Back to Journals » Journal of Inflammation Research » Volume 16
The Gut Microbial Metabolite Trimethylamine N-Oxide is Linked to Specific Complications of Systemic Sclerosis
Authors Stec A , Maciejewska M , Paralusz-Stec K, Michalska M, Giebułtowicz J, Rudnicka L, Sikora M
Received 9 March 2023
Accepted for publication 27 April 2023
Published 1 May 2023 Volume 2023:16 Pages 1895—1904
DOI https://doi.org/10.2147/JIR.S409489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Albert Stec,1 Magdalena Maciejewska,1 Karolina Paralusz-Stec,1 Milena Michalska,2 Joanna Giebułtowicz,3 Lidia Rudnicka,1 Mariusz Sikora4
1Department of Dermatology, Medical University of Warsaw, Warsaw, Poland; 2Department of General, Vascular and Transplant Surgery, Medical University of Warsaw, Warsaw, Poland; 3Department of Bioanalysis and Drugs Analysis, Faculty of Pharmacy, Medical University of Warsaw, Warsaw, Poland; 4National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland
Correspondence: Mariusz Sikora, National Institute of Geriatrics, Rheumatology and Rehabilitation, Spartańska 1, Warsaw, 02-637, Poland, Tel +48 22 670 91 00, Fax +48 22 844 77 97, Email [email protected]
Background: Systemic sclerosis (SSc) is a rare immune-mediated connective tissue disease characterized by fibrosis of the skin and internal organs, whose pathogenesis is not fully understood. Recent studies have revealed dysbiosis in patients with systemic sclerosis and have indicated the possible role of the microbiota and its metabolites in the pathogenesis of the disease. Trimethylamine N-oxide (TMAO) is a compound produced by dysbiotic microbiota observed at higher concentrations in several autoimmune diseases.
Objective: To determine concentrations of the bacteria-derived metabolite TMAO in patients with systemic sclerosis and to assess possible correlation between TMAO and a specific manifestation of the disease.
Patients and Methods: The study included 63 patients with SSc and 47 matched control subjects. The concentration of TMAO was measured with high-performance liquid chromatography.
Results: Plasma TMAO level was significantly increased in patients with SSc (283.0 [188.5– 367.5] ng/mL versus 205.5 [101.0– 318.0] ng/mL; p < 0.01). An increased concentration of TMAO was observed in patients with concomitant interstitial lung disease (ILD) (302.0 ng/mL [212.0– 385.5] ng/mL versus 204.0 [135.5– 292.0] ng/mL; p < 0.01) and esophageal dysmotility (289.75 [213.75– 387.5] ng/mL versus 209.5 ng/mL [141.5– 315.0] ng/mL; p < 0.05) compared to patients without these complications. Furthermore, TMAO concentration exhibited significant correlation with markers of heart involvement (left ventricle ejection fraction, NT-proBNP), marker of ILD severity and Scleroderma Clinical Trials Consortium Damage Index.
Conclusion: The concentration of TMAO, gut microbiota-associated metabolite, is increased in systemic sclerosis, particularly in patients with advanced organ involvement. This is the first study evaluating plasma TMAO in systemic sclerosis. Bacterial metabolites may be a link between dysbiosis and organ involvement in the course of the disease. Modulation of gut bacterial-derived metabolites may represent a new therapeutic approach in the management of systemic sclerosis.
