Back to Journals » Journal of Inflammation Research » Volume 17
The Function and Mechanism of Anti-Inflammatory Factor Metrnl Prevents the Progression of Inflammatory-Mediated Pathological Bone Osteolytic Diseases
Authors Liu N, Dong J, Li L , Zhou D, Liu F
Received 24 January 2024
Accepted for publication 7 March 2024
Published 11 March 2024 Volume 2024:17 Pages 1607—1619
DOI https://doi.org/10.2147/JIR.S455790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Nan Liu, Jinlei Dong, Lianxin Li, Dongsheng Zhou, Fanxiao Liu
Department of Orthopedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China
Correspondence: Fanxiao Liu, Department of Orthopedics, Shandong Provincial Hospital affiliated to Shandong First Medical University, No. 324, Road Jing Wu Wei Qi, Jinan, Shandong, 250021, People’s Republic of China, Tel +86-15069058755, Email [email protected]
Abstract: Metrnl, recently identified as an adipokine, is a secreted protein notably expressed in white adipose tissue, barrier tissues, and activated macrophages. This adipokine plays a pivotal role in counteracting obesity-induced insulin resistance. It enhances adipose tissue functionality by promoting adipocyte differentiation, activating metabolic pathways, and exerting anti-inflammatory effects. Extensive research has identified Metrnl as a key player in modulating inflammatory responses and as an integral regulator of muscle regeneration. These findings position Metrnl as a promising biomarker and potential therapeutic target in treating inflammation-associated pathologies. Despite this, the specific anti-inflammatory mechanisms of Metrnl in immune-mediated osteolysis and arthritis remain elusive, warranting further investigation. In this review, we will briefly elaborate on the role of Metrnl in anti-inflammation function in inflammation-related osteolysis, arthritis, and pathological bone resorption, which could facilitate Metrnl’s clinical application as a novel therapeutic strategy to prevent bone loss. While the pathogenesis of elbow stiffness remains elusive, current literature suggests that Metrnl likely exerts a pivotal role in its development.
Keywords: osteolysis, bone resorption, bone loss, Metrnl, arthritis, stiff elbow
Introduction
Imbalance of bone metabolism disrupts the physiological function of osteoblasts, osteocytes and osteomacs. This disruption transforms into a pathological condition characterized by progressive bone resorption. Inflammation plays a significant role in this impairment, particularly in various osteolytic bone diseases, where it predominantly shifts the balance towards bone resorption.1 A variety of chronic inflammatory bone diseases have been shown to be associated with physiological osteolysis, including rheumatoid arthritis (RA), spondylarthritis, osteoporosis, periodontitis, bone metastasis of malignant tumor, periprosthetic osteolysis due to aseptic loosening.2,3 Multiple immune-related molecules have been proved to play a crucial role in bone metabolism, such as IL-1β, IL-6, IL-10, IL-17 and TNF-α. These cytokines, serving as primary stimulators of osteoclastogenesis, can upregulate the expression of crucial factors involved in osteoclast differentiation, which is a key driver in the progression of pathological bone resorption.
Metrnl (IL-41, Meteorin-like, Subfatin, Cometin), a secreted protein expressed in white adipose tissue,4 barrier tissues,5,6 and activated macrophages,5 has been confirmed as a adipokine, neurotrophic, and anti-inflammatory cytokine. This factor demonstrates a multitude of physiological functions by activating various intracellular signaling pathways across different cell types including adipocytes, macrophages, myocytes and cardiomyocytes, such as the promotion of neurite outgrowth,7 improvement of cognitive dysfunction,8 inflammation inhibition,5,7 regulation of energy homeostasis,7,9 and insulin sensitivity,7,10 enhancement of the browning of white adipose tissue,7 skeletal muscle regeneration,11 and heart protection.12–14 Current research suggests that Metrnl has potential to be biomarker and therapeutic target for diseases associated with inflammation. Nonetheless, theanti-inflammatory properties and mechanism in immune inflammation-related osteolysis and arthritis remains not fully understood, and further investigation is necessary to elucidate its potential role in these conditions.
