Back to Journals » Cancer Management and Research » Volume 10
The impact of biopsy sampling errors and the quality of surgical margins on local recurrence and survival in chondrosarcoma
Authors Hodel S, Laux CJ , Farei-Campagna J, Götschi T , Bode-Lesniewska B , Müller DA
Received 30 June 2018
Accepted for publication 3 August 2018
Published 21 September 2018 Volume 2018:10 Pages 3765—3771
DOI https://doi.org/10.2147/CMAR.S178768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Raphael Catane
Sandro Hodel,1 Christoph Laux,1 Jan Farei-Campagna,1 Tobias Götschi,1 Beata Bode-Lesniewska,2 Daniel Andreas Müller1
1Orthopaedic Department, Balgrist University Hospital, Zürich, Switzerland; 2Department of Pathology, University Hospital Zurich, Zürich, Switzerland
Purpose: To examine the frequency of computed tomography (CT)-guided biopsy sampling errors in chondrosarcomas, as well as the impact of these errors and the achieved surgical margins on local recurrence-free survival (LRFS) and disease-specific survival (DSS).
Material and methods: A total of 68 consecutive patients treated for chondrosarcoma from 2000–2015 were retrospectively reviewed with a minimum follow-up duration of 2 years.
Results: The primary location was at the extremities in 46 patients (67.6%) and at the axial skeleton in 22 patients (32.4%). Seven patients underwent planned intralesional curettage. Surgical margins were assessed in the remaining 53 patients and included 21 wide (39.6%), 25 marginal (47.1%), and seven intralesional (13.2%) resections. Biopsy sampling errors occurred in ten patients (14.7%). LRFS was 82.2±7.8% at 5 years and 76.9±7.8% at 10 years. An intact anatomical barrier was associated with the most preferable LRFS of 89±10.5% after 10 years. DSS was 79.2±8.5% at 5 years and 75.5±6.4% at 10 years. The metric distance of the surgical margin and the presence of a biopsy sampling error did not affect either LRFS or DSS.
Conclusion: Even though histological grading in chondrosarcoma is difficult, sampling errors in preoperative biopsies are relatively rare and do not adversely affect outcomes. The presence of an anatomical barrier has a greater impact on LRFS than the metric distance of the surgical margins.
Keywords: bone tumor, chondrosarcoma, survival, local recurrence, surgical margin, biopsy sampling error
Introduction
Chondrosarcoma is the most common malignant bone tumor in the elderly with an estimated incidence of one in 200,000 per year.1 A spectrum of histological subtypes demonstrating various clinical behaviors has been identified, ranging from low-grade to high-grade and dedifferentiated chondrosarcoma. This primary bone tumor can affect any part of the skeleton, but it is mainly located at the pelvis, femur, and proximal humerus.2,3
Although preoperative histological grading has been associated with a high inter- and intra-rater variability in cartilaginous tumors, imaging-guided biopsy remains the standard diagnostic procedure in chondrosarcoma.4–7 In combination with clinical and radiological diagnostics, it primarily directs therapeutic decision-making. Complete surgical resection remains the gold standard of treatment since chemotherapy and radiation therapy are not effective.8 Different surgical treatments exist, from intralesional curettage in low-grade appendicular tumors to wide resection and complex bone reconstruction.9,10
Accurate preoperative grading is highly desired to select the most appropriate surgical therapy and to avoid under- or over-treatment. However, the definitive histological grading of the resected tumor can differ from that of the preoperative biopsy sample; this is referred to as a biopsy sampling error in the literature. Such errors are reported to occur in up to 41% of cases, with a reported higher incidence in pelvic tumors.11 The impact of biopsy sampling errors on disease-specific survival (DSS) and local recurrence-free survival (LRFS) has not yet been studied,11,12 despite previous reports of numerous risk factors for LRFS and DSS including histological grade, anatomical location, tumor volume, sex, age or surgical margins.13–16
In recent years, advances in surgical techniques that allow more sophisticated limb-sparing reconstructions have led to less morbidity and better functional results without compromising DSS or LRFS.17–20 Achieving complete resection with tumor-free margins has become challenging considering this development. However, the impact of metric measures or the quality of the surgical margins (such as biological barriers: fascia, periosteum) on LRFS and DSS remain unclear since various classifications exist.21,22
Overall, the clinical role of biopsy sampling errors and surgical margins has been scarcely reported in chondrosarcoma. Therefore, the purpose of the current study was to examine the frequency of computed tomography (CT)-guided biopsy sampling errors in chondrosarcomas, as well as the impact of these errors and the achieved surgical margins on DSS and LRFS.
