Back to Journals » Infection and Drug Resistance » Volume 15
The Human Papillomavirus Infection Characteristics for Patients with Cervical Intraepithelial Neoplasia in Yunnan, China: A Sampling Survey Analysis
Authors Zhi HF, Yang LF, Ge J, Yang XT
Received 2 March 2022
Accepted for publication 12 May 2022
Published 1 June 2022 Volume 2022:15 Pages 2843—2851
DOI https://doi.org/10.2147/IDR.S364763
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Hong-Fang Zhi,1,* Liu-Feng Yang,2,* Jing Ge,2 Xuan-Tao Yang3
1Department of Pathology, Kunming Kingmed Institute for Clinical Laboratory, Kunming, 650506, People’s Republic of China; 2Department of Gynecology, The First People’s Hospital of Yunnan Province/Affiliated Hospital of Kunming University of Science and Technology, Kunming, 650032, People’s Republic of China; 3Department of Pathology, The First People’s Hospital of Yunnan Province/ Affiliated Hospital of Kunming University of Science and Technology, Kunming, 650032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuan-Tao Yang, Department of Pathology, The First People’s Hospital of Yunnan Province/Affiliated Hospital of Kunming University of Science and Technology, No. 157 of Jinbi Road, Kunming, 650032, People’s Republic of China, Tel +86-871-63638441, Email [email protected] Jing Ge, Department of Gynecology, The First People’s Hospital of Yunnan Province/Affiliated Hospital of Kunming University of Science and Technology, No. 157 of Jinbi Road, Kunming, 650032, People’s Republic of China, Tel +86-871-63638701, Email [email protected]
Objective: This study aimed to analyze the status of human papillomavirus (HPV) infections in women in Yunnan in the south of China and their correlation with the grade of cervical intraepithelial neoplasia (CIN).
Methods: A total of 281 patients with CIN and HPV infection, diagnosed at Kunming Kingmed Institute for Clinical Laboratory between January 2019 and June 2021, were enrolled as the subjects of the study and underwent HPV genotyping and cervical histopathology.
Results: The mean age of the 281 patients was 42.3 years, and the median age was 42 years. There were 79 patients in the low-grade squamous intraepithelial lesion (LSIL) group, and 202 patients in the high-grade squamous intraepithelial lesion (HSIL) group. The proportion of 30– 45 years old in HSIL group was 58%. Overall, single infections accounted for 76%, and HR-HPV infections accounted for 90.1%. The most common HR-HPV subtypes in the two CIN groups were almost the same, including HPV16, HPV58 and HPV52. The most common LR-HPV subtype in the two CIN groups was HPV43. There were no significant differences in ethnic and single or multiple infection rates among different CIN groups. Single infection of HPV43 and HPV81 was found in minority HSIL patients.
Conclusion: HPV infection in Yunnan was dominated by single infection and HR-HPV. Patients aged 30 to 45 years were in the high incidence of HSIL, and the most common HR-HPV subtypes were HPV16, HPV58, and HPV52. Single LR-HPV infection exists in minority HSIL patients.
Keywords: human papillomavirus, cervical intraepithelial neoplasia, low-grade cervical intraepithelial neoplasia, high-grade cervical intraepithelial neoplasia
Introduction
Cervical cancer is the fourth most common cancer in women, and, in 2020, there were 604,000 new cases of cervical cancer and 342,000 related deaths worldwide.1 The situation in China is also discouraging, with nearly 100,000 new cases every year.2 Cervical cancer remains the most common cause of death from gynecological tumors in Chinese women.2,3 Human papillomavirus (HPV) infections are known to be closely related to cervical precancerous lesions and cervical cancer, and the main cause of cervical cancer is a persistent infection of high-risk HPV (HR-HPV).4,5 Cervical intraepithelial neoplasia (CIN) is a precancerous disease. In the long-term infection with HPV, cervical tissue may progress into low grade squamous intraepithelial lesion (LSIL) and high grade squamous intraepithelial lesion (HSIL), eventually developing into cervical cancer. However, it is also known that cervical cancer can be prevented and cured, and the World Health Organization has launched a global strategy to eliminate cervical cancer by expanding its three-level prevention strategies, which include vaccination, cervical cancer screening, and cervical cancer treatment. Very positive experiences gained in some developed countries with the three-level prevention strategies.6,7 At present, HPV vaccine in China is a non-immunization planning vaccine, that is, class II vaccine, which belongs to the principle of voluntary self-pay vaccination. A survey in economically developed provinces shows that 58.3% of people have the intention to buy HPV vaccine,8 but the actual HPV vaccine coverage rate in China is less than 1%. So cervical cancer screening is more important in the prevention and treatment of cervical cancer in China.