Keywords: gut microbiota, systemic sclerosis, trimethylamine N-oxide, bacterial metabolites, dysbiosis, damage index
Introduction
Systemic sclerosis (SSc) is an immune-mediated connective tissue disease. It is characterized by fibrosis of the skin, internal organs (eg, lungs, gastrointestinal tract) and vascular abnormalities. Based on the extent of skin involvement, SSc is commonly classified into two main subsets: diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc).1–3 To date, the pathology of systemic sclerosis remains not fully elucidated and there is a lack of established biomarkers of SSc severity and organ involvement.4,5 Early intervention with immunomodulators in patients with SSc may lead to better outcome.6,7
Recently, there has been a growing number of studies presenting alteration in intestinal microbiota (dysbiosis) in patients with systemic sclerosis and its impact on the disease. Gut microbiota profiling in systemic sclerosis revealed significant changes in its biodiversity and composition compared to healthy controls. Patients with SSc are characterized by an increased abundance of Fusobacterium, Desulfovibrio, Ruminococcus, and Lactobacillus, and a decreased abundance of Faecalibacterium.8–10 Furthermore, some of the SSc complications, such as interstitial lung disease (ILD) and esophageal dysmotility, are associated with more pronounced dysbiosis.11,12 Currently, research on modifying microbiota in SSc is emerging. Some studies have shown that probiotics or fecal microbiota transplantation may improve SSc, particularly gastrointestinal symptoms of the disease.13–16
In systemic sclerosis, metabolic proficiencies of gut microbiota also differ from the control group;9 thus, it is highly probable that patients are exposed to a different spectrum of bacterial substances. This exposure can be magnified by vascular abnormalities and intestinal involvement17 which increase intestinal permeability.18,19 The pathways by which microbiota can regulate human homeostasis have recently been intensively investigated. Among them, the impact of microbiota metabolites is the most direct and has been the best described. Some bacterial metabolites present in increased concentrations in the state of dysbiosis are considered detrimental to homeostasis. These metabolites include trimethylamine N-oxide (TMAO).20,21 TMAO is known for its pro-inflammatory properties, which exacerbate several diseases, especially atherosclerosis, chronic kidney disease and type 2 diabetes.22–24
TMAO is a small molecule that is transformed by the liver from bacterial metabolite – trimethylamine (TMA).22,25 The literature reports that the concentration of plasma TMAO is increased in conditions such as cardiovascular diseases,26–28 and renal insufficiency,29 also in rheumatic diseases such as psoriatic arthritis,30 and rheumatoid arthritis.31 Furthermore, a recent pre-clinical study has found that TMAO significantly increases the expression of extracellular matrix proteins associated with fibrosis and the major profibrotic cytokine TGF-β1, which are the key elements of SSc pathomechanism.32
The study aimed to determine concentrations of the bacteria-derived metabolite TMAO in plasma of SSc patients as well as assess potential correlation between plasma TMAO concentration and various clinical (age, disease subtype, concomitant lung, gastrointestinal, or cardiac involvement) and metabolic factors in SSc.
Materials and Methods
Subject
The study included 63 adult patients diagnosed with systemic sclerosis based on the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2013 classification criteria. Patients were recruited from the Department of Dermatology at the Medical University of Warsaw between February 2021 and January 2022.
Exclusion criteria included gastrointestinal infection during the last 3 months before the study, concomitant chronic gastrointestinal disorder (eg, Crohn’s disease, celiac disease), any gastrointestinal surgical procedures or unexplained weight loss during the last 6 months prior to the study, dietary restrictions or intake of medications modulating gut microbiota (antibiotics, probiotics or prebiotics) within the previous 3 months, chronic liver and pancreatic disease, other autoimmune diseases, estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2, pregnancy, and breastfeeding.
The control group consisted of 47 individuals who were matched for age, body mass index (BMI), and gender. Control group subjects fulfilled the same exclusion criteria.
Clinical Assessment
Detailed medical history was obtained from all participants. The extent of skin involvement was analyzed with a modified Rodnan skin score (mRSS). Microvascular abnormalities assessed in capilaroscopy were classified into early, active, or late scleroderma patterns according to Cutolo et al.33 To evaluate the gastrointestinal manifestation of systemic sclerosis a barium swallow X-ray was performed. To determine lung involvement, high-resolution computed tomography (HRCT) and pulmonary function tests, including diffusing capacity for carbon monoxide (DLCO), were performed. Cardiac involvement was assessed in echocardiography and by measuring N-terminal pro-B-type natriuretic peptide (NT-proBNP). To quantify organ damage in the course of SSc, we used Scleroderma Clinical Trials Consortium Damage Index (SCTC-DI).
Laboratory Assessment
Patients have the following tests performed: complete blood count, erythrocyte sedimentation rate glucose, lipid, kidney and liver profiles. Venous blood samples were taken for analysis following a 12-hour fast. Estimated glomerular filtration rate (eGFR) was calculated using the CDK-EPI equation. Antinuclear antibodies were detected and identified with immunoblot assay.