Recent studies have highlighted that Metrnl play a regulatory role in bone metabolism and immune inflammation. Multiple studies focused on the correlation of Metrnl and bone arthritis associated with metabolism15–17 or inflammation, including arthritis18–21 and osteolysis.3,22 This review aims to briefly elaborate on the anti-inflammatory function of Metrnl in inflammation-related osteolysis, arthritis, and pathological bone resorption, which could explore the potential of Metrnl as a novel therapeutic strategy in preventing these diseases.
Metrnl and Immune Inflammation
Metrnl is increasingly recognized as a novel immunoregulatory cytokine intricately linked to inflammatory processes.5,20 Both the expression of Metrnl and its circulating concentrations show an upsurge in inflamed tissues or in the context of inflammatory diseases. Research has documented that Metrnl is instrumental in modulating inflammatory responses and is closely associated with various inflammation-related disease, including RA, and psoriasis RA,5 airway inflammation.23
Rao et al reported that Metrnl exerted an anti-inflammatory function by increasing the expression of IL-4 and promoting alternative activation of macrophages.24 Exercise mediates its anti-inflammatory effects by upregulating Metrnl expression in various muscle depots. Additionally, recombinant Metrnl has been shown to suppress the NLRP3 inflammasome, subsequently inhibiting the secretion of IL-1β and IL-18 in BMDMs.25 This mechanism highlights the potential therapeutic value of Metrnl in modulating inflammation-related pathways. Its levels are significantly elevated in patients with asthma and in mouse models of allergic asthma induced by house dust mite (HDM) extract. Notably, Metrnl plays a critical role in attenuating the pathophysiology of airway hyperreactivity. It reduces inflammatory cell infiltration in the airways and diminishes type 2 cytokine production. This action is associated with the downregulation of dendritic cell (DC)-mediated adaptive immune responses.23
A study reported that Metrnl confers protection against LPS-induced acute lung injury (ALI) by activating ferroptosis pathway.26 In the context of LPS-induced ALI, there is a marked reduction in Metrnl levels accompanied by an upsurge in ferroptosis within the lung tissue. The overexpression of Metrnl mitigated LPS-induced ferroptosis by regulating SIRT1-P53-SLC7A11 signaling and furthermore decrease ALI severity.26
Metrnl plays a crucial role in tumour development. Akkus et al observed a histopathological increase in immunoreactivity of Metrnl in IDC samples.27 The expression of Metrnl has been observed to be significantly upregulated in colorectal cancer tissues as compared to normal tissues, indicating its potential as a prognostic marker.28 Moreover, the authors observed an association between increased Metrnl expression levels and advanced clinical stage in colorectal cancer patients, suggesting that Metrnl could serve as a predictor for poor prognosis. Kocaman et al reported an increase in Metrnl expression in malignant mesothelioma tissues, suggesting its potential as a diagnostic biomarker for this particular malignancy.29
Metrnl was possibly associated with sepsis pathogenic mechanism. Animal study confirmed that in a sepsis model induced by endotoxin, the blood concentrations of Metrnl increased sharply. A deficiency in Metrnl could heighten susceptibility to mortality in mice. Furthermore, Metrnl acts as a co-activator in mitigating inflammation in sepsis-induced renal injury, primarily through the activation of PPARδ-dependent pathways.30 A separate study revealed that serum Metrnl levels in ICU patients with sepsis are inversely correlated with the concentrations of pro-inflammatory cytokines (IL-1β, IL-6, IL-8, IL-17, TNF-α and PCT). Metrnl produced markedly and regulated the differentiation and recruitment of macrophages in sepsis patients, which significantly affected the host defense against sepsis and modulates the balance between Treg/Th17 in the immune response.31
Metrnl has been shown to inhibit the secretion of TNFα and MCP-1, and the phosphorylation of NF-κB and IκB in LPS-treated HUVECs and THP-1 cells. This suppression occurs through the enhancement of the phosphorylation of AMPK and the expression of PPARδ.32 Additionally, Metrnl mitigated myocardial ischemia/reperfusion (MI/R)-induced apoptosis in cardiomyocytes by alleviating endoplasmic reticulum stress. This protective effect is mediated via the activation of the AMPK-PAK2 signaling pathway in H9C2 cells.33