HPV infection genotyping has regional differences, and HPV16 and 18 are the most common worldwide. The most common high-risk HPV subtypes in Africa were HPV16, 52, 35, 18 and 58. The most common high-risk HPV subtypes in Asia, including China, were HPV16, 52, 58, 33 and 18, respectively.9–13 The genotyping of HPV also varies in different regions of China, and the five most common HR-HPV subtypes in Beijing are HPV52, 58, 16, 51 and 66.14
Yunnan province is an important frontier province and multi-ethnic area with unique geographical location in China. There are many ethnic minorities in Yunnan province, and their economic and cultural level is relatively backward. There are some differences in living environment, life style and customs among ethnic groups. All these factors may cause HPV infection in some areas of Yunnan Province to be different from other areas in China. Previous studies have found that HPV infection rates are high in border areas and economically developed areas of Yunnan Province, and the most common genotypes were HPV-16, 52 and 58. Meanwhile, LR-HPV has been found in cervical squamous cell carcinoma in ethnic women.15,16
In order to further understand the infection characteristics of HPV in Yunnan province and formulate more accurate prevention and control strategies accordingly, we conducted this sampling survey analysis. HPV genotyping and cervical histopathological analyses were conducted to analyze HPV infection status and its relationship with CIN in Yunnan women.
Data and Methods
Subjects
A total of 281 HPV patients who were diagnosed histopathologically with CIN at Kunming Kingmed Institute for Clinical Laboratory from January 2019 to June 2021 were selected as the study cohort. None of the patients had been vaccinated against HPV. Some went to medical institutions for physical examination or screening, and some went to medical institutions for treatment due to self-conscious symptoms. Combined with HE staining morphology and immunohistochemical results of P16 and KI-67. The diagnostic criteria for CIN were based on “The WHO classification of tumors: female genital tumors (version 5)”. CIN I: mild squamous dysplasia, koilocytic atypia, koilocytosis; CIN II: moderate squamous dysplasia; CIN III: severe squamous dysplasia, squamous cell carcinoma in situ.17
Kunming Kingmed Institute for Clinical Laboratory is a listed third-party independent medical testing institution, which cooperates with medical institutions at all levels in Yunnan Province. There were 281 patients in the study group, including 32 in municipal medical institutions, 177 in county medical institutions, 21 in township medical institutions and 51 in private medical institutions. There are 226 Han people and 55 ethnic minorities. Inclusion criteria: HPV positive and histopathologically confirmed CIN. Exclusion criteria: HPV-negative patients and histopathological patients without CIN.
Methods
The HPV Subtypes Were Determined as Follows
We use a disposable cervical cell collector, place the cervical brush at the cervical opening, gently rotate the cervical brush 4–5 times clockwise, slowly remove the cervical brush, put it into the sampling tube with a 3-mL special cell preservation solution, and send it for examination within 24 h. Some cooperative medical institutions are required to test 23 subtypes, and some are required to test 27 subtypes. HPV23 genotyping was performed using an HPV genotyping kit (Yaneng Biosciences [Shenzhen], Co., Ltd., China), and the polymerase chain reaction-reverse dot-blot method. The kit can detect 17 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 6 low-risk HPV genotypes (6, 11, 42, 43, 81, and 83). HPV27 genotyping was performed using fluorescence in situ hybridization and flow cytometry using an HPV nucleic acid genotyping kit (Shanghai Tellgen Life Science Co., Ltd., China). This kit can detect 17 high-risk HPV genotypes (6, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 10 low-risk HPV genotypes (6, 11, 40, 42, 43, 44, 55, 61, 81, and 83). The EMA9600 gene amplifying instrument was manufactured by Zhuhai Black Horse Medical Instrument Co., LTD., the thermothermic hybridization instrument (YN-H16) for HPV type 23 was manufactured by Aneng Biotechnology (Shenzhen) Co., LTD., and the Luminex 200 for HPV type 27 was manufactured by Shanghai Dijing Life Technology Co., LTD. Positive and negative controls were set conventionally.