Plasma TMAO concentration was measured using high-performance liquid chromatography (HPLC) coupled with triple-quadrupole mass spectrometry as previously described.34,35
Statistical Analysis
The statistical software STATISTICA 13.3 was used for all calculations (TIBCO, Palo Alto, CA, USA). The Shapiro–Wilk test was used to determine if the distribution of the data was normal. The mean with standard deviation were used to represent normally distributed data, while the median and interquartile range (IQR) were used to express non-normally distributed variables. A chi-squared test was used to compare categorical data that were presented as counts and percentages. For two independent samples, a Student’s t-test after assessment of the equality of variances by Levene’s test, or Mann–Whitney U-test was used to assess the continuous variables that were parametric and nonparametric, respectively. To evaluate potential linear correlations between two continuous variables, the Spearman rank test with correlation coefficient was applied. P-values <0.05 were considered to be statistically significant.
Ethics
The study was approved by the Regional Bioethical Committee at the Medical University of Warsaw (KB/136/2021). Written informed consent was obtained from all participants. The study has been carried out in accordance with the Declaration of Helsinki.
Results
Characteristics of the Study Group
The study included 63 SSc patients (57 women and 6 men) and 47 controls (42 women and 5 men) matched for age, sex and BMI. The mean age of patients and volunteers was 59.9 and 55.5 years, respectively. The SSc cohort consisted of 34 (54.0%) individuals with dcSSc and 29 (54.0%) individuals with lcSSc based on LeRoy’s criteria.
Detailed characteristics of SSc patients including demographical, clinical, and serological features are presented in Table 1.
 |
Table 1 The Main Characteristics of SSc Patients and the Control Group |
TMAO in Systemic Sclerosis
The median concentration of TMAO in the group of patients with systemic sclerosis was 283.0 ng/mL (IQR 188.5–367.5) and it was significantly higher compared to the control group (205.5 ng/mL, IQR 101.0–318.0, p = 0.006, Figure 1).
 |
Figure 1 Plasma concentration of trimethylamine N-oxide (TMAO) in patients with systemic sclerosis compared to controls (* p < 0.05). |
Furthermore, we found a significant moderate correlation between TMAO concentration and the age of patients (rho = 0.446; p = 0.0002; Figure 2).
 |
Figure 2 Correlation of TMAO with the age of patients with SSc (rho = 0.446; p < 0.001). |
When patients were categorized according to antinuclear antibodies, there were no significant differences between patients with positive anti-RNA polymerase III (298.5 ng/mL, IQR 230.5–368.0), antitopoisomerase I (262.5 ng/mL, IQR 169.0–340.0) and anticentromere antibodies (284.7 ng/mL, IQR 189.0–345.0).
TMAO and Skin Involvement
There was no significant difference between patients with lcSSc (280.7 ng/mL; IQR 183.5–330.7) and dcSSc (287.7 ng/mL; IQR 185.2–375.7). No significant correlation was found between TMAO concentration and skin involvement measured by mRSS.
TMAO level did not differ between SSc patients presenting early (284.7 ng/mL; IQR 206.2–632.0), active (253.5 ng/mL; IQR 178.0–315.0), and late (308.7 ng/mL; IQR 204.0–366.0) patterns in NVC.
TMAO and Interstitial Lung Disease
A comparison of TMAO concentration in the group of patients showed significantly higher TMAO levels in SSc patients with interstitial lung disease (Figure 3). The median concentration of TMAO in the subgroup with ILD was 302.0 ng/mL (IQR 212.0–385.5) compared to 204.0 ng/mL (IQR 135.5–292.0) in the subgroup without this complication (p = 0.001). Furthermore, DLCO, a marker of the restriction caused by ILD, showed a significant negative correlation with TMAO level (rho=−0.53; p = 0.013 (Figure 4).
 |
Figure 3 Plasma concentration of trimethylamine N-oxide (TMAO) in SSc patients with interstitial lung disease (ILD) and without this complication (* p < 0.05). |
 |
Figure 4 Correlation of trimethylamine N-oxide (TMAO) with diffusing capacity of lung for carbon monoxide (DLCO; rho = −0.53; p < 0.05). |
TMAO and Gastrointestinal Manifestations of the Disease
In SSc patients with concomitant esophageal dysmotility (confirmed in barium swallow) TMAO concentration was significantly higher (289.75 ng/mL [IQR 213.75–387.5]) than in subgroup with normal esophageal passage (209.5 ng/mL [IQR 141.5–315.0] p = 0.026, Figure 5).
 |
Figure 5 Plasma concentration of trimethylamine N-oxide (TMAO) in SSc patients subgroup with esophageal dysmotility and without this complication (* p < 0.05). |
TMAO and Cardiac Involvement of SSc
Spearman correlation coefficient revealed a significant negative correlation between TMAO plasma concentration and left ventricle (LV) ejection fraction in echocardiography (rho=−0.39; p = 0.009, Figure 6), and a significant positive correlation between TMAO and NT-proBNP concentration (rho = 0.41; p = 0.0009, Figure 7).