Studies have identified significant correlations between serum Metrnl levels and the presence and severity of type 2 diabetes mellitus (T2DM) and coronary artery disease (CAD). In patients with T2DM or CAD, serum Metrnl levels demonstrated a negative correlation with pro-inflammatory markers (high-sensitivity C-reactive protein and IL-1β), but a positive correlation with the anti-inflammatory cytokine (IL-11).34,35 Liu et al reported that exercise can increase the production of Metrnl in skeletal muscle of CAD patients by suppressing the glucose metabolic dysfunction of HUVEC and reducing the expression of NLRP3 inflammasome, and subsequently improve patient atherosclerosis.36
Ushach et al5 verified that Metrnl is derived primarily from macrophages and barrier tissues and is involved in the functions associated with macrophages, including anti-inflammatory response, wound healing and tissue remodeling. Metrnl production in macrophages is regulated by a complex interplay of multiple cytokines, such as TNF-α, IL-4, IL-12, IL-17α and IL-1β, and conversely, Metrnl influences the production of other cytokines and chemokines, such as IL-6, IL-10, CCL2, CXCL1.5,37 The study also verified that the expression of Metrnl in macrophages is strongly induced by LPS, and its levels parallel the development and resolution of inflammatory responses in vivo, which suggested that Metrnl is a new major player in inflammatory responses and is likely to play a crucial role in the pathogenesis of human inflammatory diseases. Notably, Metrnl expression in macrophages is robustly induced by LPS, and its levels are closely aligned with the progression of inflammatory responses in vivo, which suggested that Metrnl is a significant contributor to inflammatory responses and the pathogenesis of various human inflammatory diseases.37
Metrnl, Inflammation and Bone Diseases
Multiple studies have identified that Metrnl is involved in bone development, remodeling, and various bone-related pathologies. Gong et al reported that Metrnl may influence osteoblast differentiation and thus bone development.17 Further research has demonstrated that Metrnl can enhance osteoblast differentiation and mineralization in vitro, as well as facilitate bone fracture healing.15 Additional studies have observed abnormal expression of Metrnl in the cartilage tissue and synovium associated with skeletal diseases.19 Metrnl in different tissues might have different mechanisms. In patients with OA, the levels of Metrnl in serum were significantly lower than in normal healthy controls, but higher in synovial fluid.19 These findings underscored the important role of Metrnl in bone diseases. However, the precise mechanism of action of Metrnl in these contexts remains to be elucidated through further research.
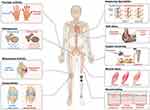
Chronic inflammation is beneficial to a catabolism, promoting osteolysis and reducing both bone formation and bone mineral density.38,39 Immune inflammation and overexpression of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) are associated with bone health issues, which have destructive impact on normal bone homeostasis through various mechanisms.40 Catabolic states associated with immune inflammation and pathological bone loss are exemplified in a range of skeletal disorders, including periprosthetic osteolysis,3,41 aseptic loosening,42,43 osteoporosis, and rheumatoid arthritis. These conditions are major contributors to disability and impose a significant financial burden globally (Figure 1). Their complex etiology and substantial impact highlight the urgency for effective therapeutic strategies and a deeper understanding of their underlying mechanisms.44–46
Metrnl and Psoriatic Arthritis
Psoriatic arthritis (PsA) is a chronic inflammatory arthropathy intricately linked to psoriasis and part of the broader Spondyloarthritis (SpA) spectrum. PsA is characterized by its ability to affect spine, peripheral joints, and entheses, presenting with distinctive clinical features, such as sacroiliitis, enthesitis, dactylitis, spinal involvement, and concurrent psoriasis. Radiologically, PsA can manifest with erosive lesions or osteolysis, as well as periarticular new bone formation, reflecting its diverse impact on bone structure.47,48 PsA is associated with inflammatory lesions at the enthesis, the site where tendons and ligaments attach to the bone. Notably, Metrnl expression is significantly elevated in both the synovial fluid and synovial tissue of PsA patients compared to those with OA. Furthermore, enthesis stromal cells have been identified as the primary source of Metrnl production in PsA. This differential expression underscores the distinct pathophysiological mechanisms underlying PsA and suggests a potential role for Metrnl in its diagnosis or treatment.20 The important role of Metrnl in PsA immunopathogenesis needs further research.
