Back to Journals » Journal of Inflammation Research » Volume 15
The Functional Mechanism of MicroRNA in Oral Lichen Planus
Authors Li Y, He Y, Xiang J, Feng L, Wang Y, Chen R
Received 4 April 2022
Accepted for publication 10 July 2022
Published 26 July 2022 Volume 2022:15 Pages 4261—4274
DOI https://doi.org/10.2147/JIR.S369304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Yunshan Li,1,* Yaodong He,1,* Junwei Xiang,1 Linfei Feng,2 Yuanyin Wang,1 Ran Chen1
1College & Hospital of Stomatology, Anhui Medical University, Key Laboratory of Oral Diseases Research of Anhui Province, Hefei, 230032, People’s Republic of China; 2Department of Oral and Maxillofacial Surgery, the First Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanyin Wang; Ran Chen, College & Hospital of Stomatology, Anhui Medical University, Key Laboratory of Oral Diseases Research of Anhui Province, Hefei, 230032, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Non-coding RNAs (ncRNAs) are transcribed from the genomes of mammals and other complex organisms, and many of them are alternately spliced and processed into smaller products. Types of ncRNAs include microRNAs (miRNAs), circular RNAs, and long ncRNAs. miRNAs are about 21 nucleotides long and form a broad class of post-transcriptional regulators of gene expression that affect numerous developmental and physiological processes in eukaryotes. They usually act as negative regulators of mRNA expression through complementary binding sequences in the 3’-UTR of the target mRNA, leading to translation inhibition and target degradation. In recent years, the importance of ncRNA in oral lichen planus (OLP), particularly miRNA, has attracted extensive attention. However, the biological functions of miRNAs and their mechanisms in OLP are still unclear. In this review, we discuss the role and function of miRNAs in OLP, and we also describe their potential functional roles as biomarkers for the diagnosis of OLP. MiRNAs are promising new therapeutic targets, but more work is needed to understand their biological functions.
Keywords: oral lichen planus, miRNA, biomarker, therapy
Introduction
Oral lichen planus (OLP) is a chronic disease that mainly affects the immune system,1 involving the oral mucosa, that features recurrence and remission.2,3 In the general population, the prevalence of OLP ranges from 0.5% to 4%, and it can appear 4–5 years after birth. In clinical practice, six clinical subgroups of OLP can be seen, singly or in combination: plaque-like, reticular, papular, erosive/ulcerative, atrophic, and bullous. The reticular, erosive/ulcerative, and plaque-like subtypes are the most common.4–6 In the literature, papular, plaque-like, and reticular OLP lesions are called non-erosive OLP (NEOLP) lesions, while atrophic/erosive, bullous, and ulcerative types are referred to as erosive OLP (EOLP).7 Because 1.63% of OLP lesions progress to oral squamous cell carcinomas (OSCC), the World Health Organization designated OLP as a potentially malignant oral condition in 2005.8–10 Thus, it is important to better understand the mechanisms of and treatments for OLP; in addition, the role of non-coding RNAs (ncRNAs) in OLP has attracted increasing attention.
NcRNAs are the most abundant cellular RNAs in all organisms.11 In contrast to mRNAs, ncRNAs, either on their own or in complex with proteins, have important cellular functions and are not translated into proteins.12 NcRNAs are necessary modulators of 90 types of gene expression and perform a signal-transduction role in cells.13 MicroRNAs (miRNAs), a type of ncRNA, are small and endogenous RNAs that regulate gene expression.14 They play an essential role in cancer; identifying the role of miRNAs in OLP as a pre-cancer disease is essential. MiR-123, miR-647, and miR-31 may, through circ_003912, have a role in the pathogenesis of EOLP.15 The apoptosis of keratinocytes may be promoted by miR-122 in OLP.16 Further, changes in the expression of miR-21 and miR-125a may lead to malignant transformation.17 In addition, a number of miRNAs show altered expression in OLP. Therefore, due to its involvement in the pathogenesis of OLP, the role of miRNA as a diagnostic and prognostic biomarker should also not be ignored.
We conducted searches on the PubMed database regarding OLP and miRNA, with the search terms “oral lichen planus”, “OLP”, “oral lichenoid disease”, “OLD”, “miRNA”, “microRNA”, and others. The Boolean operators “AND” and “OR” were used to combine different search terms. Our selection criterion was that the result must be verified in fresh tissue, embedded sample tissue, or human body fluid. Our search identified that, although some previous work has summarized the role of miRNA in OLP, the scopes of the searches have been too broad, and the mechanisms not sufficiently elaborated, so in this study, we identified miRNAs reported in several search results and elaborated the mechanisms of their roles in OLP.
The study of miRNAs is vital to understanding OLP. Therefore, in this review, we examine the role that miRNAs play in OLP and summarize their mechanisms and functions of miRNAs and future directions in therapeutic research.
Overview of Oral Lichen Planus
OLP is a chronic inflammatory disease that affects the oral mucosa and has distinct relapses and remissions. Typically, it affects the tongue, buccal mucosa, and gingiva. Oral lesions are always symmetrical on both sides, and a patient can have two or more forms of OLP simultaneously.4
Forms of OLP have also been reported that have a relationship with metabolic syndrome (MS) in addition to its usual symptoms. MS in OLP patients is higher than in controls. During treatment observation, metabolic complications should be considered. In addition, body mass index and triglyceride levels are also higher in OLP patients, and the levels of inflammatory cytokines may be increased by lipids, then occur or affect OLP. In addition, glucose tolerance and blood pressure are higher in OLP patients. The relationship between OLP and MS should not be ignored.18
The rate of malignant transformation of OLP is between 0% and 10%. A recent meta-analysis found that 1.1% of OLP lesions convert into OSCC, with a higher frequency among alcoholics, smokers, and people infected with hepatitis C. Invasive OLP appears to have the highest frequency of progression to OSCC. The most common malignant transformations occur in lesions confined to the tongue. OLP lesions were found to develop into OSCC lesions after an average of 5.5 years. In addition, compared to individuals with original OSCC, OSCC patients with previous OLP lesions had a greater probability of tumor recurrence.19
Several factors are associated with the etiology of OLP, including stress, genetic background, the stimulation of dental materials, reaction to drugs, autoimmunity, and other factors. Depression and quality of life are also closely related to the development of OLP.20 Additionally, COVID-19 vaccination may also induce OLP.21
Much controversy remains regarding the pathogenesis of OLP, but immune dysregulation is the most likely cause.22,23 Cytokines play a vital role in the initiation and maintenance of immune response and inflammatory and intercellular cross-talk, and cytokines are soluble proteins.24 Tumor necrosis factor-alpha (TNF-α), interferon (IFN)-C, and keratinocyte/T cell/antigen cell connections are thought to be generated by cell-mediated immunity induced by endogenous or external stimuli in OLP.1,2,25 A recent study found that the expression of interleukin-27 (IL-27) in severe OLP was significantly higher than in a control group, and IL-27 was positively correlated with abnormal epithelial cell proliferation, suggesting that IL-27 may have a relationship with the development of OLP.26 In the peripheral blood, IL-12 and IL-1β may affect innate lymphoid cells (ILCs), which could lead to immune dysregulation and influence the OLP.27 Inflammation-related disorders exhibit a strong link between miRNAs and cytokines. Because many miRNAs are involved in OLP with different expression levels, research on their roles in the pathogenesis of OLP is essential.28
Overview of miRNAs
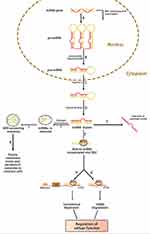
MiRNAs are ncRNAs with a length of 20–25 nucleotides that influence gene expression after transcription.29 The majority of mature miRNA sequences are found in ncRNA introns, ncRNA exons, and pre-mRNA introns, and miRNAs are processed from protein-coding gene introns or RNA polymerase II-specific transcripts of separate genes.30,31 Increasing evidence demonstrates that miRNAs act as key regulators of development and cellular homeostasis by controlling a variety of biological processes. For regulatory functions, miRNAs assemble into an RNA-induced silencing complex (RISC) to activate the complex to target mRNA. MiRNAs bind to the 3’ untranslated region (3ʹUTR) of target mRNA and play a regulatory role in post-transcriptional control. Partial base pairing causes translation inhibition and functional protein degradation, while full or near-perfect complementary base pairing causes mRNA breakdown (Figure 1).