Ethics
The study was approved by our local ethics advisory board (Kantonale Ethikkommission, Kanton Zürich; registration number: 2017 – 01666). Written informed consent was obtained from all patients.
Methods
Seventy consecutive patients treated for chondrosarcoma from 2000–2015 at a single sarcoma center were retrospectively reviewed. Inclusion criteria included a biopsy-confirmed chondrosarcoma, minimum follow-up of 2 years, and provision of written informed consent.
Patient data were retrieved from the electronic medical record system. Patient charts were reviewed for demographic data, tumor localization, tumor size, type of surgery, radio- or chemotherapy, recurrence, and survival.
The primary outcomes of the study were DSS and LRFS after 5 and 10 years. DSS was calculated from the date of surgery to the date of death from disease. LRFS was calculated from the date of surgery to the date when local recurrence occurred. Clinical and histopathological factors were analyzed for their effect on DSS and LRFS.
Histopathology reports were reviewed for histological diagnosis (in accordance with WHO guidelines 2013)23 and surgical margins. Surgical margins were classified in accordance with Enneking et al.24 The surgical margin in mm and the presence of a biological barrier (such as fascia, periosteum, corticalis) at the closest resection margin were recorded from histopathological reports. Planned intralesional curettage of appendicular low-grade tumors was excluded from the analysis of surgical margins. A biopsy sampling error was recorded when the histopathological report of the definitive resection revealed a different histological grade compared with the preoperative biopsy. Metastatic disease at diagnosis or systemic progression was defined when histologically confirmed metastasis or suspected radiological lesions were present with progression within 3 months of follow-up. Tumor volume was calculated in cm3 based on magnetic resonance imaging (MRI), in accordance with the following formula: volume = (A × B × C) / 2; A=cranio-caudal diameter, B=medio-lateral diameter, C=antero-posterior diameter.25
Follow-up was conducted at 3-month intervals for the initial 2 years, followed by 6-month intervals for another 3 years, and later annually. For the surveillance of systemic progression, chest CT was performed. Imaging of the local tumor site was performed using plain radiographs and MRI.
Statistics
Statistical analysis was performed using Stata (Release 14; StataCorp LP, College Station, TX, USA). Kaplan–Meier survival curves were used to estimate event-free survival. Categorical factor differences were tested using the log-rank test for univariate analysis. The effects of interval scaled variables were tested using a univariate Cox proportional hazards model. P-values were Bonferroni-corrected to compensate for an increase in probability for a type I error. Factors with a significant influence were subsequently included in a multivariate Cox proportional hazards model. The level of significance for all tests was set at α=0.05.
Results
Patient characteristics
Seventy consecutive patients were treated for biopsy-confirmed chondrosarcoma from 2000–2015. All patients were treated by three board-certified, fellowship-trained orthopedic onco-surgeons at our institute. Two patients were lost to follow-up as they moved abroad. A total of 68 patients were available for analysis with a mean follow-up of 5 years (range: 2–15 years). The demographic data of the included patients and tumor characteristics are listed in Table 1. A total of 31 chondrosarcomas (45.6%) were diagnosed in male patients. The mean patient age was 49 years (range: 13–85 years). The histological subtype was low-grade in 48.5% of cases (n=33), high-grade in 25% (n=17), dedifferentiated in 14.7% (n=10), mesenchymal in 2.9% (n=2), clear cell in 1.5% (n=1), myxoid extraskeletal in 3.4% (n=3), and secondary chondrosarcoma in multiple cartilaginous exostoses in 2.9% of cases (n=2). The predominant anatomical site was the proximal femur in 23 patients (33.8%), followed by the pelvis in 15 patients (22.1%). The mean tumor volume was 162.6±352.9 cm3.