Metrnl and Osteoporosis
Osteoporosis, predominantly an age-related pathology, is defined as a bone disease characterized by reduced bone mineral mass and microarchitectural degradation of bone tissue. This leads to a decrease in bone strength and significantly increases the risk of osteoporotic fractures, especially in the elderly population worldwide. Conversely, sarcopenia is characterized by progressive muscle wasting, which heightens the risk of injuries, predominantly due to falls. The concurrent development of these conditions underscores the complexity of age-related musculoskeletal decline. Osteoporosis is currently attributed to various endocrine, age, metabolic and mechanical factors. Various evidences suggest that inflammation and muscle–bone crosstalk also exert significant influence on bone metabolism, leading to bone loss. An imbalance in bone mesenchymal stem cells favoring adipogenesis over osteogenesis is detrimental to bone health, often resulting in a decrease of bone mass and the development of osteoporosis.49 Muscle and bone exhibit a closely interlinked development and growth process. Consequently, muscle disuse or atrophy often precipitates osteoporosis, highlighting the intrinsic connection between these two tissues.50 Muscle-bone crosstalk, encompassing both biomechanical and biochemical interactions, plays a crucial role in bone remodeling.51 Metrnl as an immune/metabolic regulator, plays a pivotal role in modulating the activity of diverse cell types, and in both innate and acquired immune responses. It exhibits a distinctive expression pattern primarily observed in hypertrophic chondrocytes and osteoblasts.52 Moreover, its overexpression has been shown to effectively impede mineralized nodule formation and hinder osteoblast differentiation.17 It has been proved in whole-body or macrophage-specific Metrnl knock-out (KO) mice that the lack of Metrnl could reduce the immune cell infiltration. Moreover, this deficiency impeded the transition towards an anti-inflammatory phenotype, a process that is typically mediated through STAT3 activation.11 Osteoactivin and Metrnl have been revealed to have positive correlation in regulating bone cell differentiation.53 This synergistic interaction between Metrnl and osteoactivin underscores their critical involvement in the muscle-bone crosstalk. Moreover, research focusing on genes implicated in osteoblast differentiation revealed that Metrnl exhibits a distinctive expression pattern in bone, potentially inhibiting bone cell differentiation.17 Collectively, these studies position Metrnl as a key regulator in immune inflammation and the muscle-bone crosstalk, and as a potential therapeutic target for conditions such as osteoporosis.
Metrnl and Rheumatoid Arthritis
Rheumatoid arthritis (RA) represents a chronic, systemic autoimmune disorder predominantly manifesting as progressive inflammatory pathology within synovial joints. This condition is distinguished by a hallmark immune-mediated inflammation targeting synovial tissues, leading to the degradation of articular cartilage and subsequent osseous resorption. Clinically, RA is typified by symptoms such as persistent pain, synovial swelling, joint tenderness, rigidity, and progressive deformities of the affected joints. Epidemiologically, RA has a global prevalence impacting approximately 0.5% to 1% of the population, underscoring its significance as a major rheumatologic health concern. In the pathogenesis of this articular pathology, there is a pronounced synovial hyperplasia characterized by the infiltration and activation of immune cells, notably lymphocytes and macrophages, along with synovial fibroblasts. Concurrently, the synovium witnesses the formation of inflammatory cell conglomerates, predominantly composed of activated macrophages and lymphocytes, within the osseous marrow compartment. This immunopathological cascade precipitates synovitis, which is intricately associated with localized osteopenia, manifesting as a decrement in bone mineral density.54 Metrnl is elevated in serum18 and synovial membranes5,20 of human rheumatoid arthritis. The investigation delineated a significant upregulation of serum Metrnl concentrations in patients with RA. These elevated levels of Metrnl exhibit a positive correlation with the Disease Activity Score 28 (DAS28), Rheumatoid Factor (RF), and C-Reactive Protein (CRP) levels. This correlation underscores Metrnl’s involvement in the etiology and progression of RA.18 Furthermore, the augmentation of serum Metrnl levels is intricately associated with the clinical activity of RA. This positions Metrnl not only as a crucial participant in the pathophysiological mechanisms of RA but also as a potential biomarker and therapeutic target for future interventions in RA management.