MiRNAs are implicated in various physiological and pathological processes, including critical roles in inflammation and the immune response.32 Moreover, a range of biological functions are associated with miRNAs, including organogenesis, cell differentiation, metabolism, and apoptosis; miRNAs also have an essential role in intercellular communication.30 Accumulating evidence demonstrates that human diseases such as cancer, neurological disorders, cardiovascular disease, and autoimmune illnesses are related to abnormal cellular levels of certain miRNAs.33 For example, the expression of miRNAs is different in some cancer tissues. Downregulated miRNAs can increase the expression of oncomirs, while upregulated miRNAs may lead to the suppression of tumor suppressor genes. Therefore miRNAs may play a role in the actions of both tumor suppressors and oncogenes.34
The levels of miRNAs in patients are different from those in healthy individuals. In a Canadian population, several miRNAs showed differential expression levels from those in healthy individuals; specifically, 15 miRNAs were increased, and 7 miRNAs were reduced.35 Therefore, miRNAs can be thought of as biomarkers of disease activity. To identify a new biomarker for the diagnosis of OLP and the possibility of malignant transformation, further study of the different types of miRNA expression profile is necessary.
Functional Role of miRNAs in OLP
OLP is classified as an oral potentially malignant disorder (OPMD) that has no known cause. It is a T cell-mediated autoimmune disease. During its occurrence and development, miRNA regulation cannot be ignored. Below, we summarize a few such miRNAs, including their potential targets and mechanisms, according to their expression in OLP (Table 1).
 |
Table 1 MiRNAs are Involved in Regulating Oral Lichen Planus |
miR-214
Among vertebrates, miR-214 is substantially conserved, and it is among a family of miRNA precursors.36 The human miR-214 gene is found in intron 14 of the Dynamin-3 gene in the chromosomal region 1q24.3.37 MiR-214 is an osteoclast-derived miRNA molecule that can suppress bone formation.38 Four mature miRNAs can be generated with the primary transcript of miR-214: miR-214-3p and miR-199-5p from the leading strand and miR-214-5p and miR-199-3p from the passenger strand.39 MiR-214 was first identified as a factor that was biologically linked to the death of the cell, and its expression may lower apoptosis in HeLa cells.40 It is involved in a wide range of biological processes, such as the embryonic development of the retina, muscles, neurons, and pancreas. In addition, it is implicated in controlling various critical physiological processes, such as immune tolerance of dendritic cells, hepatic gluconeogenesis, and angiogenesis.36 One study reported that the level of miR-214-3p was lower in a control group and that miR-314-3p may be a risk factor for MS.41 miR-214 has both tumor suppressor and oncogenic activities in melanoma, breast cancer, and ovarian cancer.42 In the oral cavity, by targeting the β-catenin gene CTNNB1, miR-214 may regulate the Wnt/β-catenin signaling pathway, thus participating in mechanisms underlying the differentiation of periodontal ligament stem cells.43 In addition, a recent study explored the influence of miR-214 on the proliferation and apoptosis of oral cancer cells.44 By sponging miR-214, circFOXO3 may upregulate the expression of lysine demethylase 2A (KDM2A), which promotes tumor progression in OSCC.45 LncRNA HOXA11-AS promotes cisplatin resistance and the proliferation of OSCC by suppressing miR-214-3p expression,46 which indicates that miR-214 may play an essential role in the development of oral illnesses. CD44 is a multifunctional cell receptor that regulates vascular gene activation in proatherogenic environments and inflammatory gene production in macrophages and conveys a cancer phenotype.47 In mucosa samples of patients with OLP, a direct link has been found between CD44 expression and miR-214 expression; that study also found that CD44 is the direct target of miR-214, confirming that low levels of miR-214 are associated with OLP and are possible candidate molecules for drug development.48
miR-146a
Dr. David Baltimore’s lab published the first report on human miR-146a in 2006. The miR-146a gene is found on chromosome 11 in mice, and it is found on chromosomes 5, 10, and 19 in humans.49,50 MiR-146a is implicated in the differentiation, inflammation, and function of adaptive and innate immune cells,49 and it also has substantial regulatory involvement in a number of immune cells, including T cells, B cells, and dendritic cells. The physiopathology of various immunologically based inflammatory diseases may be influenced by miR-146a.51,52 MiR-146a works as a differentiation inhibitor for human T helper type 1 cells (Th1-cells) by interacting with the protein kinase C epsilon (PRKC), and it controls Th1-cell development in human CD4+T cells as part of a functional complex composed of signal transducers and transcriptional activators 4 and PRKC.50 Its involvement in the beginning and progress of numerous kinds of cancers has been reported, including prostate and esophageal cancer,53 and it promotes the proliferation, migration, and invasion by OSCC cells.54 One study found that miR-146a-5p exhibits upregulated expression in OSCC, and inhibits transcriptional activity, performing a carcinogenic function in OSCC.55 In another study, the expression of miR-146a was not obviously different between peripheral blood T cells and a control group; however, it was higher in local lesions of OLP patients. As a necessary regulator of Treg cells, forkhead box P3 (FOXP3) acts as a mediator of inflammation and immune response and is overexpressed in OLP.56 Wang et al57 demonstrated that by regulating miR-146a, FOXP3 influenced cell proliferation and the apoptosis of lipopolysaccharide (LPS)-incubated HaCaT cells and raised Treg cells in CD4+T cells derived from individuals with OLP. By modulating its target gene, TNF receptor-associated factor 6 (TRAF6), miR-146a alone can control LPS-incubated HaCaT cells. These findings suggest that in OLP, miR-146a may be necessary for the local immune response and may be usable as a biomarker to evaluate the severity of OLP.
miR-155
MiR-155 is derived from an exon of the B-cell integration cluster gene,58,59 miR-155 host gene.60 It is one of the most multifunctional and conservative miRNAs, and it has a variety of functions and is connected to tumor development, immunological modulation, and inflammation.61,62 miR-155 deficiency can cause a disorder of immune system response.63 In MS, insulin resistance and poor glycemic control are linked to the dysregulation of miR-155.64 MiR-155 is elevated in oral submucous fibrosis tissues, and its suppression may help alleviate persistent myofibroblast activation by upregulating its direct target, mammalian target of rapamycin complex 1.65 In oral cancer cells, the expression of FOXO3a is upregulated by the inhibition of miR-155 expression, which also increases cell apoptosis and inhibits cell growth.66
MiR-155 may be linked to OLP. Liang et al67 found that miR-155 might play an important role in the pathogenesis of OLP. Through transcriptional control of immunologically relevant genes, IFN-γ coordinates a vast variety of cellular activities.68 IFN-γ may limit the synthesis of anti-inflammatory cytokines, such as IL-10 and IL-4, while boosting the secretion of proinflammatory cytokines such as IL-2.69,70 In Hu et al, miR-155 was closely linked to the severity of OLP, and ELOP patients’ peripheral blood had a high level of expression of miR-155. miR-155 may be upregulated by inflammatory cytokines such as INF-γ, TNF-α, TGF-β1, and IL-1β. The expression of miR-155 is upregulated by IL-2 and IL-15. Other proinflammatory triggers, such as LPS—which activates Toll-like receptor 4 in macrophages and dendritic cells, as well as bleomycin—can also activate miR-155. Conversely, IL-10 is a powerful inhibitor of miR-155. In addition, in EOLP CD4+T cells, positive feedback loops for miR-155 and IFN-γ have been discovered that could lead to Th1-dominated immune responses; in the feedback loop, miR‑155 may negatively regulate suppressor of cytokine signaling 1 (SOCS1). The most likely target of miR-155 is SOCS1.71 Further, it has been found that the deregulation of miR-155/endothelial nitric oxide synthase may be responsible for an elevated risk for OLP.72 Therefore, miR-155 is vital for investigating pathogenesis in OLP.