Histopathology
The specimens were fully fixed with 4% neutral formaldehyde solution, embedded with paraffin, and consecutively sliced into 4-μm sections, which were stained with hematoxylin and eosin (H&E). In cases which H&E morphology is difficult to distinguish between HSIL and LSIL, immunohistochemical tests for P16 and Ki67 were performed. The expression of p16 and ki-67 was detected using streptavidin-peroxidase immunohistochemistry, the antibodies used were all purchased from Fuzhou Maxim Biotechnology Co., Ltd. (China), and the operations were carried out strictly in accordance with the kit instructions. A CIN grading diagnosis of the combined H&E and immunohistochemical staining results was then carried out.
Statistical Analysis
Data were statistically analyzed using the SPSS Version 26.0 (SPSS Inc., Chicago, IL). Measurement data were expressed as mean ± standard deviation (SD). The chi-square test was used to compare the HPV characteristic differences between the 2 groups. P < 0.05 was considered statistically significant.
Results
General Patient Information
Two hundred and eighty-one patients were divided into two groups: 79 in the LSIL group and 202 in the HSIL group. The normality test indicates that age is normally distributed. Overall, the patients ranged in age from 17 to 71 years, with an average age of 41.5 ± 0.59 years. Based on HE morphology and immunohistochemical results, 281 patients were divided into LSIL group and HSIL group. Women diagnosed with LSIL were aged from 17 to 67, with an average age of 42.3 ± 1.24 years, while those in the HSIL group ranged from 18 to 71 years of age, with an average age of 41.1 ± 0.66 years. Sixteen percent of LSIL patients were <30 years old. There were 34 patients above 45 years of age in the LSIL group, which accounted for 43% of the patients, and this was the age group with a high incidence. Twelve percent of HSIL patients were <30 years old, and there were 60 patients aged 30–45 years in the HSIL group, accounting for 58% of the group, and this was the age group with a high incidence. The age distribution is shown in detail in Table 1.
 |
Table 1 Distribution of Patients’ Age in Different CINs |
H&E and Immunohistochemical Results
HSIL: H&E morphology showed an atypical cell extending above the lower third of the mucosa (Figure 1). LSIL: H&E morphology showed that atypical cells were involved in no more than a lower third of the mucosa. Immunohistochemical P16 expression was block-positive reactivity in HSIL patients (Figure 2), Ki67 index >30% (Figure 3). Morphological LSIL patients showed negative or mottled expression of P16 and Ki67 index <30%.
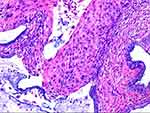 |
Figure 1 33-year-old female with HPV35 infection in cervical H&E. Atypical cell extended above the lower third of the mucosa. |
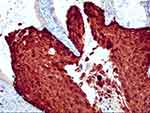 |
Figure 2 Streptavidin–peroxidase method. P16 was block-positive reactivity. |
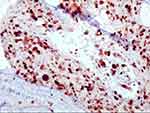 |
Figure 3 Streptavidin–peroxidase method. High Ki-67 index. |
Single and Multiple HPV Subtype Infections in the CIN Patients
All patients were HPV positive. Among the 281 cases, 231 had single infections and 68 cases had multiple infections (68 cases). Single infection was dominant, accounting for 76% of all cases. The proportion of patients with single subtype and multiple subtype infections in the two groups differed according to the age groups, but most of the patients in both groups had a single subtype infection (75% LSIL and 76% HSIL), followed by those with a double subtype infection, and as the number of subtypes increased, the infection rate decreased. In the LSIL group, there were 27 patients (34%) with a single subtype infection in the age range from 30 to 45 years, which was the age group with the highest proportion of single-type infections, and in the HSIL group, there were 83 patients (41%) with a single subtype infection in the age range from 30 to 45 years old, which was also the main single subtype infection age group. Single and multiple infection rates of different ages in LSIL group showed no significant difference (X2 = 2.797, P = 0.258). There was also no significant difference in single and multiple infection rates of different ages in HSIL group (X2 = 5.037, P = 0.081) (Table 2).
