu, Xicai Sun, Dehui Wang
Department of Otolaryngology - Head and Neck Surgery, Affiliated Eye, Ear, Nose, and Throat Hospital, Fudan University, Shanghai, 200031, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xicai Sun; Dehui Wang, Department of Otolaryngology - Head and Neck Surgery, Affiliated Eye, Ear, Nose, and Throat Hospital, Fudan University, 83 Fen Yang Road, Shanghai, People’s Republic of China, Email [email protected]; [email protected]
Objective: To investigate the effects of endoscopic endonasal surgery (EES) on longitudinal quality of life (QoL) in patients with malignant tumors of the anterior skull base.
Methods: Eligible patients prospectively completed the Anterior Skull Base Surgery Questionnaire (ASBQ) and the 22-item Sino-Nasal Outcome Test (SNOT-22) questionnaires in referring to 3 different periods throughout their treatment and recovery.
Results: Forty patients were included. The median volume coronal maximum length of the tumor was 3.6 cm (95% CI 2.7– 4.1cm). Overall QoL significantly worsened at 1 month postoperatively but returned to baseline after 1 year. Unrelieved symptoms in specific domains prompted further evaluation of individual items. Transient worsening of taste (p=0.011) and olfaction (p=0.004) lasted for 1 month but gradually relieved within the first postoperative year, but vision consistently worsened over the course of the treatment (p=0.126). Age> 50 years (p< 0.001), comorbidities (p< 0.001), tumor necrosis (p< 0.001) and recurrence (p=0.001) were associated with worse preoperative QoL. Poor long-term QoL was noted in those undergoing adjuvant therapy (p=0.032). Overall ASBQ scores (p=0.024), subdomain scores in specific symptoms (p=0.016), and vision scores (p=0.009) were worse only in patients with the greater coronal maximum diameter at 1-month postoperatively. Greater coronal maximum diameter was related to worse preoperative subdomain scores regarding specific symptoms (p=0.030) and decreased postoperative long-term decreased vision scores (p=0.014).
Conclusion: Long-term site-specific and sinonasal QoL eventually stabilized after EES. Greater coronal maximum diameter was significantly associated with worsened vision function. Temporarily worse olfactory, vision, and taste function may be tied to decreased short-term QoL.
Keywords: quality of life, endoscopic endonasal surgery, anterior skull base, malignant tumors, prospective study
Introduction
According to several international centers, minimally invasive endoscopic endonasal surgery (EES) can be an effective alternative to traditional open surgery and microscope-based approaches for treating malignant tumors of the anterior skull base upon adequate assessment and under expert guidance because of its shorter recovery period, better wound healing, increased illumination, and improved visualization of the operating field.1–5 Numerous malignant tumors that affect the anterior skull base can even extend to the olfactory region, orbit, cavernous sinus, and frontal lobe, all of which are closely linked to iatrogenic morbidity.6,7 Growing evidence suggests that the EES can entirely remove tumors affecting the skull base and dura despite a higher risk of cerebrospinal leakage, anosmia, and dysgeusia.8–12 In the literature, evaluation of the therapeutic effect of EES in the treatment of malignant tumors involving the anterior skull base has generally concentrated on surgical resection, complications, survival rate, and other characteristics. From a clinical viewpoint, the type of treatment causing the lowest possible impact on the patient’s quality of life (QoL) should be chosen. In addition, QoL measures may eventually become reportable indicators of the success of a surgical intervention. QoL instruments can be generalized or site- or disease-specific. It is crucial to use reliable tools to evaluate the QoL of patients with malignant tumors of the anterior skull base treated with EES. The Anterior Skull Base Questionnaire (ASBQ) is the most widely used disease-specific health-related QoL tool.13 The psychometric properties of the questionnaire have been preliminarily explored and tested for the endoscopic approach.5,14 As a characteristic QoL measurement tool in the rhinology literature, the 22-item Sino-Nasal Outcome Test (SNOT-22) was designed to assess chronic rhinosinusitis requiring functional sinus surgery. While the SNOT-22 lacks pertinent skull base problems, it can be used as a complementary instrument for the evaluation of patients with malignant tumors of the anterior skull base.