miR-27a/b
The structures of miR-27a and miR-27b are generated from subtypes of miR-27 encoded on various chromosomes.73 miR-27 participates in the proliferation of various cells, including myocytes, neuroblastoma cells, and T cells.74–76 By degrading mRNA, miR-27a can inhibit both protein and mRNA levels of the target genes and can control gene expression.77 MiR-27b functions differently in pathological and physiological environments.73 A large body of evidence suggests that miR-27b is mainly regulated through transcriptional regulation of multiple molecules and signaling pathways through epigenetic changes and genomic loss (including DNA methylation and histone modification).78 MiR-27b-3p is a crucial mediator of human adipogenesis, so it is also crucial to MS.79
In OSCC, F-box and WD repeat domain containing 7 inhibit miR-27a-regulated cell proliferation and invasion through the epithelial-mesenchymal transition (EMT) and the PI3K/AKT signaling pathway.80 In tongue squamous cell carcinoma (TSCC) tissues and cell lines, miR-27b expression is considerably reduced, and the expression of miR-27b has been linked to cancer status. MiR-27b hampers TSCC migration and proliferation by suppressing the EMT process and targeting integrin subunit alpha 5 (ITGA5).81 In studies on OLP, the expression of miR-27a/b was lower than in controls, suggesting that signaling suppression may be the reason for the reduction of miR-27a/b, and that miR-27a/b may take part in the immune response.82,83 Polo-like kinase 2 (PLK2) is related to cell proliferation and apoptosis.84 When miR-27b targets PLK2, it stimulates the proliferation of oral keratinocytes.85 Peptidyl prolyl isomerase F, also called CypD, is associated with apoptosis. The reduction of miR-27b-3p may be the reason for increased CypD protein levels and lead to OLP basal epithelial apoptosis.86 One study noted its potential as a biomarker for miR-27b.87 Therefore, investigation of miR-27a/b is vital for comprehending the pathogenesis of OLP.
miR-26a/b
The human miR-26 family includes miR-26a, miR-26b, miR-4465, and miR-1297, which are all highly conserved miRNAs.88 Two distinct loci produce the mature version of miR-26a: on human chromosome 3p21.3, miR-26a-1 is found at the intron of the C-terminal domain (CTD) small phosphatase-like (CTDSPL) gene; on human chromosome 12q14.1, miR-26a-2 is located in the intron of the CTD small phosphatase (CTDSP)2 gene.89 On chromosome 2, miR-26b is found in the fourth intron of the CTDSP1 gene.90 In addition, miR-26 has been implicated in various developmental and physiological functions, including neuronal differentiation, muscle development, and hepatic glucose and lipid metabolism.91–95 It is significantly downregulated, and PAK1 (PAKs are a family of serine/threonine kinases that function at downstream nodes in a variety of oncogenic signaling pathways) is highly expressed in TSCC tissues and cells. In addition, in vitro, acquiring miR-26a or miR-26b blocks cell glycolysis, invasion, migration, and cycle, but the inhibition of PAK1 promotes apoptosis, and miR-26a/miR-26b overexpression inhibits the proliferation of TSCC tumors.
The above results indicate that miR-26 takes part in tumor inhibition and may be the therapeutic target of TSCC patients.96 Furthermore, recent investigations have demonstrated that miR-26b inhibits the IL-6, TNF-α, and NF-kB pathways in bronchial epithelial cells, suggesting that it has a role in regulating inflammatory responses, which can reduce the number of biopsies taken from patients with OLP.97,98 Du et al99 found that OLP patients have lower levels of miR-26, and this may be due to the inhibition of the vitamin D/vitamin D receptor (VDR) pathway. In the oral keratinocyte microenvironment, PKCδ is an essential regulator of apoptosis action, while CD38 is an inflammatory marker, and PKCδ is related to the activity of CD38. Thus, by targeting CD38, miR-26 may also reduce inflammation. Therefore, higher miR-26 levels tend to result in better anti-OLP results. However, that conclusion is not definitive and further studies should be conducted to ensure the efficacy of miR-26 for the treatment of OLP.
Other miRNAs also play a vital role in OLP. In OLP mucosa, the expression of the putative target cyclin D1 (CCND1) is increased when miR-138 is suppressed, and by targeting CCND1, miR-23a-3p might be a regulator of proliferation and inflammatory response. This suggests that miR-138 and miR-23a-3p may play critical roles in pathogenesis and thus, for patients with OLP, be promising new therapeutic targets.100,101 Aghbari et al102 reported that in the oral mucosa of OLP patients, there is a negative association between the tissue expression of miR-137 (reduced) and that of CD8 (elevated). In addition, in CD4+ T cells, increased miR-29b interacts with IFN-γ through a regulatory feedback loop, causing widespread DNA hypomethylation, which alters the immunological response of Th1-cells. This ultimately contributes to the immune dysregulation of OLP.103
Signaling Pathway in the OLP
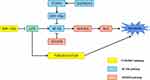
The biological functions of miRNAs have not yet been fully identified. However, many studies of miRNAs associated with human diseases have suggested that these molecules play an essential role in controlling cellular pathways. They are known to be involved in various signaling pathways, such as vitamin D/VDR, NF-κB, and PI3K/AKT/mTOR that may also be critical therapeutic targets for OLP (Figure 2).
Vitamin D/VDR Signaling Pathway
Vitamin D is an essential factor involved in the regulation of inflammation, immune response, and carcinoma inhibition via the action of its receptor VDR.104 The VDR endocrine system has been discovered in nearly all nucleated cells.105 Hypoxia inducible factor 1 subunit alpha (HIF-1α) is a heterodimeric transcription factor.106 LPS promotes the HIF-1α state by activating the nuclear factor-κB (NF-κB) pathway in myeloid cells and increasing the level of succinic acid in macrophages;107,108 the induced HIF-1α is thought to boost transcription of cytokines, including IFN-γ and IL-1β in immune cells, thus speeding up inflammatory response.109,110 Von Hippel-Lindau (VHL) and prolyl hydroxylase (PHD) are critical factors required for HIF-1 α proteasome degradation.106 LPS inhibits the expression of VHL in human oral keratinocyte (HOK), but not PHD1 or PHD2, suggesting that VHL may play a protective role in inflammatory diseases.111 Vitamin D is a critical regulator that attenuates endotoxin-induced HIF-1α in a VHL-dependent manner and inhibits lips-induced HIF-1α, thereby blocking IFN-γ and IL-1β production. Mechanically, the NF-κB pathway is inactivated by vitamin D, which increases VHL levels, resulting in a decrease in HIF-1α.111 In oral keratinocytes, vitamin D/VDR signaling protects cells by suppressing cytokine production and apoptosis in OLP.111,112 By decreasing VDR expression, miR-122 can improve the apoptosis of oral keratinocytes.16 In addition, the vitamin D/VDR signaling pathway inhibits the expression of miR-802 in human oral keratinocytes that is stimulated or activated by lip-polysaccharides, thereby inhibiting the apoptosis of OLP cells by blocking the NF-κB signaling pathway.112 Ge et al82 found that the expression of miR-27 in OLP is accelerated by vitamin D/VDR signaling. Vitamin D enhances VDR in oral epithelial cells when paired with VDREs, improving miR-27 transcription. Therefore, vitamin D/VDR signaling suppression may be at least partially responsible for the lowering of miR-27 in OLP. Although the mechanisms of miR-27 reduction have been elucidated, its involvement in the development of OLP requires extensive investigation to explain the etiology of OLP.