Metrnl and Osteoarthritis
Osteoarthritis (OA) is recognized as a highly prevalent and progressively debilitating articular disorder, exerting a substantial impact on individual health and quality of life.55,56 Recent evidence has redefined OA as a metabolic disorder, transcending the traditional view of it being merely a degenerative “wear and tear” arthritis.57,58 While a myriad of pharmacological interventions are necessary to alleviate pain and enhance life quality in OA patients, none have demonstrated efficacy in arresting or reversing the disease’s progression. Furthermore, these symptomatic treatments, often associated with adverse effects like nephrotoxicity and gastrointestinal complications, pose a significant economic burden over long-term usage.59,60 Obesity is universally acknowledged as a primary and modifiable risk factor for the development of OA.61 Recent research has shed light on the importance of Metrnl, an emerging adipokine, in the pathophysiology of OA. This novel adipokine has been identified to have significant associations with the onset and progression of OA, suggesting a potential mechanistic link between adipose tissue dysregulation and osteoarthritic changes.62 Metrnl has been identified as a key agent in counteracting obesity-induced insulin resistance and enhancing glucose tolerance. This is predominantly mediated through the activation of PPARγ, a factor critical to the pathophysiology of OA. Activation of PPARγ exerts anti-inflammatory effects and diminish the synthesis of cartilage degradation markers, both in vitro and in vivo studies. Notably, PPARγ activation has also been associated with a reduction in the development of cartilage lesions in OA animal models.63 A recent study delineated a distinctive pattern in the distribution of Metrnl levels among obese individuals with and without OA. Findings indicated that, in obese patients afflicted with OA, serum levels of Metrnl were observed to be lower compared to those in obese individuals without OA. Contrastingly, Metrnl concentrations were higher in the synovial fluid of OA patients. Additionally, a notable decrease in Metrnl levels was observed in serum of subjects with advanced-stage OA, as opposed to those with early-stage OA, suggesting a potential inverse correlation between Metrnl levels and OA severity19 Liu et al conducted pivotal research demonstrating that Metrnl mitigates inflammation through the inhibition of the PI3K/Akt/NF-κB signaling pathway in IL-1β-stimulated OA chondrocytes. Additionally, Metrnl was shown to curtail chondrocyte pyroptosis by impeding the nod-like receptor protein-3 (NLRP3)/caspase-1/gasdermin D cascade.64 These seminal findings not only position Metrnl as a promising therapeutic target for Osteoarthritis but also pave the way for further exploration into its mechanistic role in the pathology of OA.