At present, numerous studies have explored the changes in QoL after EES for benign diseases of the anterior skull base,11,15,16 but for malignant tumors of the anterior skull base, the literature is limited and heterogeneous. In a prospective study involving 66 sinonasal malignant patients undergoing endoscopic skull base surgery, Glicksman et al found that sinonasal QoL, as measured by the SNOT-22, appeared to significantly improve at 3 months following resection when compared with the preoperative baseline, and this improvement was sustained throughout a 2-years follow-up.17 However, in a recent review, researchers determined that patients with malignant anterior skull base tumors experienced an initial decrease in QoL after EES followed by a slow improvement over several months, while stabilizing at a lower QoL than initially.18 In addition, a typical concern is the recent use of artificial repair materials and autografts for skull base reconstructions, which may have negative implications for patient’s QoL.19–21 Therefore, we sought to assess QoL during the recovery periods after EES for malignant tumors of the anterior skull base using the ASBQ and the SNOT-22 and then used the patient’s preoperative QoL score as an internal control. Moreover, we further investigated demographic, clinical, surgical, and oncological factors associated with QoL change.
Methods
Patient Selection and Information Retrieval
We prospectively recruited patients with malignant tumors of the anterior skull base who were prepared to undergo EES from January 2021 to July 2021 at the Departments of Otorhinolaryngology of the Fudan University of the ENT and Eye Hospital. The surgical procedure was performed by two technical teams with similar conceptions of clinical and surgical strategies. All patients who underwent EES for pathologically confirmed malignant tumors of the anterior skull base were identified. Demographic and clinical data, pathologic findings, surgical reports, data on adjuvant therapies and complications, and follow-up information were retrieved from a clinical database and telephone interview. Patients were selected based on the following criteria: age > 18 years of age; malignant tumors involving at least the bone of the anterior skull base; treated via EES with curative intent; able to read and write, and without severe psychopathological or cognitive impairment. Patients unable to complete more than 1 year of follow-up were excluded. All patients whose enhanced magnetic resonance imaging (MRI) showed lesions < 1.8 mm away from the internal carotid artery (ICA) were defined as ICA invasion. We defined orbital invasion and intracranial invasion as tumor invasion of at least the extraconal fat and dura, respectively. Tumor volume was calculated by the length × width × height on T1-weighted enhanced MRI. Comorbidity was defined as underlying disease, mainly including hypertension, diabetes, and cerebral infarction, and was diagnosed before or during hospitalizations. Data were collected from patients anonymously and voluntarily with a verbal agreement, which was accepted and approved by the Institutional Review Board of the Affiliated Eye, Ear, Nose, and Throat Hospital at Fudan University. We confirmed that our study complied with the Declaration of Helsinki.
Surgical Procedures
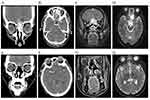
Choosing to undergo EES depended on tumor location, disease degree, the preferences of patients, and consultation with radiation oncologists and surgeons. The surgery is mainly performed by otolaryngologists, but the resection of intracranial tumors often requires the assistance of neurosurgeons. The extent of lesion involvement was determined by the endoscopic findings and imaging results (Figure 1A–D). Upon completion of the survey, endoscopic examination and intranasal debridement were performed during each postoperative visit. Imaging examinations were performed every 3 months within 1 year after surgery (Figure 1E–H). The primary surgical procedures are briefly described as follows. If possible, intranasal lesions were excised for pathologic examination. While expanded excision was considered, the Draf III approach was performed on the anterior skull base, and the bilateral lamina papyracea was observed (Figure 2A and B). After blocking both the anterior and posterior ethmoidal arteries (Figure 2C), the anterior skull base bone was removed and the dura was cut along the safe surgical margin (Figure 2D). The frontal lobe was examined and if invaded, the affected portion was also resected to the safe margin. Artificial repair materials and autografts were harvested for skull base reconstruction. The dural defect was repaired with the artificial dura or fascia lata of the thigh (Figure 2E). Extracranial restoration required a Hadad-Bassagasteguy flap or a free mucosa flap of the middle turbinate (Figure 2F).