  | Table 1 Patient characteristics and tumor information |
Treatment
All patients were reviewed by a multidisciplinary sarcoma board prior to surgery. Surgical treatment was performed in a total of 60 patients and included limb-sparing surgery in 55 patients (91.7%), resection and biological reconstruction with allograft or autograft in 31 patients (51.7%), implantation of a modular tumor prosthesis in 17 patients (28.3%), and planned intralesional curettage in seven patients (11.7%). All patients who were treated using planned intralesional curettage were alive at the latest follow-up and no local recurrence occurred. An amputation was performed in five patients (8.3%) (Table 2). Eight patients did not undergo surgery (11.7%). In four of those patients (5.9%) watchful waiting was preferred in the presence of an asymptomatic, appendicular low-grade chondrosarcoma. Primary palliative chemotherapy was the first-line treatment for four patients (5.9%) in the presence of metastatic disease at the time of diagnosis.
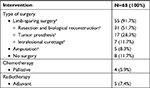  | Table 2 Treatment overview for biopsy-confirmed chondrosarcoma (n=68) Note: aPercentages apply to 60 patients (=100%) who underwent surgery. |
Adjuvant radiotherapy was conducted in five patients (7.4%) based on the consensus of the multidisciplinary sarcoma board (Table 1). The reasons were the presence of a high-grade or dedifferentiated lesion with marginal resection (≤1 mm without a biological barrier) (n=2), a planned intralesional resection at the spine or pelvis (n=2), and one unplanned intralesional resection of a mesenchymal chondrosarcoma of the mandibula.
Biopsy sampling error
A biopsy sampling error occurred in a total of ten patients (14.7%). The tumor was located at the axial skeleton in two patients and at the appendicular skeleton in eight patients. In four patients with a low-grade lesion according to the preoperative biopsy, a high-grade lesion was found in the definitive histopathological assessment after surgical resection. In three patients with a high-grade lesion and one patient with a low-grade lesion according to the preoperative biopsy, a dedifferentiated portion was found in the definitive histopathological assessment of the resected specimen. Two patients had a chondrogenous neoplasm of unclear malignancy according to the preoperative biopsy and a low-grade chondrosarcoma was present in the definitive histological assessment. The low-grade malignant part of the lesion could not be detected despite repeated CT-guided biopsy. The tumors were localized around the knee and the shoulder joint, respectively. A secondary resection was necessary in both cases to provide adequate treatment. Consequently, sufficient resection could be obtained. In the remaining patients in whom a biopsy sampling error occurred, neither changes in decision-making nor revision surgeries were necessary.
Surgical margins
Surgical margins were assessed in 53 patients in accordance with Enneking et al,24 and included: 21 wide (39.6%), 25 marginal (47.1%), and seven unplanned intralesional (13.2%) resections. A biological barrier at the resection margin was present in nine patients (15%). A wide resection was not feasible or not reconcilable with the patient’s wishes because of associated morbidity at the thoracolumbar spine (n=1, 1.7%) or pelvis (n=1, 1.7%). Two patients (3.3%) were admitted for further diagnostic assessment and treatment at our tertiary center after inadequate surgical resection at an external institution (“whoops” lesion). Additionally, under an assumption of enchondroma, two patients (3.3%) underwent an intralesional curettage and secondary resection was performed (“Biopsy sampling error” section). In one mesenchymal chondrosarcoma of the mandibula (1.7%) an adequate surgical resection could not be achieved and therefore the patient underwent adjuvant radiotherapy.