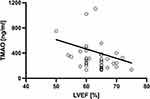 |
Figure 6 Correlation of trimethylamine N-oxide (TMAO) with left ventricular ejection fraction (LVEF; rho = −0.39; p < 0.01). |
 |
Figure 7 Correlation of trimethylamine N-oxide (TMAO) with N-terminal pro B-type natriuretic peptide (NT-proBNP; rho = 0.41; p < 0.001). |
TMAO and Organ Damage in SSc
There was a significant correlation (rho = 0.78; p = 0.0001) between TMAO concentration and the Scleroderma Clinical Trials Consortium Damage Index (SCTC-DI) as shown in Figure 8.
 |
Figure 8 Correlation of trimethylamine N-oxide (TMAO) with the Scleroderma Clinical Trials Consortium Damage Index (SCTC-DI; rho = 0.78; p < 0.001). |
Discussion
This work provides evidence that intestinal microbiota metabolite TMAO is associated with some specific complications of systemic sclerosis. To the best of our knowledge, this is the first human study investigating microbiota metabolite TMAO in SSc. We found that this compound is present in a greater concentration in patients with SSc compared to the age-, sex-, and BMI-matched control subjects. The possible explanation for this could be that bacterial genera which are more abundant in the dysbiotic microbiota of SSc patients (ie, Fusobacterium, Desulfovibrio, Ruminococcus) have been observed to be linked with greater plasma TMAO concentrations.36 Moreover, dysbiosis with an abundance of these bacteria has been observed in conditions such as end-stage renal disease,37 pulmonary arterial hypertension,38 and cardiac failure.39
Additionally, SSc patients with concomitant interstitial lung disease and reflux disease were characterized by an increased level of TMAO compared to patients without mentioned comorbidities. Significant correlations between the TMAO level and markers of cardiac involvement were also revealed. The observed results are corresponding to the previous preclinical studies. Endothelial injury is an early feature of systemic sclerosis and plays a key role in pathogenesis.40 Recent preclinical studies have shown that TMAO can potentially impact the pathogenesis of systemic sclerosis by inducing endothelial injury, promoting profibrotic cytokine signaling and collagen deposition, suppressing endothelium-dependent dilation of arteries, and inhibiting nitric oxide release in the epithelium.32,41,42 It was observed that TMAO can induce the differentiation of fibroblasts into myofibroblasts and endothelial-mesenchymal transition.32 The presence of myofibroblasts in involved organs is one of the histopathological hallmarks of systemic sclerosis.33
The available literature reveals that the incidence and increased severity of SSc-associated complications may be linked to an increased level of TMAO. An elevated level of this metabolite has been observed in pulmonary arterial hypertension (PAH)43,44 which is a common comorbidity in systemic sclerosis and affects about 15% of patients.1 Additionally, higher TMAO level was related to the worse course of PAH.44,45 In heart involvement, microbiota metabolites could be also an important factor in prognosis. Literature reports that TMAO can aggravate heart fibrosis and therefore decrease ejection fraction and contractility of the myocardium.46 More severe gastrointestinal symptoms, including esophageal reflux, were observed in patients with higher circulating TMAO.47 Despite these encouraging findings, it is still unknown whether a rise in TMAO is a causative factor of the mentioned conditions or just an effect of the disease. To accurately evaluate the pathogenic effect of TMAO on the complications of systemic sclerosis more studies are needed, especially prospective and multi-centered, that would evaluate the long-term exposition to TMAO and its potential effects. However, several studies reported the advantages of an approach based on reducing the TMAO level. Lowering TMAO by 3,3-dimethyl-1-butanol (DMB), an inhibitor of the bacterial synthesis of TMA, improved hemodynamic parameters and pulmonary vascular remodeling in mouse and rat models of PAH.44,45 In a mouse model of cardiac fibrosis lowering TMAO was associated with the decreased gene expression level of collagen I and TGF-β, and decreased fibrosis in a microscopic examination of cardiomyocytes.48 According to the mentioned studies, patients with systemic sclerosis may benefit from adjuvant medication intended to reduce harmful bacteria metabolites and possibly experience reduced disease activity. A growing number of evidences suggest that TMAO concentration can be modulated by several bioactive compounds or dietary patterns. The low-fat diet, resveratrol, capsanthin and allicin decrease TMAO concentrations, whereas high-fat and high protein diet promote overproduction of TMAO. Diet can affect TMAO in several pathways, including the metabolism of its precursors, modulating intestinal permeability as well as shifting gut microbiota.49,50
Understanding the mechanisms of how dysbiotic microbiota and its metabolites may influence systemic sclerosis seems to be valuable in clinical practice. Our study suggests that TMAO may serve as a useful marker for identifying the severity of multi-organ involvement in SSc due to exhibiting a strong positive correlation with SCTC-DI. Therefore, measurement of TMAO may help to stratify patients into different disease severity categories, which could help to optimize the treatment of the disease.