Metrnl and Axial Spondyloarthritis
Axial spondyloarthritis (AxSpa), known as ankylosing Spondylitis, is a chronic inflammatory rheumatic disease that often causes back pain and limited mobility. Assessing clinical manifestations and monitoring disease activity is important for early diagnosis and effective treatment. A study showed that the serum Metrnl concentration of AxSpa patients was lower compared with healthy individuals. However, the mechanism was still undiscovered. Metrnl as a novel biomarkers is related with autoimmune inflammatory rheumatic diseases, which could be used to measure clinical disease activity and assess treatment efficacy.65
Metrnl and Elbow Stiffness
Elbow stiffness is a challenging and common problem in surgical treatment. Long-term joint fixation is generally recognized as a basic risk factor for posttraumatic motor disability. This pathological state precipitates a cascade of physical and biochemical dysfunctions within and surrounding joint structures. Key manifestations include the erosion of articular cartilage, a marked reduction in the content of articular proteoglycans, and the development of soft tissue contractures, particularly affecting the joint capsule.66,67 Heterotopic ossification (HO) initiated by inflammation and the ectopic bone formation within soft tissues is the primary cause of elbow stiffness, Fan et al reported that the IL-17 plays a critical role in the formation of HO. At the early stage of HO, IL-17 overexpressed and released into the tissue, and enhanced ectopic bone formation.68 Metrnl is confirmed to inhibit the production of IL-17 and attenuate the progress of inflammation,31 which is a significant potential of Metrnl in the research and treatment of elbow stiffness. Limited mobility is the major symptom of elbow stiffness. However, numerous studies showed that exercise could promote the expression of Metrnl, which in return suppress the inflammation and fibrosis.25,64 These persuasive evidences suggested that there may be an important link with Metrnl and elbow stiffness.
Metrnl and Aseptic Loosening Cased by Periprosthetic Osteolysis
Aseptic loosening is a prevalent complication that frequently occurs after total joint arthroplasty, primarily attributed to periprosthetic inflammatory osteolysis. It stands as the primary cause for revision procedures.40 The loosening of joint implants due to wear debris incurs a significant need for revision surgery, leading to severe complications and substantial economic burden.69 The release of wear particles from the prosthetic materials triggers a persistent local inflammatory response, and inflammatory factors such as IL-1β, IL-6, TNF-α, RANKL and PGE2 were generated and secreted by macrophages, thereby stimulating osteoclast activities and resulting in bone loss around the implant.70 Metrnl inhibited the secretion of TNF-α, IL-1β and other inflammatory factors and promoted the production of anti-inflammatory factors in macrophages, thus suppressed the inflammation response and osteolysis.5,32,37
Metrnl and Muscle Injury
Sarcopenia, alongside other muscular injuries, represents a prevalent health concern, frequently resulting in significant disability, chronic pain, and an elevated economic burden.71 Skeletal muscle possesses remarkable regenerative capabilities, and the interaction between muscle and the immune system during muscle repair is crucial for successful regeneration.72 Baht et al confirmed Metrnl facilitate skeletal muscle repair.11 Metrnl was high expressed in macrophages in the injured muscle, and in turn, Metrnl stimulated macrophages to produce growth factors functions in muscle regeneration through STAT3/IGF-1 signaling pathway.11 Another research showed that the immune responsiveness is enhanced by Metrnl to counteract a pro-fibrotic program through inducing apoptosis of fibro/adipogenic progenitor (FAP), which improved muscle homeostasis and repair in aged muscle.73 Obesity and metabolic disorders development are companied with skeletal muscle inflammation, which characterized by the activation of proinflammatory responses and the infiltration of immune cells in intramyocellular and perimuscular adipose tissues.74 Several studies suggested that exercise-induced Metrnl performed anti-inflammatory effects on by inhibiting NLRP3 inflammasome activation and downregulating the expression of pro-inflammatory cytokines.25,75 Metrnl has anti-inflammatory and healing effects on skeletal muscle injury, suggesting a new therapeutic target on skeletal muscle inflammation.