Prognostic factors for DSS
A total of 16 patients (23.5%) died of disease-related causes and one patient (1.5%) died of another cause. The DSS of the total 68 patients was 79.2±8.5% at 5 years and 75.5±6.4% at 10 years. DSS varied significantly among histological types: dedifferentiated types showed the worst survival rates (10±9.3% at 10 years) while low-grade tumors showed the best survival rates (93.8±4.0% at 10 years; P<0.001) (Figure 1, Table 3). Histological grade remained a significant risk factor in multivariate analysis and the HR for DSS was 1.2 (95% CI: 0.73–2.00). Furthermore, metastatic disease at the time of diagnosis was identified as a risk factor with an HR for DSS of 7.95 (95% CI: 2.08–30.38).
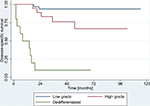  | Figure 1 Disease-specific survival according to histological grading. |
A wide resection was associated with a better DSS of 88±8.3% at 10 years compared with the DSS associated with an unplanned intralesional resection (37.5±17.1%). This factor did not remain significant after Bonferroni correction (Table 3). A complete list of the reviewed risk factors is highlighted in Table 3. Rare histological subtypes (n<2) were not illustrated.
Prognostic factors for LRFS
Local recurrence occurred in 12 patients (17.6%) and the median time to local recurrence was 1.8 years (range, 0.4–7.9 years). The rate of LRFS was 82.2±7.8% at 5 years and 76.9±7.8% at 10 years. In univariate analysis, a lower histological grade was associated with a better LRFS (86±10.5% at 10 years, P<0.001) (Table 4). In multivariate analysis, a higher histological grade remained an independent prognostic factor for poor LRFS (P<0.001) with an HR of 1.58 (95% CI: 0.99–2.51) (Table 4). An intact biological barrier was associated with the most preferable LRFS of 89±10.5% at 10 years compared with that of an unplanned intralesional resection of 57.1±18.7% (Table 4, Figure 2). This trend showed no statistical significance after Bonferroni correction (Table 4). Further risk factors are shown in Table 4.
  | Figure 2 Local recurrence-free survival according to surgical margins. |
Intended intralesional curettage in low-grade appendicular chondrosarcoma was not included (n=7).
Discussion
To the best of our knowledge, this is the first study to examine the role of biopsy sampling errors and the impact of resection margin quality on DSS and LRFS in chondrosarcomas. The limitations of this study include its retrospective design and the relatively small sample size owing to the low incidence of this disease. The intra- and inter-observer reliability of histological grading could not be assessed because of the retrospective design and may potentially have influenced the reported outcomes. However, all pathological specimens were assessed by experienced pathologists, who specialize in sarcomas. Treatment protocols may have varied among the cohort as the study was conducted over a 15-year period. However, the applied diagnostic and therapeutic approaches remained largely unchanged.
Although the histological features of cartilaginous tumors have been described in detail, they remain a diagnostic challenge in terms of preoperative biopsies, and diagnosis relies on clinical and radiological findings.4–7 Biopsy sampling errors were relatively rare and did not affect DSS and LRFS in our cohort. However, a secondary resection was required in two cases with a previously assumed low-grade lesion. This underlines the importance of the preoperative biopsy and its possible far-reaching consequences. The incidence of biopsy sampling errors in our cohort was smaller than that reported by Roitman et al, who reported biopsy sampling errors in up to 41% of cases.11 Furthermore, we did not reproduce findings of a higher incidence at the axial skeleton or pelvis or a correlation with tumor size. Because of considerable inter-observer variability in histological grading in cartilaginous tumors, the role of preoperative biopsies remains controversial, especially in high-volume axial tumors, and should always be assessed in combination with clinical and radiological findings.4 This is most likely the reason that biopsy sampling errors were not an independent risk factor for DSS and LRFS, since therapeutic decisions are also based on clinical and radiological findings. However, an evaluation of the diagnostic value of a CT-guided biopsy is beyond the scope of this paper. The impact of such biopsies needs to be elucidated, especially in low-grade lesions, if an intended intralesional curettage is planned.