Questionnaires and Assessments
QoL outcomes were routinely assessed using the ASBQ13 and SNOT-22.22 The ASBQ complete questionnaire consists of 35 questions ranging from general domains of pain, energy, and mood to specific questions covering smell, taste, and nasal function. All answers on a Likert scale range from 1 to 5 with a higher score indicating a better QoL. The SNOT-22 consists of 22 questions with a higher score indicating a worse QoL. Primary assessments were performed on the overall scores of ASBQ and SNOT-22. Six subscale scores of the ASBQ for performance, physical function, emotions, vitality, pain, and specific symptoms were also assessed. Malignant tumors of the anterior skull base are inclined to involve the olfactory groove and orbit, affecting the taste, smell, and vision functions of the patients before and after EES treatment. Therefore, Questions 10–11 and 15 of the ASBQ were extracted to assess the taste, smell, and vision subdomains, respectively. The questionnaires were administered in person or by telephone interview. An independent doctor conducted all interviews to avoid any bias from doctor–patient interactions. Patients completed the questionnaires referring to 3 different periods throughout their treatment and recovery. The preoperative period was assessed to establish a baseline for QoL. The 1-month period was helpful to investigate how much the resection and its extensiveness had affected the patient’s QoL, and the 1-year period was used to evaluate the long-term effect on QoL.
Statistical Analysis
A commercially available computer software package (IBM SPSS for Windows, version 22.0) was applied. Each case has its own historical control. Outcome parameters were summarized using simple descriptive statistics. After confirming the normality test, we used the paired t-test to compare mean preoperative and postoperative overall scores and subdomain scores. Since the normality test was not passed, we adopted a more appropriate Wilcoxon rank test for preoperative and postoperative single-item scores of taste, smell, and vision. In addition, a distribution-based method was used to assess the minimal clinically important difference (MCID). The calculation formula was as follows: MCID = 0.5 × SDbaseline; SDbaseline means standard deviation of preoperative overall scores. Pearson’s correlation was used to analyze the correlation between the ASBQ and SNOT-22 scores. After confirming the normality test, univariate analysis of the ASBQ scores, subdomain scores, and single-item scores was performed for potentially relevant variables associated with demographic, clinical, surgical and oncological parameters at 3 different time points using unpaired t-tests. A p value < 0.05 was considered statistically significant.
Results
Patients
After the initial screening, 56 patients met the inclusion criteria, of which 7 patients declined to participate in the study, 5 patients were lost to follow-up (3 at 1 month after surgery and 2 at 1 year after surgery), and 4 patients were excluded due to missing data. Available clinical/demographic variables of refusal and attrition patients are basically consistent with that of eligible patients. Finally, forty patients were recruited, with a mean age of 53.0 years (range, 26–76 years) and male preponderance (60.0%). The most common symptoms at presentation were nasal obstruction (52.5%) and nasal hemorrhage (35.0%). Tumor necrosis was observed in 9 (22.5%) patients. Lymph node metastasis was noted in 5 (12.5%) patients. The majority of patients (82.5%) underwent gross-total resection (GTR) of their tumor. Anterior skull base tumors frequently invade the orbit (52.5%) but comparatively less frequently invade the intracalvarium (30.0%). Few patients (15.0%) with large tumors reported ICA involvement. The median tumor volume was 16.1 cm³ (95% CI 3.0–42.1 cm³). The median coronal maximum length was 3.6 cm (95% CI 2.7–4.1 cm). Postoperative cerebral edema was seen in 5 (12.5%) patients and was transient. Cerebrospinal fluid leakage occurred in 2 (5.0%) patients postoperatively and was managed successfully with subsequent repair. Seventeen (42.5%) patients received postoperative adjuvant therapies. The most commonly used reconstruction materials were artificial dura (25.0%) and fascia lata of the thigh (25.0%). Other preoperative and postoperative information on the recruited patients, including detailed pathological types are summarized in Table 1 and Table 2, respectively.