Metrnl and Wound Healing
Skin wound healing is a complex process associated with endothelial-mediated angiogenesis and anti-inflammatory actions.76 Impaired wound healing is a significant contributor to morbidity in patients with diabetes with increased inflammation and poor angiogenesis, often leading to foot ulcers, amputation, and even death.77,78 Studies showed that Metrnl overexpressed and released by endothelial cells and then promoted the proliferation, migration and tube formation of endothelial cells through AKT/eNOS signal pathway.79,80 Another study reported Metrnl overexpressed during the process of physiological wound healing, and thus promoted M2 macrophage polarization and increased endothelial cell proliferation. Activated M2 macrophages further secreted more Metrnl to promote angiogenesis, ultimately enhancing the repair of skin injuries.81
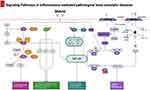
Pathways of Metrnl Contributing to Anti-Inflammatory Effects
During the past years, Metrnl as a new adipokine has garnered considerable interest for its anti-inflammatory functions in various disorders.5,82 Presently, Metrnl is also recognized for its significant role in the pathogenesis and progression of orthopedic diseases (Figure 2). It can influence these conditions by inducing or inhibiting catabolic or apoptotic pathways, and by directly or indirectly affecting bone metabolism.3,15,19 However, the mechanism of Metrnl regulating anti-inflammatory responses and bone diseases is still unclear.
AKT/eNOS Signaling Pathway
Xu et al reported that Metrnl participates skin wound healing by regulating angiogenesis through AKT/eNOS signaling pathway,79 a key pathway downstream of angiogenic factors VEGFA, which can increase the production of NO(*) in endothelial cell to promote angiogenesis.83 In Metrnl-/- mice, the expression of VEGFA was suppressed and further inhibited the phosphorylation of p-AKT(S473) and p-eNOS(S1177) in HUVECs and skin wound tissues, leading to attenuated angiogenesis and consequently retarding wound healing. When Metrnl-deficient HUVECs were co-incubated with SC79, an AKT activator, a marked restoration in their angiogenic activity was observed.79
PI3K/Akt/NF-κB Signaling Pathway
Nuclear factor κB (NF-κB) plays a pivotal role in the pathogenesis of inflammatory-mediated pathological orthopedic diseases. Chronic activation of NF-κB in chondrocytes is a primary factor driving pathological changes in OA.84 It was also involved in the process of trauma-induced HO development.85 The PI3K/Akt signaling pathway activates NF-κB and facilitates its nuclear translocation through phosphorylation. It has been implicated to inducing inflammation in chondrocytes, accompanied by upregulation of matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), as well as the decrease of collagen II.86 Metrnl exerts its anti-inflammatory effects by inhibiting the activation and nuclear translocation of NF-κB.64 IL-1β induction increased the phosphorylation levels of PI3K, Akt, NF-κB p65, and IkBα. However, the administration of Metrnl can suppress these changes, thereby mitigating inflammation and pyroptosis in OA chondrocytes.
STAT3/IGF-1 Signaling Pathway
Metrnl directly signals to macrophages through STAT3 pathway, leading to their differentiation into an anti-inflammatory phenotype and induces expression and secretion of growth factor IGF-1 functioning on muscle regeneration.11 When the muscle was injured, Metrnl was high expressed in macrophage populations, which is crucial for muscle regeneration. Phosphorylation of STAT3 was increased in bone marrow-derived macrophages after Metrnl treatment. Phosphorylated STAT3 further induced the expression of several growth factors (IL-6, IL-10 and IGF-1) in macrophages associated with muscle regeneration, which were eliminated by the STAT3 inhibitor. The secretion of IGF-1 stimulated the proliferation of muscle satellite cells, which is crucial for muscle regeneration.11 Aberrant STAT3 signaling was experimentally linked to a wide range of intracellular processes.87 In chondrocytes, STAT3 activation has been observed to induce degradation and apoptosis.88 However, the interrelation of STAT3 with Metrnl appears complicated and far from being fully elucidated.