The presence of a biological barrier was associated with the most favorable DSS and LRFS. The vast majority of the patients in our cohort could be treated using limb-sparing surgery. One single local recurrence occurred when a biological barrier was present at the closest resection margin. Neither histological grading in accordance with Enneking et al,24 nor the distance in mm were significant risk factors for LRFS or DSS in a univariate Cox proportional hazards model. The definition of an adequate resection margin is still a matter of debate and various classifications have been proposed.21,22,24,26 For example, the assessment of a circumferential resection margin in mm has been suggested by Wittekind et al.26 However, no study has yet demonstrated the significance of an anatomical barrier at the closest margin for LRFS or DSS in chondrosarcoma of the bone.27–29 Our findings support the importance of the quality of resection (biological barriers, including fascia and the periosteum) rather than an absolute numeric value. This finding can aid orthopedic tumor surgeons in preoperative planning. The inclusion of an anatomical barrier at the closest resection margin should be aimed for, if possible. However, the ultimate role of the distance to the closest resection margin could not be elucidated reliably in the current study. Uniform criteria with histopathological assessment of the distance in mm and the presence of biological barriers are necessary for the reproducibility of future research. Further large studies are needed to examine the role of quantitative vs qualitative criteria to assess resection margins.
Histological grade is a significant prognostic risk factor for DSS and LRFS, with a worse outcome for dedifferentiated lesions and the best outcome for low-grade chondrosarcoma in our cohort. Metastatic disease at the time of diagnosis was the biggest risk factor for DSS with an HR of 7.95 (95% CI: 2.08–30.38). These findings are in accordance with previously published studies.1,15,30,31 The overall DSS in the current study was slightly higher than that reported Giuffrida et al and Damron et al, who analyzed risk factors in the largest cohort studies to date, despite a higher rate of dedifferentiated lesions in our cohort compared with Beauchamp et al (14.7%, n=10 vs 1.4%, n=40).30,31 The overall rate of LFRS was within the range of that reported in previously published cohort studies.30,31
Some of the previously described prognostic factors in chondrosarcoma, such as tumor volume, tumor localization or age, did not impact DSS or LRFS in our overall cohort, or in a subgroup analysis of exclusively localized conventional chondrosarcoma (low- and high-grade). This might have been caused by the relatively small sample size, as well as the relatively high percentage of included dedifferentiated lesions.
Conclusion
Even though histologic grading in chondrosarcoma is difficult, sampling errors in preoperative biopsies are relatively rare and do not affect outcomes. The presence of an anatomical barrier has a higher impact on LRFS than the metric distance of the surgical margins. Moreover, we confirmed that histological grade and stage of disease are significant risk factors for DSS and LRFS in chondrosarcoma.
Acknowledgment
The abstract of this paper was presented at the Swiss Orthopedics Conference 2018 (Montreux) as a conference talk with interim findings.
Disclosure
The authors report no conflicts of interest in this work.
References
Grimer RJ, Carter SR, Tillman RM, Mangham DC, Abudu A, Fiorenza F. Chondrosarcoma of bone. J Bone Joint Surg Am. 2000;82-A(8):1203–1204. | ||
Hodel S, Seeli F, Fuchs B. Demographic Analysis of Patients with Osteosarcoma, Chonddrosarcoma, Ewing’s Sarcoma from one Sarcoma Center in Switzerland. Praxis. 2015;104(13):673–680. | ||
Gitelis S, Bertoni F, Picci P, Campanacci M. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1981;63(8):1248–1257. | ||
Eefting D, Schrage YM, Geirnaerdt MJ, et al. Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol. 2009;33(1):50–57. | ||