 |
Table 1 Preoperative Characteristics of Recruited Patients with Malignant Tumors Involving Anterior Skull Base |
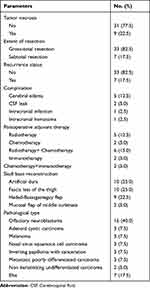 |
Table 2 Postoperative Characteristics of Recruited Patients with Malignant Tumors Involving Anterior Skull Base |
QoL
The mean values of overall ASBQ scores started at 3.68 (95% CI 3.53–3.82) in the preoperative period, significantly decreased to 3.39 (95% CI 3.20–3.59, p < 0.001) in the 1-month posttreatment period, and then stabilized at 3.75 (95% CI 3.58–3.93, p=0.073) in the 1-year postoperative period. At the one-year follow-up, 6 (15.0%) patients deteriorated, 10 (25.0%) patients improved, and 24 (60.0%) patients returned to preoperative level based on the ASBQ scores. Similarly, the mean values of the overall SNOT-22 score started at 2.11 (95% CI 1.99–2.23) during the preoperative period, significantly increased to 2.33 (95% CI 2.18–2.48, p< 0.001) during the 1-month posttreatment period, and stabilized at 2.14 (95% CI 2.00–2.28, p=0.493) during the 1-year postoperative period. Moreover, both the difference in mean overall ASBQ (0.2857 > 0.2117) and the SNOT-22 (0.2250 > 0.1387) scores at the 1-month posttreatment period reached their respective MCID when compared with baseline (Table 3). The ASBQ and the SNOT-22 scores showed a significant inverse correlation preoperatively and at 1 month and 1 year postoperatively (r= - 0.7754, r= - 0.6300, r= - 0.6162, respectively) (Supplementary Table 1). At 1 month following surgery, independent analysis of the ASBQ scores’ two subdomains regarding physical function (p=0.001) and specific symptoms (p=0.008) revealed significantly lower scores. At 1 year following surgery, five subdomains returned to baseline, except for specific symptoms (Figure 3). Furthermore, significantly worse smell component ASBQ scores were noted at the 1-month posttreatment period (mean 2.50, 95% CI 2.12–2.88, p=0.004) when compared with baseline (mean 3.15, 95% CI 2.72–3.58) and were still lower than baseline at the 1-year postoperative period (mean 2.60, 95% CI 2.21–3.00, p=0.019). Olfaction was closely related to taste and showed a similar tendency. Significantly worse taste component ASBQ scores were detected at the 1-month posttreatment period (mean 3.13, 95% CI 2.77–3.48, p=0.011) compared with baseline (mean 3.63, 95% CI 3.31–3.94), but returned to baseline at 1 year after surgery (mean 3.43, 95% CI 3.09–3.76, p=0.333). Vision component ASBQ scores gradually decreased as the time from surgery increased, but there were no significant differences at 1 month after surgery (mean 3.20, 95% CI 2.95–3.45, p=0.136) and at 1 year after surgery (mean 3.08, 95% CI 2.77–3.38, p=0.072) compared with baseline (mean 3.40, 95% CI 2.93–3.61) (Figure 4). In addition, the ASBQ and SNOT-22 demonstrated the largest symptom burden in items of the sense of smell, taste, and eyesight at 1 year postoperatively (Supplementary Table 2).
 |
Table 3 Overall ASBQ and SNOT-22 Scores in Patients with Malignant Tumors Involving Anterior Skull Base |
 |
Figure 3 Plots illustrating preoperative and postoperative scores for each of the six domains assessed using the ASBQ. A higher score indicates better quality of life. *p < 0.05 (paired t-test). |
Univariate Analysis
Univariate analysis was performed for several variables at each time point (Figure 5). Age (≤50 years) (p<0.001), non-comorbidity (p<0.001), non-tumor necrosis (p<0.001), and non-recurrence (p=0.001) were associated with better preoperative overall ASBQ scores. A coronal maximum diameter > 3 cm was associated with lower overall ASBQ scores at 1 month postoperatively (p=0.024). Overall, ASBQ scores were worse in those with tumor necrosis (p=0.002) and those undergoing adjuvant therapies at 1 year postoperatively (p=0.032). There were no significant differences in overall ASBQ scores in terms of sex, the extent of resection, orbit invasion, intracranial invasion, tumor volume, complications, or skull base reconstruction. Univariate analysis of subdomain scores for variables was further assessed. Patients over 50 years of age had lower scores in the four preoperative subdomains (performance, vitality, emotional impact, and specific symptoms). ICA involvement (p=0.044) and orbital invasion (p=0.040) were related to worse preoperative subdomain scores regarding the emotional impact and specific symptoms, respectively. A coronal maximum diameter > 3 cm was associated with worse subdomain scores regarding specific symptoms preoperatively (p=0.030) and 1-month postoperatively (p=0.016). A long-term decline in vitality score was observed only in those with ICA involvement (p=0.010) (Table 4). Univariate analysis was used to examine the ASBQ scores for relevant items, including smell, vision, and taste. Age (≤50 years) was related to better baseline taste (p=0.005). Preoperative vision (p=0.012) and taste (p=0.032) were worse in patients with comorbidities. Patients with a coronal maximum diameter > 3 cm registered poorer vision at the 1-month (p=0.009) and 1-year (p=0.014) postoperative assessments (Table 5).