NF-κB Signaling Pathway
NF-κB, which was identified in 1986, is a transcription factor that binds to enhancer elements of the activated B cell immunoglobulin kappa light chain. It is made up of a group of transcription factors that play a vital role in controlling a broad range of biological responses.113 Due to its function in regulating pro-inflammatory genes, such as cytokines, chemokines, and adhesion molecules, the NF-κB pathway is traditionally considered to be a typical pro-inflammatory signaling pathway.114,115 The relative abundances of bacteria in the genera Derxia, Haemophilus, and Pseudomonas tend to be lower, and the expression levels of TLR4, NF-κB p65, IL-6, and TNF-α are higher, in OLP patients. Changes in microbial composition ratio may initiate the TLR4-NF-κB inflammatory signaling pathway and play a role in OLP lesions.116,117 In addition, as stated earlier, through the FOXP3/miR-146a/NF-κB axis, lncRNA DQ786243 regulates the induction and function of CD4+ Treg cells in OLP.118 DQ786243 and Foxp3 expression are highly upregulated in OLP patients compared to controls. This indicates that DQ786243 and Foxp3 dysregulation may play a role in the development of OLP. In addition, there is a positive association between DQ786243 and Foxp3, which implies that DQ786243 influences the expression of Foxp3, and dysregulation of DQ786243 is implicated in the progression of OLP by altering Foxp3+ Treg cells. Foxp3+ Treg cells keep the immune system in check by inhibiting various inflammatory responses; DQ786243 overexpression-induced Foxp3+ Treg cells show an extraordinary inhibitory ability to restrict T cells from proliferating. In addition, Foxp3 regulates miR-146a, which governs the course of OLP; the overexpression of DQ786243 increases miR-146a expression, which is inhibited by Foxp3 reduction. MiR-146a reduces the expression of interleukin 1 receptor associated kinase 1 and TRAF6, inhibiting NF-kB activation. In addition, the expressions of pIκB-α and p-p65 are dramatically reduced by DQ786243 overexpression.
The above findings suggest that DQ786243 dysregulation plays a crucial role in regulating Treg cells in OLP through the Foxp3/miR-146a/NF-kB axis, which implies a new way of looking at how OLP develops and a prospective therapeutic method for OLP. In addition, NF-κB can be restrained by total glucosides of paeony (TGP) and influence the production of inflammatory cytokines; additionally, TGP can increase the expression of miR-214 and p-STAT3 and improve the immunomodulatory function of mesenchymal stem cells. The connection between these observations is also worth looking into.119,120
PI3K/AKT/mTOR Signaling Pathway
In numerous human malignancies, the PI3K/AKT/mTOR signaling pathway is typically activated, and metastasis, angiogenesis, metabolism, growth, proliferation, and survival are only a few of the cellular mechanisms it controls.121,122 Many autoimmune and inflammatory illnesses have also been found to feature IGF1 as a critical regulator.123 A previous study reported elevated IGF1 mRNA expression in OLP peripheral T cells and activation of the AKT/mTOR pathway in local OLP lesions.124,125 The activation of the IGF1 and PI3K/AKT/mTOR pathways and aberrant autophagy may govern crosstalk between T cells and keratinocytes and contribute to the pathogenesis of OLP.126 In LPS-incubated HaCaT cells, a reduction of miR-125b may enhance the phosphorylation of Akt and its downstream target mTOR protein, underlining the role of the dysfunctional PI3K/Akt/mTOR signaling pathway in the malignant potential of OLP.127 Lc3B is found in the membranes of autophagosomes, plays a crucial function in autophagosome production, and is a reliable marker of autophagy. MiR-122 and miR-199 directly target AKT1 and mTOR, miR-122 overexpression inhibits the expression of AKT1 and Lc3B, and miR-199 overexpression reduces the levels of Lc3B and mTOR. Hence, miR-199 and miR-122, by modulating the expression of mTOR and AKT1, may have a role in the pathogenesis of OLP, and miR-122 and miR-199 may be potential targets for use in the treatment of OLP.128 Bioinformatics analyses have suggested that miR-34a-5p can modulate OLP progression through the PI3K/Akt signaling pathway and may be a potential biomarker.129
Although the cellular biology of these pathways is well documented regarding cell growth and the pathophysiology of many diseases, the mechanics of these pathways in OLP are poorly understood. Although the exact regulatory mechanisms involved in miRNAs and these proteins remain unclear, miRNAs play a vital part in the progression of OLP, and more research is needed to understand how miRNA networks influence the complexity of OLP through signal transduction pathways.
Future Expectations
In a previous article, we reported on the activity of a few miRNAs in OLP and their potential function in pathogenesis. There is no doubt that miRNAs play a significant role in OLP; the study and exploration of miRNAs may be crucial for diagnosis and treatment.
The clinical characteristics of oral squamous moss (including its symmetry distribution) have led some researchers to suggest that making a correct diagnosis is enough, particularly in the case of common skin lesions. However, to validate clinical diagnoses and rule out hyperplasia and malignant tumors, oral biopsy is usually recommended, combined with a histopathological examination.130 Some OLP patients refuse to undergo biopsy, so there is an urgent need for a noninvasive and accurate tool to aid clinical diagnosis.
OLP is caused by an immune system malfunction that produces precancerous lesions. Therefore, despite the availability of traditional biopsy methods, new identification procedures are necessary.131 Among cytokines, TNF-α and IL are thought to be feasible biomarkers for OLP diagnosis and prognosis.132 In contrast with other potential biomarkers that predict OLP, there is growing evidence that misregulated miRNAs can cause (or can be associated with) carcinogenesis in OLP. A biomarker is a molecule that can be utilized to diagnose and predict the prognosis of a disease. Sensitivity and durability are among the most critical features of a useful biomarker. Biomarkers can be obtained in a relatively painless manner. Recent findings show that miRNAs can be used as biomarkers in the diagnosis of some diseases and are viable therapeutic targets. Therefore, they may become valuable biomarkers in OLP diagnosis. However, it should be noted that most studies have been single-center retrospective studies and have generally been underpowered cohort studies. As a result, many non-overlapping and even conflicting reports have been published. These differences are largely due to biological and technical differences between the starting materials (purified cell types of cells using control population RNA extraction) and technology platforms (microarray qRT-PCR and next-generation sequencing) used in the studies, as well as differences in the different statistical methods used.133 One study that performed microarray analysis of miRNAs in patients with OLP and healthy controls found significant changes (more than two-fold differences) in the expression of about 70 miRNAs.28 However, clinical studies have involved only a small number of patients or healthy controls, so larger study cohorts and live animal models are needed to identify the best new biomarkers.