 |
Table 2 Analysis of Single and Multiple HPV Infections in CIN Patients at Different Age Groups |
The Distribution Characteristics of the HPV Subtypes in the Different CIN Groups
The HPV subtypes of single and multiple subtype infections in the two CIN groups were counted one by one, and there was a total of 384, including 38 LR-HPVS and 346 HR-HPVs. HR-HPV accounted for 90.1%, indicating that HR-HPV infection was predominant. The main HR-HPV subtypes were 52 (25.3%), 58 (16.1%) and 16 (16.1%) in the LSIL group, and 16 (37.8%), 58 (15.4%), and 52 (11.2%) in the HSIL group. In other words, the most common HR-HPV subtypes in the CIN of the two groups were basically the same, namely HPV16, HPV58, and HPV52. HPV16 ranked third (16.1%) in LSIL group and ranked the first (37.8%) in HSIL group, indicating that the positive rate of HPV16 increased with the increase in the grade of CIN. The single subtype infection rate of LR-HPV was low in both of the CIN groups, and multiple subtype infections of HR-HPV subtypes were dominant. The main LR-HPV subtypes were 43 (23.8%), 42 (19.0%), and 40 (4.8%) in the LSIL group, and 43 (29.4%), 81 (29.4%), and 11 (17.6%) in the HSIL group. Thus, although the common LR-HPV subtypes in CIN differed in the two groups, HPV43 was the main subtype in both groups. In the different CIN groups, the single infection rate of LR-HPV was low, and the co-infection of HR-HPV subtype was dominant (Table 3).
 |
Table 3 Distribution Characteristics of HPV Subtypes in Different CINs |
The Infection Characteristics in Different Nations
In the LSIL group, there were 39 cases of single infection and 15 cases of multiple infection in han nationality, 19 cases of single infection and 6 cases of multiple infection in minority nationality, χ2= 0.0063, P > 0.05, the difference was not statistically significant. In HISL group, there were 131 cases of single infection and 41 cases of multiple infection in han nationality, 22 cases of single infection and 8 cases of multiple infection in minority nationality (χ2=0.0105, P>0.05). It showed that there was no significant difference between ethnic group and single or multiple infection in different CIN groups (Table 4). However, it should be noted that two ethnic HSIL patients had a single LR-HPV infection. A 43-year-old Jingpo nationality case was positive for HPV81. A 44-year-old Yi nationality case was positive for HPV43.
 |
Table 4 The Infection Characteristics of HPV in Different Nations |
Discussion
Yunnan is a frontier province with many ethnic minorities and relatively backward economic and cultural levels. Li et al18 recruited 28,457 individuals aged 17–84 from 13 clinical hospitals in 10 different regions of Yunnan Province. Age subgroup analysis showed that there were two peak ages, one group <25 years old and the other group >56 years old. This study showed that LSIL group >45 years old belonged to the high-risk age range, while HSIL group 30–45 years old belonged to the high-risk age range. There is only one peak age group in our study, which is lower than Li’s cohort. The disease in young women may be related to early sexual behavior, unprotected sex and other unhealthy sexual habits.15 Therefore, it is suggested that women ≤30 years old should also be included in the focus of cervical precancerous lesions or cervical cancer screening objects. Some even recommend a colposcopy for all HR-HPV patients aged ≥21 years.19
Moreover, in the study by Li et al,18 among 28,457 women in Yunnan province, the overall HPV infection rate was 12.9%, the single HPV infection rate was 10.6%, and the multitype HPV infection rate was 2.3%. The three most common HR-HPV subtypes were 52, 16 and 58. In our study, 281 patients with CIN combined with HPV infection were mainly single infection (76%) and HR-HPV infection (90.1%). The common HR-HPV subtypes in CIN patients were basically the same in two groups, and the 3 subtypes with the highest infection rate were HPV16, HPV52 and HPV58, respectively, which was consistent with the literature reports.12–19 There was no significant difference between single and multiple infection rates in different age groups. The single LR-HPV infection was low, and multiple HR-HPV subtype infection was dominant.