 |
Table 4 Univariate Analysis of Subdomain Scores of the ASBQ Inpatients with Malignant Tumors Involving Anterior Skull Base |
 |
Table 5 Univariate Analysis of Smell, Vision and Taste Scores Inpatients with Malignant Tumors Involving Anterior Skull Base |
Discussion
The majority of research outcomes for malignant tumors of the anterior skull based have traditionally focused on survival and complications. Despite improvements in multimodal treatment and surgical techniques, survival rates have plateaued, and thus, efforts have been directed toward incorporating QoL as a key outcome parameter, balancing oncologic management with functional disability.23–25 A patient’s QoL has become increasingly for improving preoperative counseling, surgeon willingness, surgical outcomes, and subsequent recovery.26 The evaluation of QoL using appropriate questionnaires in patients with malignant tumors of the anterior skull base undergoing EES treatment comprises broad confounding variables, including demographic, clinical, and surgical factors. However, studies specifically addressing the QoL of patients with malignant tumors of the anterior skull base via EES are lacking and inconsistent. Chow et al reported that the QoL of patients with malignant tumors of the anterior skull base improved within a few months after surgery but still stabilized at a lower level than the initial QoL mainly related to postoperative adjuvant therapy.18 Our results showed that the overall ASBQ scores significantly worsened at 1 month postoperatively but slowly recovered to at least preoperative values at the 1-year follow-up. QoL can be affected for several years after treatment.27,28 Therefore, long-term follow-up is required to monitor any decline in QoL. In this study, postoperative overall SNOT-22 scores indicated a decline in early postoperative sinonasal-related QoL, which may be associated with nasal edema, crusting, and nasal secretions. Subsequent debridement and nasal irrigation helped to alleviate these symptoms. Additionally, a substantial inverse correlation between the ASBQ and SNOT-22 indicated that assessment using several instruments would yield Supplementary Information. It was recommended that the use of several instruments might provide more additional data that cover multiple fields.
In our study, the univariate analysis demonstrated that worse preoperative QoL was associated with age (>50 years) and comorbidities. However, low preoperative QoL was significantly associated with female sex and diabetes in a prospective cohort with pituitary lesions.15 Therefore, comorbidities appeared to have a general effect on the preoperative QoL of patients undergoing anterior skull base surgery. Worse postoperative QoL was significantly correlated with tumor necrosis, larger coronal maximum diameter, and postoperative adjuvant therapy in univariate analysis. Our previous study reported by Li et al29 found that tumor necrosis was not significantly related to postoperative QoL in patients with nasopharyngeal carcinoma. Radiotherapy as a common postoperative adjuvant treatment was noted in 11 of 17 (64.7%) patients. Long-term outcomes suggested that patients receiving radiotherapy had impairments across multiple memory fields. Visual memory deteriorated over time, but verbal memory remained stable These findings highlighted the severity of neurocognitive deficits in anterior skull base patients treated with radiotherapy. Impaired QoL associated with a larger coronal maximum diameter was first reported 1 month after surgery, likely due to extensive dissection. Our GTR rate (82.5%) is basically consistent and even superior to that in a series of benign patients (78.0%).11 Considering the surgical advances in optic nerve decompression30 and internal carotid artery embolization,31 GTR is likely achieved in most patients who undergo EES by experienced surgeons. A study suggested that incomplete resection may result in initially higher QoL scores postoperatively but could lead to further surgery or adjuvant radiotherapy that would result in worsening of long-term QoL.32 Although we found no significant difference between the GTR and subtotal resection groups at the 1-year of follow-up, a longer follow-up may shed light on differences in QoL between the two groups. Cerebrospinal fluid leakage is a frequent and feared complication after EES, which often triggers tension pneumocephalus and meningitis. Our rate of postoperative cerebrospinal fluid leakage (5.0%) is similar to the rate from meningioma surgery (6.0%). The main possible explanation for the lower rate is careful manipulation by the surgeon and timely skull base repair during the operation. Despite retraction of brain tissue or resection of the affected tissue, the majority of postoperative brain edema (12.5%) was transient with postoperative excellent fluid management, and brain edema appeared to have diminished during the imaging examination several months following surgery. The use of artificial and autogenous flaps reduced the risk of cerebrospinal leak rates in many series,33–35 highlighting the importance of this reconstruction technique for closing high-grade skull base defects. Interestingly, we obtained the above results based on whether skull base reconstruction was performed, and in practice, we used a variety of reconstruction materials. The impact of each reconstruction material on QoL should be further evaluated.