Rosenthal DI, Schiller AL, Mankin HJ. Chondrosarcoma: correlation of radiological and histological grade. Radiology. 1984;150(1):21–26. | ||
Saifuddin A, Mann BS, Mahroof S, et al. Dedifferentiated chondrosarcoma: use of MRI to guide needle biopsy. Clin Radiol. 2004;59(3):268–272. | ||
Yoshimura Y, Isobe K, Arai H, Aoki K, Kito M, Kato H. Preoperative radiographic and histopathologic evaluation of central chondrosarcoma. Arch Orthop Trauma Surg. 2013;133(9):1225–1231. | ||
van Maldegem AM, Bovée JV, Gelderblom H. Comprehensive analysis of published studies involving systemic treatment for chondrosarcoma of bone between 2000 and 2013. Clin Sarcoma Res. 2014;4(4):11. | ||
Abdikarimov KG, Gafur-Akhunov MA, Islamov U, et al. Endoprostheses in Treatment of Bone Tumors. European Journal of Surgical Oncology. 2012;38(9):874–875. | ||
Hanna SA, Whittingham-Jones P, Sewell MD, et al. Outcome of intralesional curettage for low-grade chondrosarcoma of long bones. Eur J Surg Oncol. 2009;35(12):1343–1347. | ||
Roitman PD, Farfalli GL, Ayerza MA, et al. Is Needle Biopsy Clinically Useful in Preoperative Grading of Central Chondrosarcoma of the Pelvis and Long Bones? Clin Orthop Relat Res. 2017;475(3):808–814. | ||
Trieu J, Schlicht SM, Choong PF. Diagnosing musculoskeletal tumours: How accurate is CT-guided core needle biopsy? Eur J Surg Oncol. 2016;42(7):1049–1056. | ||
Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84(1):93–99. | ||
Donati D, El Ghoneimy A, Bertoni F, di Bella C, Mercuri M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J Bone Joint Surg Br. 2005;87(11):1527–1530. | ||
Angelini A, Guerra G, Mavrogenis AF, et al. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106(8):929–937. | ||
Bindiganavile S, Han I, Yun JY, Kim HS. Long-term Outcome of Chondrosarcoma: A Single Institutional Experience. Cancer Res Treat. 2015;47(4):897–903. | ||
Brewer P, Riddell Z, Grimer RJ, Jeys L. Perioperative mortality following above-knee amputations indicated for bone and soft tissue tumours. Eur J Surg Oncol. 2012;38(8):706–710. | ||
Chauhan A, Joshi GR, Chopra BK, Ganguly M, Reddy GR. Limb salvage surgery in bone tumors: a retrospective study of 50 cases in a single center. Indian J Surg Oncol. 2013;4(3):248–254. | ||
Witte D, Bernd L, Bruns J, et al. Limb-salvage reconstruction with MUTARS hemipelvic endoprosthesis: a prospective multicenter study. Eur J Surg Oncol. 2009;35(12):1318–1325. | ||
Puri A, Pruthi M, Gulia A. Outcomes after limb sparing resection in primary malignant pelvic tumors. Eur J Surg Oncol. 2014;40(1):27–33. | ||
Hoang K, Gao Y, Miller BJ. The Variability in Surgical Margin Reporting in Limb Salvage Surgery for Sarcoma. Iowa Orthop J. 2015;35:181–186. | ||
Dürr HR, Bakhshai Y, Rechl H, Tunn PU. Resection margins in bone tumors: what is adequate? Unfallchirurg. 2014;117(7):593–599. | ||
Fletcher CD. WHO Classification of Tumours of Soft Tissue and Bone, fourth ed. Lyon: International Agency for Research on Cancer; 2013. | ||
Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. | ||
Monga SP, Wadleigh R, Sharma A, et al. Intratumoral therapy of cisplatin/epinephrine injectable gel for palliation in patients with obstructive esophageal cancer. Am J Clin Oncol. 2000;23(4):386–392. | ||
Wittekind C, Compton C, Quirke P, et al. A uniform residual tumor (R) classification. Cancer. 2009;115(15):3483–3488. | ||
Mckee MD, Liu DF, Brooks JJ, et al. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol. 2004;85(2):68–76. | ||
Beebe KS, Bertrand TE, Cruz A, Binitie O. CORR Insights(®): Do Surgical Margins Affect Local Recurrence and Survival in Extremity, Nonmetastatic, High-grade Osteosarcoma? Clin Orthop Relat Res. 2016;474(3):677–683. | ||
Sadoski C, Suit HD, Rosenberg A, Mankin H, Efird J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52(4):223–230. | ||
Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91(5):1063–1072. | ||
Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