In recent years, cytokine treatment has become a prominent research focus, and preliminary studies have demonstrated its efficacy. At present, many miRNA-related therapies are being investigated.134 Several immune-related proteins—including vascular endothelial growth factor, FOXP3, toll-like receptors, matrix metalloproteinase 9, and others—have been documented in patients with OLP.28,135 MiRNAs have been linked to these immune-related proteins in basic tests for clinical inflammatory diseases or research in molecular biology. In addition, due to the human body’s high tolerance for it and its assistance in confirming the targeting of specific cells, miRNA may be a more ideal drug carrier than liposomes.136 OLP is a complex, multifactorial disease that leaves us with many unanswered questions. The radical treatment of OLP requires intensive treatment, but complications can still occur because there is currently no way to block multi-signal carcinogenic pathways and the adverse reactions caused by drugs. Therefore, new therapeutic strategies need to be developed to improve clinical outcomes and quality of life. Targeting oncogenes and downstream signaling proteins with miRNAs could prove to be a breakthrough in the treatment of OLP.
Although many experimental investigations incorporating miRNA therapies have been undertaken over the years, only a few miRNA therapeutics have reached clinical development. Choosing the most appropriate miRNA candidates or miRNA targets for each type of illness is one of the most challenging issues in creating miRNA-based treatments. Among the other obstacles are developing miRNA delivery vehicles that provide better stability to the treatment candidate and enable tissue-specific targeting, as well as minimizing potential toxicities and off-target effects.137 Many questions concerning the pathogenesis of OLP remain unanswered. Obtaining a deeper understanding of the role of the miRNA-mRNA-cytokine pathway in the pathogenesis of OLP will be a milestone for future treatments. In addition, such discoveries may be helpful in single-gene therapy.
Conclusion
Numerous cytokines have been implicated in the development of OLP, and miRNAs may play a role in the normal inflammatory response and immune function. However, the regulatory pathways of miRNA-mRNA in OLP remain unclear, and a great deal of work needs to be done to understand the molecular mechanisms of miRNAs and OLP fully. We reviewed research on several important miRNAs, and also examined the role of miRNA in several signaling pathways. This review may serve as a foundation for future research and contribute to the study of OLP pathogenesis. Further in vitro and animal model studies are needed to explore miRNA therapies for OLP.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Anhui Medical University School of Stomatology Discipline Construction Follow-up Project (2020kqsy02) and Natural Science Research Project of Higher Education Institutions in Anhui Province (KJ2021A0304).
Disclosure
The authors declare that they have no competing interests.
References
1. Eisen D, Carrozzo M, Bagan Sebastian JV, et al. Number V Oral lichen planus: clinical features and management. Oral Dis. 2005;11(6):338–349. doi:10.1111/j.1601-0825.2005.01142.x
2. Lodi G, Scully C, Carrozzo M, et al. Current controversies in oral lichen planus: report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(1):40–51. doi:10.1016/j.tripleo.2004.06.077
3. Scully C, Beyli M, Ferreiro MC, et al. Update on oral lichen planus: etiopathogenesis and management. Crit Rev Oral Biol Med. 1998;9(1):86–122. doi:10.1177/10454411980090010501
4. Alrashdan MS, Cirillo N, McCullough M. Oral lichen planus: a literature review and update. Arch Dermatol Res. 2016;308(8):539–551.
5. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28(1):100–108.
6. Scully C, Carrozzo M. Oral mucosal disease: lichen planus. Br J Oral Maxillofac Surg. 2008;46(1):15–21.
7. Chiang CP, Yu-Fong Chang J, Wang YP, et al. Oral lichen planus - Differential diagnoses, serum autoantibodies, hematinic deficiencies, and management. J Formos Med Assoc. 2018;117(9):756–765.
8. Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36(10):575–580.
9. van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45(4–5):317–323.
10. Chen J, Du G, Wang Y, et al. Integrative analysis of mRNA and miRNA expression profiles in oral lichen planus: preliminary results. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(4):390–402 e317. doi:10.1016/j.oooo.2017.05.513
11. Byeon B, Bilichak A, Kovalchuk I. Computational characterization of ncRNA fragments in various tissues of the brassica rapa plant. Noncoding RNA. 2017;3(2). doi:10.3390/ncrna3020017
12. Jochl C, Rederstorff M, Hertel J, et al. Small ncRNA transcriptome analysis from Aspergillus fumigatus suggests a novel mechanism for regulation of protein synthesis. Nucleic Acids Res. 2008;36(8):2677–2689. doi:10.1093/nar/gkn123
13. Heo MJ, Yun J, Kim SG. Role of non-coding RNAs in liver disease progression to hepatocellular carcinoma. Arch Pharm Res. 2019;42(1):48–62. doi:10.1007/s12272-018-01104-x
14. Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. doi:10.1016/j.jaci.2017.08.034
15. Huang Z, Liu F, Wang W, et al. Deregulation of circ_003912 contributes to pathogenesis of erosive oral lichen planus by via sponging microRNA-123, −647 and −31 and upregulating FOXP3. Mol Med. 2021;27(1):132. doi:10.1186/s10020-021-00382-4
16. Ge X, Xie H, Wang L, et al. MicroRNA-122 promotes apoptosis of keratinocytes in oral lichen planus through suppressing VDR expression. J Cell Mol Med. 2021;25(7):3400–3407. doi:10.1111/jcmm.16418
17. Mehdipour M, Shahidi M, Manifar S, et al. Diagnostic and prognostic relevance of salivary microRNA-21, −125a, −31 and −200a levels in patients with oral lichen planus - a short report. Cell Oncol. 2018;41(3):329–334. doi:10.1007/s13402-018-0372-x
18. Daye M, Temiz SA, Isık B. The relationship between lichen planus and metabolic syndrome. J Cosmet Dermatol. 2021;20(8):2635–2639. doi:10.1111/jocd.13905
19. Tampa M, Caruntu C, Mitran M, et al. Markers of oral lichen planus malignant transformation. Dis Markers. 2018;2018:1959506. doi:10.1155/2018/1959506
20. Fiocco Z, Kupf S, Patzak L, et al. Quality of life and psychopathology in lichen planus: a neglected disease burden. Acta Derm Venereol. 2021;101(12):adv00619. doi:10.2340/actadv.v101.442
21. Sharda P, Mohta A, Ghiya BC, et al. Development of oral lichen planus after COVID-19 vaccination - a rare case report. J Eur Acad Dermatol Venereol. 2022;36(2):e82–e83. doi:10.1111/jdv.17718
22. Kurago ZB. Etiology and pathogenesis of oral lichen planus: an overview. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(1):72–80. doi:10.1016/j.oooo.2016.03.011
23. Roopashree MR, Gondhalekar RV, Shashikanth MC, et al. Pathogenesis of oral lichen planus - a review. J Oral Pathol Med. 2010;39(10):729–734. doi:10.1111/j.1600-0714.2010.00946.x