Single subtype infection and multiple subtype infection have different impacts on CIN. Kim et al20 compared the HPV genotypes of 236 patients with a multiple subtype infection and 180 patients with a single subtype infection, and they found that the correlation between multiple subtype infections and HSIL was closer than that between a single subtype infection and HSIL. The infections were also seen to last longer in patients with multiple subtype infections than in patients with a single subtype infection. Brant et al21 conducted a ribonucleic acid sequencing analysis and found that in invasive cervical cancer with an infection of multiple HPV genotypes, a single HPV genotype was preferentially expressed, which supports the hypothesis that a single HPV genotype is associated with the development of the cancer. However, it was also previously reported that multiple subtype infections had no impact on cervical lesions.22 Wang et al observed that women with multiple HR-HPV infections with HPV16/18 also had a higher risk of CIN2+, whereas multiple HR-HPV infections without HPV16/18 did not have a significantly greater risk.23
In this study, HPV16 ranked third in the LSIL group, but first in HSIL, indicating that the positive rate of HPV16 increased with the increase of CIN level, and it is the main infection type for HSIL. It is well-known that the probability of CIN patients with HPV16 and HPV18 developing invasive cervical cancer within two years was three times and two times higher, respectively, than it was in CIN patients with other HR-HPV subtypes and that CIN II and CIN III can gradually become cancerous with continuous infections involving the HPV16, HPV18, and HPV58 subtypes.4,5
Baloch et al24 found that the overall HR-HPV and single HPV infection rates of Tibetan women were significantly higher than those of Naxi and Han women. Our study found no correlation between ethnicity and single or multiple infection rates in different CIN groups. However, a single LR-HPV infection was found in two minority HSIL patients. A 43-year-old Jingpo nationality was positive for HPV81. One case was Yi nationality, 44 years old, positive for HPV43. Interestingly, Yunnan scholars Yuanyue et al12 also found that the prevalence of LR-HPV81 in cervical squamous cell carcinoma in women of Han ethnicity was very high. In addition, 4 patients with LR-HPV (2 patients with HPV6, 1 patient with HPV11, and 1 patient with HPV42) were detected among 18 patients from ethnic minorities with squamous cell carcinoma. Therefore, HPV infection in HSIL or squamous cell carcinoma of ethnic minorities in Yunnan may be different from that in other regions. This is related to the different susceptibility and clearance rates of different types of HPV in different regions, different nationalities and different physical hosts.25 However, the sample size of this study is too small, which requires further investigation and research with a large sample.
Conclusion
In summary, this sampling survey analysis found that 30–45 years old patients in Yunnan area had a high incidence of HSIL. Patients were mainly characterized by single or multiple infections. The three subtypes of HR-HPV with the highest infection rate were HPV16, HPV52 and HPV58. HPV43 is the main subtype of LR-HPV. There was no correlation between ethnicity and single or multiple infections in different CIN groups. A single infection of HPV43 and HPV81 was found in HSIL group for minority. Therefore, timely screening of HPV subtypes is of great significance in the primary prevention of cervical cancer. The findings also suggest that the pathogenicity of LR-HPV43 and LR-HPV81 should be investigated further and references for the formulation of HPV vaccine strategies in Yunnan should be provided.
Ethical Statement
This study was conducted with approval from the Ethics Committee of the Kunming Kingmed Institute for Clinical Laboratory. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Funding
2020 health scientific research project of Kunming City “Ten Hundred Thousand” Project NO.2021-SW(reserve)-310.44.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. Jiang X, Tang H, Chen T. Epidemiology of gynecologic cancers in China. J Gynecol Oncol. 2018;29(1):e7. doi:10.3802/jgo.2018.29.e7
3. Wang Z, Guo E, Yang B, et al. Trends and age-period-cohort effects on mortality of the three major gynecologic cancers in China from 1990 to 2019: cervical, ovarian and uterine cancer. Gynecol Oncol. 2021;163(2):358–363. PMID: 34507827. doi:10.1016/j.ygyno.2021.08.029
4. Boda D, Docea AO, Calina D, et al. Human papilloma virus: apprehending the link with carcinogenesis and unveiling new Research avenues (Review). Int J Oncol. 2018;52(3):
5. de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. doi:10.1016/j.bpobgyn.2017.08.015
6. Das M. WHO launches strategy to accelerate elimination of cervical cancer. LancetOncol. 2021;22:20–21.
7. Rebolj M, Pesola F, Mathews C, Mesher D, Soldan K, Kitchener H. The impact of catch-up bivalent human papillomavirus vaccination on cervical screening outcomes: an observational study from the English HPV primary screening pilot. Br J Cancer. 2022. PMID: 35347326. doi:10.1038/s41416-022-01791-w.