Severe symptom assessment and subdomain analysis simultaneously revealed the significant impact of taste, smell, and vision on patients with malignant tumors of the anterior skull base after EES. At long-term follow-up, smell and taste scores of the ASBQ were below preoperative baseline but gradually recovered. Similarly, according to the ASBQ, poor smell symptoms affected most patients (40% of patients reported scores of 1 or 2) in the malignant cohort.36 Taste is inextricably linked to smell, and combined deficits are closely related to worse QoL. Routine procedures involve removal of the olfactory mucosa, destruction of the lamina cribrosa, and partial sectioning of the olfactory fibers and predictably lead to a considerable reduction or loss of olfactory capacity. In addition, the continuous decline in vision scores of the ASBQ is of concern. Although orbital invasion may lead to blindness and decreased QoL, it was not found to be associated with decreased survival.37 We believe that a combined analysis of QoL and survival rates would be more important. Owning to the short follow-up period, these data are not presented here, and long-term follow-up data may explain the pertinence between QoL and survival. Furthermore, remedies for postoperative taste, visual and olfactory defects in patients with anterior skull base malignancies need to be further explored.
Several limitations of this study must be considered in guiding future research. First, these are exploratory analyses, thus the findings are only preliminary. Second, the ASBQ was not validated for comparisons of single items as opposed to subscale scores, so findings regarding taste, smell, and vision were merely impressionistic. Third, there is sample heterogeneity in terms of diseases and adjuvant treatments. Fourth, only selected aspects of QoL were evaluated (ie, many relevant dimensions are not incorporated within the ASBQ and SNOT-22). Fifth, some variables used to stratify QoL may show no significant difference due to the small number included, especially for the extent of resection, intracranial invasion, orbital invasion, and skull base reconstruction, so a large sample size and longer follow-up time should be considered. In addition, we believe that future studies would be more meaningful if we could establish associations between QoL and survival parameters.
Conclusions
Long-term site-specific and sinonasal QoL eventually stabilized after EES. Greater coronal maximum diameter was significantly associated with worsened vision function. Temporarily worse olfactory, vision, and taste function may be tied to decreased short-term QoL. In addition, only certain aspects of QoL were assessed, and there is a need for further research given the small, underpowered sample and exploratory nature of the analyses.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author of reasonable request.
Acknowledgments
We thank all the participants for their contribution and participation.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81870703), Shanghai Shen Kang Hospital Development Center (SHDC12018118), Science and Technology Commission of Shanghai Municipality (20Y11902000, 21ZR1411700, 23YF1404600), and Shanghai Municipal Health Commission (201940143). This work was also funded by the “Outstanding Doctor & Outstanding Clinical Researcher” project titled “Effectiveness of radiotherapy versus endoscopic surgery in the treatment of olfactory neuroblastoma sensitive to chemotherapy: A randomized controlled clinical trial” (No. SYB202006) supported by the Eye, Ear, Nose and Throat Hospital of Fudan University to Xicai Sun.