24. Nibali L, Fedele S, D’Aiuto F, et al. Interleukin-6 in oral diseases: a review. Oral Dis. 2012;18(3):236–243. doi:10.1111/j.1601-0825.2011.01867.x
25. Payeras MR, Cherubini K, Figueiredo MA, et al. Oral lichen planus: focus on etiopathogenesis. Arch Oral Biol. 2013;58(9):1057–1069. doi:10.1016/j.archoralbio.2013.04.004
26. Wang QM, Huang XY, Guan WQ. Expressions of Interleukin-27 in oral lichen planus, oral leukoplakia, and oral squamous cell carcinoma. Inflammation. 2022;45(3):1023–1038. doi:10.1007/s10753-021-01599-5
27. Wang ZM, Zhang J, Wang F, et al. The tipped balance of ILC1/ILC2 in peripheral blood of oral lichen planus is related to inflammatory cytokines. Front Cell Dev Biol. 2021;9:725169. doi:10.3389/fcell.2021.725169
28. Ma H, Wu Y, Yang H, et al. MicroRNAs in oral lichen planus and potential miRNA-mRNA pathogenesis with essential cytokines: a review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(2):164–173. doi:10.1016/j.oooo.2016.03.018
29. Otmani K, Lewalle P. Tumor suppressor miRNA in cancer cells and the tumor microenvironment: mechanism of deregulation and clinical implications. Front Oncol. 2021;11:708765. doi:10.3389/fonc.2021.708765
30. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, et al. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi:10.1002/jcp.27486
31. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi:10.1038/nrg2843
32. Zhu H, Xiao X, Shi Y, et al. Inhibition of miRNA-29a regulates intestinal barrier function in diarrhea-predominant irritable bowel syndrome by upregulating ZO-1 and CLDN1. Exp Ther Med. 2020;20(6):155. doi:10.3892/etm.2020.9284
33. Wostenberg C, Quarles KA, Showalter SA. Dynamic origins of differential RNA binding function in two dsRBDs from the miRNA “microprocessor” complex. Biochemistry. 2010;49(50):10728–10736. doi:10.1021/bi1015716
34. Mishra S, Yadav T, Rani V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol. 2016;98:12–23. doi:10.1016/j.critrevonc.2015.10.003
35. Mazumder S, Datta S, Ray JG, et al. Liquid biopsy: miRNA as a potential biomarker in oral cancer. Cancer Epidemiol. 2019;58:137–145. doi:10.1016/j.canep.2018.12.008
36. Sun Y, Kuek V, Liu Y, et al. MiR-214 is an important regulator of the musculoskeletal metabolism and disease. J Cell Physiol. 2018;234(1):231–245. doi:10.1002/jcp.26856
37. Penna E, Orso F, Taverna D. miR-214 as a key hub that controls cancer networks: small player, multiple functions. J Invest Dermatol. 2015;135(4):960–969. doi:10.1038/jid.2014.479
38. Hrdlicka HC, Lee S-K, Delany AM. MicroRNAs are critical regulators of osteoclast differentiation. Curr Mol Biol Rep. 2019;5(1):65–74. doi:10.1007/s40610-019-0116-3
39. Stevens HC, Deng L, Grant JS, et al. Regulation and function of miR-214 in pulmonary arterial hypertension. Pulm Circ. 2016;6(1):109–117.
40. Zhao Y, Ponnusamy M, Zhang L, et al. The role of miR-214 in cardiovascular diseases. Eur J Pharmacol. 2017;816:138–145.
41. Avgeris M, Kokkinopoulou I, Maratou E, et al. Blood-based analysis of 84 microRNAs identifies molecules deregulated in individuals with type-2 diabetes, risk factors for the disease or metabolic syndrome. Diabetes Res Clin Pract. 2020;164:108187.
42. Cagle P, Niture S, Srivastava A, et al. MicroRNA-214 targets PTK6 to inhibit tumorigenic potential and increase drug sensitivity of prostate cancer cells. Sci Rep. 2019;9(1):9776.
43. Cao F, Zhan J, Chen X, et al. miR-214 promotes periodontal ligament stem cell osteoblastic differentiation by modulating Wnt/betacatenin signaling. Mol Med Rep. 2017;16(6):9301–9308.
44. Zhang H, Sun P, Wang YL, et al. MiR-214 promotes proliferation and inhibits apoptosis of oral cancer cells through MAPK/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(7):3710–3716.
45. Ai Y, Wu S, Zou C, et al. Circular RNA circFOXO3 regulates KDM2A by targeting miR-214 to promote tumor growth and metastasis in oral squamous cell carcinoma. J Cell Mol Med. 2021:26(6):1842–1852.
46. Wang X, Li H, Shi J. LncRNA HOXA11-AS promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by suppression of miR-214-3p expression. Biomed Res Int. 2019;2019:8645153.
47. Miletti-Gonzalez KE, Murphy K, Kumaran MN, et al. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J Biol Chem. 2012;287(23):18995–19007.
48. Zheng H, Li S. Reduced miRNA214 expression in oral mucosa contributes to the pathogenesis of oral lichen planus by targeting CD44. Mol Med Rep. 2018;17(1):1919–1925.
49. Nahand JS, Karimzadeh MR, Nezamnia M, et al. The role of miR-146a in viral infection. IUBMB Life. 2020;72(3):343–360.
50. Yang JG, Sun YR, Chen GY, et al. Different expression of MicroRNA-146a in peripheral blood CD4(+) T cells and lesions of oral lichen planus. Inflammation. 2016;39(2):860–866.
51. Mortazavi-Jahromi SS, Aslani M, Mirshafiey A. A comprehensive review on miR-146a molecular mechanisms in a wide spectrum of immune and non-immune inflammatory diseases. Immunol Lett. 2020;227:8–27.
52. Setién-Olarra A, Gainza-Cirauqui ML, Aguirre-Urizar JM, et al. The role of microRNAs in oral lichenoid disorders. Systematic review. Med Oral Patol Oral Cir Bucal. 2017;22(5):e548–e553.
53. Shomali N, Mansoori B, Mohammadi A, et al. MiR-146a functions as a small silent player in gastric cancer. Biomed Pharmacother. 2017;96:238–245.
54. Wang F, Ye LJ, Wang FJ, et al. miR-146a promotes proliferation, invasion, and epithelial-to-mesenchymal transition in oral squamous carcinoma cells. Environ Toxicol. 2020;35(10):1050–1057.
55. Zhu FY, Gan CW, Wang MX, et al. MiR-146a-5p inhibits proliferation and promotes apoptosis of oral squamous cell carcinoma cells by regulating NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(7):3717–3723.
56. Shen Z, Gao X, Ma L, et al. Expression of Foxp3 and interleukin-17 in lichen planus lesions with emphasis on difference in oral and cutaneous variants. Arch Dermatol Res. 2014;306(5):441–446.
57. Wang J, Yang L, Wang L, et al. Forkhead box p3 controls progression of oral lichen planus by regulating microRNA-146a. J Cell Biochem. 2018;119(11):8862–8871.
58. Gao X, Zhou J, Wang J, et al. Mechanism of exosomal miR-155 derived from bone marrow mesenchymal stem cells on stemness maintenance and drug resistance in myeloma cells. J Orthop Surg Res. 2021;16(1):637.
59. Hefzy EM, Hassuna NA, Shaker OG, et al. miR-155 T/A (rs767649) and miR-146a A/G (rs57095329) single nucleotide polymorphisms as risk factors for chronic hepatitis B virus infection among Egyptian patients. PLoS One. 2021;16(8):e0256724.
60. Bayraktar R, Van Roosbroeck K. miR-155 in cancer drug resistance and as target for miRNA-based therapeutics. Cancer Metastasis Rev. 2018;37(1):33–44.
61. Emami N, Mohamadnia A, Mirzaei M, et al. miR-155, miR-191, and miR-494 as diagnostic biomarkers for oral squamous cell carcinoma and the effects of Avastin on these biomarkers. J Korean Assoc Oral Maxillofac Surg. 2020;46(5):341–347.