8. Lin Y, Lin Z, He F, et al. HPV vaccination intent and willingness to pay for 2-,4-, and 9-valent HPV vaccines: a study of adult women aged 27–45 years in China. Vaccine. 2020;38(14):3021–3030. PMID: 32127227. doi:10.1016/j.vaccine.2020.02.042
9. Okoye JO, Chukwukelu CF, Okekpa SI, Ogenyi SI, Onyekachi-Umah IN, Ngokere AA. Racial disparities associated with the prevalence of vaccine and non-vaccine HPV types and multiple HPV infections between Asia and Africa: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2021;22(9):2729–2741. PMID: 34582640; PMCID: PMC8850889. doi:10.31557/APJCP.2021.22.9.2729
10. Li K, Yin R, Li Q, Wang D. Analysis of HPV distribution in patients with cervical precancerous lesions in Western China. Medicine. 2017;96(29):e7304. PMID: 28723743; PMCID: PMC5521883. doi:10.1097/MD.0000000000007304
11. Wang S, Li L, Yang J, Han N, Bao H, Wang HJ. Comparison of different HPV-based strategies and cytology in routine cervical cancer screening programme in china: a population-based study. Cancer Prev Res. 2022;15(1):45–54. PMID: 34556493. doi:10.1158/1940-6207.CAPR-21-0104
12. Zhang L, Bi Q, Deng H, et al. Human papillomavirus infections among women with cervical lesions and cervical cancer in Eastern China: genotype-specific prevalence and attribution. BMC Infect Dis. 2017;17(1):107. PMID: 28143439; PMCID: PMC5282745. doi:10.1186/s12879-017-2223-1
13. Yan X, Shen L, Xiao Y, Wang Q, Li F, Qian Y. Prevalence, characteristics, and distribution of HPV genotypes in women from Zhejiang Province, 2016–2020. Virol J. 2021;18(1):208. PMID: 34670576; PMCID: PMC8527678. doi:10.1186/s12985-021-01676-z
14. Zhu X, Wang Y, Lv Z, Su J. Prevalence and genotype distribution of high-risk HPV infection among women in Beijing, China. J Med Virol. 2021;93(8):5103–5109. PMID: 33847386. doi:10.1002/jmv.27013
15. Yuanyue L, Baloch Z, Yasmeen N, Tao Y, Xiaomei W, Xueshan X. The distribution of human papillomavirus genotypes in cervical cancer and intraepithelial neoplasia lesions among Chinese women in Yunnan Province. J Infect Public Health. 2018;11(1):105–110. PMID: 28697900. doi:10.1016/j.jiph.2017.06.012
16. Li Z, Shi L, Yan ZL, et al. Analysis of human papillomavirus infection status in six regions of Yunnan Province. J Guizhou Med Univ. 2017;42(4):396–399.
17. WHO Classification of Tumours Editorial Board. WHO Classification of Tumors: Female Genital Tumors. Lyon (France): IARC Publications; 2020:342–346.
18. Li Z, Liu F, Cheng S, et al. Prevalence of HPV infection among 28,457 Chinese women in Yunnan Province, southwest China. Sci Rep. 2016;6:21039. PMID: 26868772; PMCID: PMC4751528. doi:10.1038/srep21039
19. Gu L, Hong Z, Gao H, Qiu L, Di W. Incidence of cervical high-grade squamous intraepithelial lesions and squamous cell carcinoma in women with high-risk human papillomavirus and normal cervical cytology: a retrospective analysis of 1858 cases stratified by age and human papillomavirus genotype. Cytopathology. 2019;30(4):419–425. PMID: 31069857. doi:10.1111/cyt.12717
20. Kim M, Park NJ, Jeong JY, Park JY. Multiple Human Papilloma Virus (HPV) infections are associated with HSIL and persistent HPV infection status in Korean patients. Viruses. 2021;13(7):1342. PMID: 34372548; PMCID: PMC8310096. doi:10.3390/v13071342
21. Brant AC, Menezes AN, Felix SP, Almeida LM, Moreira MAM. Preferential expression of a HPV genotype in invasive cervical carcinomas infected by multiple genotypes. Genomics. 2020;112(5):2942–2948. PMID: 32437850. doi:10.1016/j.ygeno.2020.05.009
22. Mazarico E, Gómez-Roig MD, Miñano J, Cortes L, Gonzalez-Bosquet E. Relationship of human papilloma virus multiple genotype infection with patient’s age and type of cervical lesion. Eur J Gynaecol Oncol. 2014;35(4):378–381. PMID: 25118477.
23. Wang Y, Xue J, Dai X, et al. Distribution and role of high-risk human papillomavirus genotypes in women with cervical intraepithelial neoplasia: a retrospective analysis from Wenzhou, southeast China. Cancer Med. 2018;7(7):3492–3500. PMID: 29851256; PMCID: PMC6051158. doi:10.1002/cam4.1559
24. Baloch Z, Yuan T, Wang B, et al. Ethnic and geographic variations in HPV prevalence and genotype distribution in north-western Yunnan, China. J Med Virol. 2016;88(3):532–540. PMID: 26266484. doi:10.1002/jmv.24352
25. BalochZ, YasmeenN, LiY, et al.Prevalence and risk factors for human papillomavirus infection among Chinese ethnic women in southern of Yunnan, China. Braz J Infect Dis. 2017;21(3):325–332. PMID: 28284657. doi:10.1016/j.bjid.2017.01.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