Limitations of our study include small sample size, single-center type, and cross-sectional character. To assess the causative effects of prolonged exposure to increased plasma levels of bacterial metabolites on the development of complications of SSc prospective, multicenter studies are needed.
Conclusion
Our cross-sectional study showed that increased plasma TMAO level was associated with increased prevalence and severity of interstitial lung disease and heart involvement in systemic sclerosis. Also, a higher concentration of TMAO was observed in patients with concomitant gastroesophageal dysmotility. This is the first study evaluating plasma TMAO in systemic sclerosis. The mentioned findings indicate the possible usefulness of TMAO as a biomarker of incidence, and severity of complications of the disease. A more profound knowledge of the causal link between microbiota metabolites and SSc may point to pathophysiological pathways that may be addressed for targeted therapeutics to improve disease severity and complications.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
Publication financed by the Medical University of Warsaw as part of the Time 2 MUW project (agreement number: POWR.03.05.00-00-Z040/18-00).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. doi:10.1016/s0140-6736(17)30933-9
2. Bobeica C, Niculet E, Craescu M, et al. CREST syndrome in systemic sclerosis patients - is dystrophic calcinosis a key element to a positive diagnosis? J Inflamm Res. 2022;15:3387–3394. doi:10.2147/JIR.S361667
3. Bernero E, Sulli A, Ferrari G, et al. Prospective capillaroscopy-based study on transition from primary to secondary Raynaud’s phenomenon: preliminary results. Reumatismo. 2013;65(4):186–191. doi:10.4081/reumatismo.2013.186
4. Pawlik KK, Bohdziewicz A, Chrabaszcz M, et al. Biomarkers of disease activity in systemic sclerosis. Wiad Lek. 2020;73(10):2300–2305. doi:10.36740/WLek202010137
5. Utsunomiya A, Oyama N, Hasegawa M. Potential biomarkers in systemic sclerosis: a literature review and update. J Clin Med. 2020;9(11):3388. doi:10.3390/jcm9113388
6. Smith V, Pizzorni C, Riccieri V, et al. Stabilization of microcirculation in patients with early systemic sclerosis with diffuse skin involvement following rituximab treatment: an open-label study. J Rheumatol. 2016;43(5):995–996. doi:10.3899/jrheum.151018
7. Cheng H, Yu Z, Yan CL, Yang HD, Gao C, Wen HY. Long-term efficacy and low adverse events of methylprednisolone pulses combined to low-dose glucocorticoids for systemic sclerosis: a retrospective clinical study of 10 years’ follow-up. J Inflamm Res. 2022;15:4421–4433. doi:10.2147/JIR.S373387
8. Patrone V, Puglisi E, Cardinali M, et al. Gut microbiota profile in systemic sclerosis patients with and without clinical evidence of gastrointestinal involvement. Sci Rep. 2017;7(1):14874. doi:10.1038/s41598-017-14889-6
9. Volkmann ER, Chang YL, Barroso N, et al. Association of systemic sclerosis with a unique colonic microbial consortium. Arthritis Rheumatol. 2016;68(6):1483–1492. doi:10.1002/art.39572
10. Volkmann ER, Hoffmann-Vold AM, Chang YL, et al. Systemic sclerosis is associated with specific alterations in gastrointestinal microbiota in two independent cohorts. BMJ Open Gastroenterol. 2017;4(1):e000134. doi:10.1136/bmjgast-2017-000134
11. Andreasson K, Lee SM, Lagishetty V, et al. Disease features and gastrointestinal microbial composition in patients with systemic sclerosis from two independent cohorts. ACR Open Rheumatol. 2022;4(5):417–425. doi:10.1002/acr2.11387
12. Andréasson K, Alrawi Z, Persson A, Jönsson G, Marsal J. Intestinal dysbiosis is common in systemic sclerosis and associated with gastrointestinal and extraintestinal features of disease. Arthritis Res Ther. 2016;18(1):278. doi:10.1186/s13075-016-1182-z
13. Frech TM, Khanna D, Maranian P, Frech EJ, Sawitzke AD, Murtaugh MA. Probiotics for the treatment of systemic sclerosis-associated gastrointestinal bloating/ distention. Clin Exp Rheumatol. 2011;29(2 Suppl 65):S22–S25.