Disclosure
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
1. Schaberg M, Anand V, Schwartz T, Cobb W. Microscopic versus endoscopic transnasal pituitary surgery. Curr Opin Otolaryngol Head Neck Surg. 2010;18(1):8–14. doi:10.1097/MOO.0b013e328334db5b
2. DeKlotz T, Chia S, Lu W, Makambi K, Aulisi E, Deeb Z. Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope. 2012;122(3):511–518. doi:10.1002/lary.22479
3. McCoul ED, Anand VK, Bedrosian JC, Schwartz TH. Endoscopic skull base surgery and its impact on sinonasal-related quality of life. Int Forum Allergy Rhinol. 2012;2(2):174–181. doi:10.1002/alr.21008
4. McCoul ED, Anand VK, Schwartz TH. Improvements in site-specific quality of life 6 months after endoscopic anterior skull base surgery: a prospective study. J Neurosurg. 2012;117(3):498–506. doi:10.3171/2012.6.JNS111066
5. Abergel A, Cavel O, Margalit N, Fliss D, Gil Z. Comparison of quality of life after transnasal endoscopic vs open skull base tumor resection. Arch Otolaryngol Head Neck Surg. 2012;138(2):142–147. doi:10.1001/archoto.2011.1146
6. Cook SW, Smith Z, Kelly DF. Endonasal transsphenoidal removal of tuberculum sellae meningiomas: technical note. Neurosurgery. 2004;55(1):239–244; discussion 244–236. doi:10.1227/01.NEU.0000126952.51782.4D
7. Fahlbusch R, Schott W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96(2):235–243. doi:10.3171/jns.2002.96.2.0235
8. Wang E, Zanation A, Gardner P, et al. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol. 2019;9:S145–S365. doi:10.1177/1945892418817221
9. Griffiths C, Cutler A, Duong H, et al. Avoidance of postoperative epistaxis and anosmia in endonasal endoscopic skull base surgery: a technical note. Acta Neurochir. 2014;156(7):1393–1401. doi:10.1007/s00701-014-2107-8
10. Ottenhausen M, Rumalla K, Alalade A, et al. Decision-making algorithm for minimally invasive approaches to anterior skull base meningiomas. Neurosurg Focus. 2018;44(4):E7. doi:10.3171/2018.1.FOCUS17734
11. Castle-Kirszbaum M, Kam J, Dixon B, Goldschlager T, King J, Wang YJ. Surgical outcomes and longitudinal quality of life after endoscopic endonasal surgery for anterior skull base meningioma. J Neurosurg. 2022;137:1–8.
12. Hura N, Orlov CP, Khalafallah AM, Mukherjee D, Rowan NR. Impact of routine endoscopic skull base surgery on subjective olfaction and gustation outcomes. Oper Neurosurg. 2021;21(3):137–142. doi:10.1093/ons/opab137
13. Gil Z, Abergel A, Spektor S, Shabtai E, Khafif A, Fliss DM. Development of a cancer-specific anterior skull base quality-of-life questionnaire. J Neurosurg. 2004;100(5):813–819. doi:10.3171/jns.2004.100.5.0813
14. Fliss D, Abergel A, Cavel O, Margalit N, Gil Z. Combined subcranial approaches for excision of complex anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2007;133(9):888–896. doi:10.1001/archotol.133.9.888
15. Buchlak Q, Esmaili N, Bennett C, Wang Y, King J, Goldschlager T. Predictors of improvement in quality of life at 12-month follow-up in patients undergoing anterior endoscopic skull base surgery. PLoS One. 2022;17(7):e0272147. doi:10.1371/journal.pone.0272147
16. Ahn J, Cho S, Kim D, et al. Recovery period of sinonasal quality of life and its associated factors after endoscopic endonasal approach for anterior skull base tumors. Acta Otolaryngol. 2019;139(5):461–466. doi:10.1080/00016489.2019.1574982
17. Glicksman J, Parasher A, Brooks S, et al. Sinonasal quality of life after endoscopic resection of malignant sinonasal and skull base tumors. Laryngoscope. 2018;128(4):789–793. doi:10.1002/lary.26833
18. Chow V, Tsetsos N, Poutoglidis A, Georgalas C. Quality of life in sinonasal tumors: an up-to-date review. Curr Opin Otolaryngol Head Neck Surg. 2022;30(1):46–57. doi:10.1097/MOO.0000000000000774
19. Battaglia P, Turri-Zanoni M, De Bernardi F, et al. Septal flip flap for anterior skull base reconstruction after endoscopic resection of sinonasal cancers: preliminary outcomes. Acta Otorhinolaryngol Ital. 2016;36(3):194–198. doi:10.14639/0392-100X-748