62. Liu Y, Wan X, Yuan Y, et al. Opposite effects of miR-155 in the initial and later stages of lipopolysaccharide (LPS)-induced inflammatory response. J Zhejiang Univ Sci B. 2021;22(7):590–598.
63. Tao Y, Ai R, Hao Y, et al. Role of miR-155 in immune regulation and its relevance in oral lichen planus. Exp Ther Med. 2019;17(1):575–586.
64. Cerda A, Amaral AA, de Oliveira R, et al. Peripheral blood miRome identified miR-155 as potential biomarker of MetS and cardiometabolic risk in obese patients. Int J Mol Sci. 2021;22(3):1468.
65. Chou MY, Fang CY, Hsieh PL, et al. Depletion of miR-155 hinders the myofibroblast activities and reactive oxygen species generation in oral submucous fibrosis. J Formos Med Assoc. 2022;121(2):467–472.
66. Li X, Liu K, Zhou W, et al. MiR-155 targeting FoxO3a regulates oral cancer cell proliferation, apoptosis, and DDP resistance through targeting FoxO3a. Cancer Biomark. 2020;27(1):105–111.
67. Liang XY, Hu JY, Zhou G. [Detection of miR-155, miR-146a in PBNCs and tissues from patients with oral lichen planus]. Shanghai Kou Qiang Yi Xue. 2015;24(6):729–734. Chinese.
68. Schroder K, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189.
69. Ilia S, Goulielmos GN, Samonis G, et al. Polymorphisms in IL-6, IL-10, TNF-alpha, IFN-gamma and TGF-beta1 genes and susceptibility to acute otitis media in early infancy. Pediatr Infect Dis J. 2014;33(5):518–521.
70. Gein SV, Sharavieva IL. Effect of rotation and immobilization stress on IL-1beta, IL-2, IL-4, and IFN-gamma production by splenocytes under opiate receptor blockade in vivo. Dokl Biol Sci. 2014;454:69–71.
71. Hu JY, Zhang J, Ma JZ, et al. MicroRNA-155-IFN-gamma Feedback Loop in CD4(+)T Cells of Erosive type Oral Lichen Planus. Sci Rep. 2015;5:16935.
72. Wang L, Wu W, Chen J, et al. MicroRNA microarray-based identification of involvement of miR-155 and miR-19a in development of Oral Lichen Planus (OLP) by modulating Th1/Th2 balance via targeting eNOS and Toll-Like Receptor 2 (TLR2). Med Sci Monit. 2018;24:3591–3603.
73. Lan X, Li G, Liu H, et al. MiR-27a/b regulates liver regeneration by posttranscriptional modification of Tmub1. Dig Dis Sci. 2018;63(9):2362–2372.
74. Lee JJ, Drakaki A, Iliopoulos D, et al. MiR-27b targets PPARgamma to inhibit growth, tumor progression and the inflammatory response in neuroblastoma cells. Oncogene. 2012;31(33):3818–3825.
75. Lozano-Velasco E, Contreras A, Crist C, et al. Pitx2c modulates Pax3+/Pax7+ cell populations and regulates Pax3 expression by repressing miR27 expression during myogenesis. Dev Biol. 2011;357(1):165–178.
76. Rameshwar P, Chen K-D, Goto S, et al. Identification of miR-27b as a novel signature from the mRNA profiles of adipose-derived mesenchymal stem cells involved in the tolerogenic response. PLoS One. 2013;8(4):e60492.
77. Kang T, Lu W, Xu W, et al. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem. 2013;288(48):34394–34402.
78. Ding L, Ni J, Yang F, et al. Promising therapeutic role of miR-27b in tumor. Tumour Biol. 2017;39(3):1010428317691657.
79. Wu H, Pula T, Tews D, et al. microRNA-27a-3p but Not −5p Is a crucial mediator of human adipogenesis. Cells. 2021;10(11):3205.
80. Li C, Lin XF, Wang JN, et al. FBXW7 inhibited cell proliferation and invasion regulated by miR-27a through PI3K/AKT signaling pathway and epithelial-to-mesenchymal transition in oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2020;24(7):3701–3709.
81. Li T, Wu Q, Liu D, et al. miR-27b suppresses tongue squamous cell carcinoma epithelial-mesenchymal transition by targeting ITGA5. Onco Targets Ther. 2020;13:11855–11867.
82. Ge X, Yuan L, Wei J, et al. Vitamin D/VDR signaling induces miR-27a/b expression in oral lichen planus. Sci Rep. 2020;10(1):301.
83. Stasio DD, Mosca L, Lucchese A, et al. Salivary mir-27b expression in oral lichen planus patients: a series of cases and a narrative review of literature. Curr Top Med Chem. 2019;19(31):2816–2823.
84. Cozza G, Salvi M. The acidophilic kinases PLK2 and PLK3: structure, substrate targeting and inhibition. Curr Protein Pept Sci. 2018;19(8):728–745.
85. Chen J, Du G, Chang Y, et al. Downregulated miR-27b promotes keratinocyte proliferation by targeting PLK2 in oral lichen planus. J Oral Pathol Med. 2019;48(4):326–334.
86. Chen J, Wang Y, Du G, et al. Down-regulation of miRNA-27b-3p suppresses keratinocytes apoptosis in oral lichen planus. J Cell Mol Med. 2019;23(6):4326–4337.
87. Aghbari SM, Zayed SO, Shaker OG, et al. Evaluating the role of tissue microRNA-27b as a diagnostic marker for oral lichen planus and possible correlation with CD8. J Oral Pathol Med. 2019;48(1):68–73.
88. Li C, Li Y, Lu Y, et al. miR-26 family and its target genes in tumorigenesis and development. Crit Rev Oncol Hematol. 2021;157:103124.
89. Kim H, Huang W, Jiang X, et al. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci U S A. 2010;107(5):2183–2188.
90. Arora H, Qureshi R, Park AK, et al. Coordinated regulation of ATF2 by miR-26b in gamma-irradiated lung cancer cells. PLoS One. 2011;6(8):e23802.
91. Acharya A, Berry DC, Zhang H, et al. miR-26 suppresses adipocyte progenitor differentiation and fat production by targeting Fbxl19. Genes Dev. 2019;33(19–20):1367–1380.
92. Dill H, Linder B, Fehr A, et al. Intronic miR-26b controls neuronal differentiation by repressing its host transcript, ctdsp2. Genes Dev. 2012;26(1):25–30.
93. Dey BK, Gagan J, Yan Z, et al. miR-26a is required for skeletal muscle differentiation and regeneration in mice. Genes Dev. 2012;26(19):2180–2191.
94. Icli B, Wara AK, Moslehi J, et al. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ Res. 2013;113(11):1231–1241.
95. Fu X, Dong B, Tian Y, et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J Clin Invest. 2015;125(6):2497–2509.
96. Wei Z, Chang K, Fan C, et al. MiR-26a/miR-26b represses tongue squamous cell carcinoma progression by targeting PAK1. Cancer Cell Int. 2020;20:82.
97. Danielsson K, Ebrahimi M, Wahlin YB, et al. Increased levels of COX-2 in oral lichen planus supports an autoimmune cause of the disease. J Eur Acad Dermatol Venereol. 2012;26(11):1415–1419.
98. Chen CY, Chang JT, Ho YF, et al. MiR-26 down-regulates TNF-alpha/NF-kappaB signalling and IL-6 expression by silencing HMGA1 and MALT1. Nucleic Acids Res. 2016;44(8):3772–3787.
99. Du J, Gao R, Wang Y, et al. MicroRNA-26a/b have protective roles in oral lichen planus. Cell Death Dis. 2020;11(1):15.