14. Low AHL, Teng GG, Pettersson S, et al. A double-blind randomized placebo-controlled trial of probiotics in systemic sclerosis associated gastrointestinal disease. Semin Arthritis Rheum. 2019;49(3):411–419. doi:10.1016/j.semarthrit.2019.05.006
15. Fretheim H, Chung BK, Didriksen H, et al. Fecal microbiota transplantation in systemic sclerosis: a double-blind, placebo-controlled randomized pilot trial. PLoS One. 2020;15(5):e0232739. doi:10.1371/journal.pone.0232739
16. Hoffmann-Vold A-M, Fretheim H, Chung BK, et al. OP0327 Fecal microbiota transplantation in systemic sclerosis: a double-blind, placebo-controlled randomized pilot trial. Ann Rheum Dis. 2019;78(Suppl2):246–247. doi:10.1136/annrheumdis-2019-eular.4684
17. Sakkas LI, Simopoulou T, Daoussis D, Liossis SN, Potamianos S. Intestinal involvement in systemic sclerosis: a clinical review. Dig Dis Sci. 2018;63(4):834–844. doi:10.1007/s10620-018-4977-8
18. Caserta L, de Magistris L, Secondulfo M, et al. Assessment of intestinal permeability and orocecal transit time in patients with systemic sclerosis: analysis of relationships with epidemiologic and clinical parameters. Rheumatol Int. 2003;23(5):226–230. doi:10.1007/s00296-003-0286-3
19. Catanoso M, Lo Gullo R, Giofré MR, et al. Gastro-intestinal permeability is increased in patients with limited systemic sclerosis. Scand J Rheumatol. 2001;30(2):77–81. doi:10.1080/03009740151095303
20. Taguchi K, Fukami K, Elias BC, Brooks CR. Dysbiosis-related advanced glycation endproducts and trimethylamine N-oxide in chronic kidney disease. Toxins. 2021;13(5):361. doi:10.3390/toxins13050361
21. Chen YY, Chen DQ, Chen L, et al. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J Transl Med. 2019;17(1):5. doi:10.1186/s12967-018-1756-4
22. Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398. doi:10.3390/nu10101398
23. Zhou Z, Jin H, Ju H, Sun M, Chen H, Li L. Circulating trimethylamine-N-oxide and risk of all-cause and cardiovascular mortality in patients with chronic kidney disease: a systematic review and meta-analysis. Front Med. 2022;9:828343. doi:10.3389/fmed.2022.828343
24. Zhuang R, Ge X, Han L, et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: a systematic review and dose-response meta-analysis. Obes Rev. 2019;20(6):883–894. doi:10.1111/obr.12843
25. Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition. 2015;31(11–12):1317–1323. doi:10.1016/j.nut.2015.05.006
26. Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–1914. doi:10.1016/j.jacc.2014.02.617
27. Qi J, You T, Li J, et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22(1):185–194. doi:10.1111/jcmm.13307
28. Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi:10.1016/j.cell.2016.02.011
29. Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. doi:10.1161/circresaha.116.305360
30. Coras R, Kavanaugh A, Boyd T, et al. Choline metabolite, trimethylamine N-oxide (TMAO), is associated with inflammation in psoriatic arthritis. Clin Exp Rheumatol. 2019;37(3):481–484.