20. Lee S, Ha C, Hong S, et al. Clinical impact of hydroxyapatite on the outcome of skull base reconstruction for intraoperative high-flow CSF leak: a propensity score matching analysis. Front Oncol. 2022;12:906162. doi:10.3389/fonc.2022.906162
21. Castelnuovo P, Lepera D, Turri-Zanoni M, et al. Quality of life following endoscopic endonasal resection of anterior skull base cancers. J Neurosurg. 2013;119(6):1401–1409. doi:10.3171/2013.8.JNS13296
22. Kennedy J, Hubbard M, Huyett P, et al. Sino-nasal outcome test (SNOT-22): a predictor of postsurgical improvement in patients with chronic sinusitis. Ann Allergy Asthma Immunol. 2013;111(4):246–251.e242. doi:10.1016/j.anai.2013.06.033
23. Shah JP. Quality of life after skull base surgery: the patient’s predicament. Skull Base. 2010;20(1):3–4. doi:10.1055/s-0029-1242977
24. Kirkman M, Borg A, Al-Mousa A, Haliasos N, Choi D. Quality-of-life after anterior skull base surgery: a systematic review. J Neurol Surg B Skull Base. 2014;75(2):73–89. doi:10.1055/s-0033-1359303
25. Schaberg MR. Quality of life outcomes after endoscopic approaches to intracranial tumors. Curr Opin Otolaryngol Head Neck Surg. 2018;26(1):58–64. doi:10.1097/MOO.0000000000000427
26. Gil Z, Fliss DM. Quality of life in patients with skull base tumors: current status and future challenges. Skull Base. 2010;20(1):11–18. doi:10.1055/s-0029-1242979
27. Deckard N, Harrow B, Barnett S, Batra PS. Comparative analysis of quality-of-life metrics after endoscopic surgery for sinonasal neoplasms. Am J Rhinol Allergy. 2015;29(2):151–155. doi:10.2500/ajra.2015.29.4137
28. Derousseau T, Manjunath L, Harrow B, Zhang S, Batra PS. Long-term changes in quality of life after endoscopic resection of sinonasal and skull-base tumors. Int Forum Allergy Rhinol. 2015;5(12):1129–1135. doi:10.1002/alr.21608
29. Li W, Lu H, Liu J, et al. Quality of life following salvage endoscopic nasopharyngectomy in patients with recurrent nasopharyngeal carcinoma: a prospective study. Front Oncol. 2020;10:437. doi:10.3389/fonc.2020.00437
30. Elshazly K, Kshettry V, Farrell C, Nyquist G, Rosen M, Evans JJ. Clinical outcome after endoscopic endonasal resection of tuberculum sella meningiomas. Oper Neurosurg. 2018;14(5):494–502. doi:10.1093/ons/opx165
31. Li W, Liu Q, Wang H, et al. Innovative application of internal carotid artery embolization in salvage endoscopic nasopharyngectomy for recurrent nasopharyngeal carcinoma: a case-matched comparison. Int Forum Allergy Rhinol. 2022;12(6):838–848. doi:10.1002/alr.22927
32. Jozaghi Y, Phan J, Hanna E, Kupferman M, Su SY. Functional outcomes and quality of life in patients with sinonasal, nasopharyngeal, and anterior skull base tumors. Curr Oncol Rep. 2022;24(6):775–781. doi:10.1007/s11912-022-01214-2
33. Riley C, Tabaee A, Conley L, et al. Long-term sinonasal outcomes after endoscopic skull base surgery with nasoseptal flap reconstruction. Laryngoscope. 2019;129(5):1035–1040. doi:10.1002/lary.27637
34. Koutourousiou M, Fernandez-Miranda J, Stefko S, Wang E, Snyderman C, Gardner PA. Endoscopic endonasal surgery for suprasellar meningiomas: experience with 75 patients. J Neurosurg. 2014;120(6):1326–1339. doi:10.3171/2014.2.JNS13767
35. Ottenhausen M, Banu M, Placantonakis D, et al. Endoscopic endonasal resection of suprasellar meningiomas: the importance of case selection and experience in determining extent of resection, visual improvement, and complications. World Neurosurg. 2014;82(3–4):442–449. doi:10.1016/j.wneu.2014.03.032
36. Tyler M, Mohamed A, Smith J, et al. Long-term quality of life after definitive treatment of sinonasal and nasopharyngeal malignancies. Laryngoscope. 2020;130(1):86–93. doi:10.1002/lary.27849
37. Kilic S, Kilic S, Baredes S, Liu J, Eloy JA. Survival, morbidity, and quality-of-life outcomes for sinonasal and ventral skull base malignancies. Otolaryngol Clin North Am. 2017;50(2):467–480. doi:10.1016/j.otc.2016.12.018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.