100. Ghallab NA, Kasem RF, El-Ghani SFA, et al. Gene expression of miRNA-138 and cyclin D1 in oral lichen planus. Clin Oral Investig. 2017;21(8):2481–2491. doi:10.1007/s00784-017-2091-5
101. Wang J, Hu M, Li L. Clinical values of miR-23a-3p in oral lichen planus and its role in keratinocyte proliferation and inflammatory response. J Inflamm Res. 2021;14:5013–5021. doi:10.2147/JIR.S325986
102. Aghbari SMH, Abushouk AI, Shakir OG, et al. Correlation between tissue expression of microRNA-137 and CD8 in oral lichen planus. Clin Oral Investig. 2018;22(3):1463–1467. doi:10.1007/s00784-017-2252-6
103. Zhang J, Chen GY, Wang F, et al. MiR-29b interacts with IFN-gamma and induces DNA hypomethylation in CD4(+) T cells of oral lichen planus. Int J Biol Macromol. 2020;147:1248–1254. doi:10.1016/j.ijbiomac.2019.09.252
104. Shang M, Sun J. Vitamin D/VDR, probiotics, and gastrointestinal diseases. Curr Med Chem. 2017;24(9):876–887. doi:10.2174/0929867323666161202150008
105. Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–776. doi:10.1210/er.2008-0004
106. Palazon A, Goldrath AW, Nizet V, et al. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41(4):518–528. doi:10.1016/j.immuni.2014.09.008
107. Mills EL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457–470 e413. doi:10.1016/j.cell.2016.08.064
108. Fan D, Coughlin LA, Neubauer MM, et al. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21(7):808–814. doi:10.1038/nm.3871
109. Corcoran SE, O’Neill LA. HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest. 2016;126(10):3699–3707. doi:10.1172/JCI84431
110. Lee JH, Elly C, Park Y, et al. E3 ubiquitin ligase VHL regulates hypoxia-inducible Factor-1alpha to maintain regulatory T cell stability and suppressive capacity. Immunity. 2015;42(6):1062–1074. doi:10.1016/j.immuni.2015.05.016
111. Ge X, Wang L, Li M, et al. Vitamin D/VDR signaling inhibits LPS-induced IFNgamma and IL-1beta in Oral epithelia by regulating hypoxia-inducible factor-1alpha signaling pathway. Cell Commun Signal. 2019;17(1):18. doi:10.1186/s12964-019-0331-9
112. Zhao B, Xu N, Li R, et al. Vitamin D/VDR signaling suppresses microRNA-802-induced apoptosis of keratinocytes in oral lichen planus. FASEB J. 2019;33(1):1042–1050. doi:10.1096/fj.201801020RRR
113. Dolcet X, Llobet D, Pallares J, et al. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446(5):475–482. doi:10.1007/s00428-005-1264-9
114. Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651. doi:10.1101/cshperspect.a001651
115. Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17(9):545–558. doi:10.1038/nri.2017.52
116. Deng S, Xu Y, Wang X, et al. Study on the role of salivary flora and NF-kappaB inflammatory signal pathway in oral lichen planus. Inflammation. 2020;43(3):994–1008. doi:10.1007/s10753-020-01185-1
117. Rusanen P, Marttila E, Uittamo J, et al. TLR1-10, NF-kappaB and p53 expression is increased in oral lichenoid disease. PLoS One. 2017;12(7):e0181361. doi:10.1371/journal.pone.0181361
118. Wang J, Zhai X, Guo J, et al. Long non-coding RNA DQ786243 modulates the induction and function of CD4(+) Treg cells through Foxp3-miR-146a-NF-kappaB axis: implications for alleviating oral lichen planus. Int Immunopharmacol. 2019;75:105761. doi:10.1016/j.intimp.2019.105761
119. Wang Y, Zhang H, Du G, et al. Total glucosides of paeony (TGP) inhibits the production of inflammatory cytokines in oral lichen planus by suppressing the NF-κB signaling pathway. Int Immunopharmacol. 2016;36:67–72. doi:10.1016/j.intimp.2016.04.010
120. Zhao Z, Han Y, Zhang Z, et al. Total glucosides of paeony improves the immunomodulatory capacity of MSCs partially via the miR-124/STAT3 pathway in oral lichen planus. Biomed Pharmacother. 2018;105:151–158. doi:10.1016/j.biopha.2018.05.076
121. Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11(7):1946–1954. doi:10.1039/C5MB00101C
122. Aoki M, Fujishita T. Oncogenic roles of the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 2017;407:153–189. doi:10.1007/82_2017_6
123. Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62(2):199–236. doi:10.1124/pr.109.002469
124. Tan YQ, Zhang J, Du GF, et al. Altered autophagy-associated genes expression in T cells of oral lichen planus correlated with clinical features. Mediators Inflamm. 2016;2016:4867368. doi:10.1155/2016/4867368
125. Zhang N, Zhang J, Tan Y-Q, et al. Activated Akt/mTOR-autophagy in local T cells of oral lichen planus. Int Immunopharmacol. 2017;48:84–90. doi:10.1016/j.intimp.2017.04.016
126. Ma RJ, Tan YQ, Zhou G. Aberrant IGF1-PI3K/AKT/MTOR signaling pathway regulates the local immunity of oral lichen planus. Immunobiology. 2019;224(3):455–461. doi:10.1016/j.imbio.2019.01.004
127. Wang J, Luo H, Xiao Y, et al. miR-125b inhibits keratinocyte proliferation and promotes keratinocyte apoptosis in oral lichen planus by targeting MMP-2 expression through PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2016;80:373–380. doi:10.1016/j.biopha.2016.02.043
128. Wang LWW, Chen J, Li Y, Xu M, Cai Y, Cai Y. miR‑122 and miR‑199 synergistically promote autophagy in oral lichen planus by targeting the Akt/mTOR pathway. Int J Mol Med. 2019;43(3):1373–1381. doi:10.3892/ijmm.2019.4068
129. Peng Q, Zhang J, Zhou G. Differentially circulating exosomal microRNAs expression profiling in oral lichen planus. Am J Transl Res. 2018;10(9):2848–2858.
130. Al-Hashimi I, Schifter M, Lockhart PB, et al. Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(S25):e21–12. doi:10.1016/j.tripleo.2006.11.001
131. Nosratzehi T. Oral lichen planus: an overview of potential risk factors, biomarkers and treatments. Asian Pac J Cancer Prev. 2018;19(5):1161–1167. doi:10.22034/APJCP.2018.19.5.1161
132. Bindakhil M, Akintoye S, Corby P, et al. Influence of topical corticosteroids on malignant transformation of oral lichen planus. J Oral Pathol Med. 2022;51(2):188–193. doi:10.1111/jop.13257
133. Sole C, Larrea E, Di Pinto G, et al. miRNAs in B-cell lymphoma: molecular mechanisms and biomarker potential. Cancer Lett. 2017;405:79–89. doi:10.1016/j.canlet.2017.07.020
134. Wang H, Jiang Y, Peng H, et al. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv Drug Deliv Rev. 2015;81:142–160. doi:10.1016/j.addr.2014.10.031
135. Sinon SH, Rich AM, Parachuru VPB, et al. Downregulation of toll-like receptor-mediated signalling pathways in oral lichen planus. J Oral Pathol Med. 2016;45(1):28–34. doi:10.1111/jop.12319
136. Li C, He H, Wang J, et al. Possible roles of exosomal miRNAs in the pathogenesis of oral lichen planus. Am J Transl Res. 2019;11(9):5313–5323.
137. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.