31. Chan MM, Yang X, Wang H, Saaoud F, Sun Y, Fong D. The microbial metabolite trimethylamine N-oxide links vascular dysfunctions and the autoimmune disease rheumatoid arthritis. Nutrients. 2019;11(8):1821. doi:10.3390/nu11081821
32. Kim SJ, Bale S, Verma P, et al. Gut microbe-derived metabolite trimethylamine N-oxide activates PERK to drive fibrogenic mesenchymal differentiation. iScience. 2022;25(7):104669. doi:10.1016/j.isci.2022.104669
33. Cutolo M, Sulli A, Smith V. Assessing microvascular changes in systemic sclerosis diagnosis and management. Nat Rev Rheumatol. 2010;6(10):578–587. doi:10.1038/nrrheum.2010.104
34. Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30(12):1700–1705. doi:10.1016/j.cjca.2014.09.010
35. Sikora M, Kiss N, Stec A, et al. Trimethylamine N-oxide, a gut microbiota-derived metabolite, is associated with cardiovascular risk in psoriasis: a cross-sectional pilot study. Dermatol Ther. 2021;11(4):1277–1289. doi:10.1007/s13555-021-00547-3
36. Fu BC, Hullar MAJ, Randolph TW, et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am J Clin Nutr. 2020;111(6):1226–1234. doi:10.1093/ajcn/nqaa015
37. Lohia S, Vlahou A, Zoidakis J. Microbiome in chronic kidney disease (CKD): an omics perspective. Toxins. 2022;14(3):176. doi:10.3390/toxins14030176
38. Wu P, Zhu T, Tan Z, Chen S, Fang Z. Role of gut microbiota in pulmonary arterial hypertension. Front Cell Infect Microbiol. 2022;12:812303. doi:10.3389/fcimb.2022.812303
39. Wang Z, Cai Z, Ferrari MW, et al. The correlation between gut microbiota and serum metabolomic in elderly patients with chronic heart failure. Mediators Inflamm. 2021;2021:5587428. doi:10.1155/2021/5587428
40. Zanin-Silva DC, Santana-Goncalves M, Kawashima-Vasconcelos MY, Oliveira MC. Management of endothelial dysfunction in systemic sclerosis: current and developing strategies. Front Med. 2021;8:788250. doi:10.3389/fmed.2021.788250
41. Brunt VE, Gioscia-Ryan RA, Casso AG, et al. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension. 2020;76(1):101–112. doi:10.1161/HYPERTENSIONAHA.120.14759
42. Querio G, Antoniotti S, Geddo F, Levi R, Gallo MP. Trimethylamine N-oxide (TMAO) impairs purinergic induced intracellular calcium increase and nitric oxide release in endothelial cells. Int J Mol Sci. 2022;23(7):3982. doi:10.3390/ijms23073982
43. Kim S, Rigatto K, Gazzana MB, et al. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75(4):1063–1071. doi:10.1161/hypertensionaha.119.14294
44. Huang Y, Lin F, Tang R, et al. Gut microbial metabolite trimethylamine N-oxide aggravates pulmonary hypertension. Am J Respir Cell Mol Biol. 2022;66(4):452–460. doi:10.1165/rcmb.2021-0414OC
45. Yang Y, Zeng Q, Gao J, et al. High-circulating gut microbiota-dependent metabolite trimethylamine N-oxide is associated with poor prognosis in pulmonary arterial hypertension. Eur Heart J Open. 2022;2(5):oeac021. doi:10.1093/ehjopen/oeac021
46. Li X, Geng J, Zhao J, et al. Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front Physiol. 2019;10:866. doi:10.3389/fphys.2019.00866
47. Sikora M, Stec A, Chrabaszcz M, et al. Clinical implications of intestinal barrier damage in psoriasis. J Inflamm Res. 2021;14:237–243. doi:10.2147/JIR.S292544
48. Nanto-Hara F, Kanemitsu Y, Fukuda S, et al. The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol Dial Transplant. 2020;35(2):250–264. doi:10.1093/ndt/gfz126
49. Simo C, Garcia-Canas V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020;11(8):6745–6776. doi:10.1039/d0fo01237h
50. Coutinho-Wolino KS, de FC, de Oliveira Leal V, Mafra D, Stockler-Pinto MB. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur J Nutr. 2021;60(7):3567–3584. doi:10.1007/s00394-021-02491-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