AMPK or PPARδ-Dependent Pathways
AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor δ (PPARδ) play pivotal roles in cellular energy homeostasis. AMPK phosphorylation and PPARδ activation exert significant anti-inflammatory effects and immunosuppressive effects by suppressing the NF-κB signaling pathway.89,90 Jung et al reported that Metrnl could induce AMPK phosphorylation and PPARδ expression independently and consequently decreased inflammation. In vivo experiments conducted on mice fed with a high-fat diet demonstrated that treatment with Metrnl effectively mitigated NF-κB signaling and reduced the production of pro-inflammatory cytokines (TNFα and MCP-1).44 In addition, Metrnl increased the expression of peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) through AMPK and PPARδ-mediated signaling.44 PGC-1α has been proved protective effects in muscle biology by suppressing chronic inflammation and muscle catabolism.91 Another study confirmed that Metrnl can suppress LPS-induced inflammation in HUVECs and THP-1 cells via AMPK and PPARδ-mediated signaling pathways.32 Treatment with Metrnl on HUVECs, THP-1 cells, and primary mouse monocytes exhibited a concentration-dependent mitigation of LPS-induced phosphorylation of NF-κB and IκB, as well as the translocation of NF-κB into the nucleus. Furthermore, the administration of Metrnl effectively attenuated the elevated levels of pro-inflammatory cytokines in HUVECs and THP-1 cells.32 Metrnl regulates AMPK or PPARδ-dependent signaling pathway simultaneously, thereby suppressing inflammatory diseases.
NLRP3/Caspase-1/GSDMD Signaling Pathway
Metrnl can regulate the activation of NLRP3 inflammasome and inhibit inflammatory response.25,64 NLRP3 is a cytoplasmic immune factor in response to cellular stress signals. As a member of the NOD-like receptor family, it is normally activated in response to infection or inflammation, forming the NLRP3 inflammasome (composed of NLRP3, adapter protein ASC, and inflammatory protease caspase-1), which induces apoptosis.92 Exercise-induced Metrnl could ameliorate pyroptosis in chondrocytes by inhibiting the NLRP3/caspase-1/GSDMD pathway. This leads to improved chondrocyte morphology and recovery of nuclear integrity.5 Another research reported that exogenous Metrnl treatment suppressed NLRP3 inflammasome activation and completely abolished IL-1β secretion in BMDMs induced by LPS and ATP. Moreover, this effect was partly mediated by activation of both ERK and p38 MAPK signaling.93 The NLRP3 inflammasome primarily functions in inducing gasdermin D (GSDMD)-dependent pyroptosis, significantly influencing the pathophysiology of OA.64 Based on the aforementioned reports, it can be postulated that Metrnl represents a promising target for effectively attenuating NLRP3-mediated inflammation in adipose tissues. Consequently, this highlights its therapeutic potential in addressing metabolic and inflammatory disorders.
Discussion and Future Perspectives
Metrnl, as an emerging adipokine, plays a vital role in both pathological and physiological processes and shows regulatory functions in metabolic and inflammatory diseases.9,37 This study reported the important link of Metrnl and skeletal development, remodeling, and some bone-related diseases, contributing to understanding the role of Metrnl in inflammatory orthopedic diseases and demonstrating its potential in orthopedic disease therapy.15
Metrnl has been proved to participate the development of orthopedic disease by regulating immune inflammation and bone-muscle crosstalk, including periprosthetic osteolysis, aseptic loosening, osteoporosis, rheumatoid arthritis and axial spondyloarthritis.64,73,82 The expression of Metrnl can be induced by exercise, like elbow stiffness,25,64 the mechanism of which is still indistinct. Metrnl regulates the expression of cytokines related to inflammation and fibrosis, which are participated in the regulatory pathway of heterotopic ossification and joint contracture, showing the potential of Metrnl in the occurrence of elbow stiffness. Exercise induced the production of Metrnl and it relieves the symptoms of elbow stiffness, further indicating it might be used in elbow stiffness therapy.
Despite clinical evidence linking Metrnl to a number of orthopedic inflammatory diseases, the function of Metrnl still require further validation, and its mechanisms are unexplored. Clinical investigations of Metrnl and possible orthopedic disorders remain inadequate. The function and mechanism of Metrnl in human orthopedic diseases continue to be areas needing more comprehensive exploration and understanding. More clinical studies are needed to verify the relationship between Metrnl and various orthopedic diseases and inflammatory diseases, as well as other underlying conditions that Metrnl may be involved in. This research will help improve our understanding of the role of Metrnl in human orthopedic health and disease.
In conclusion, these studies have prompted us to acknowledge the significant contribution of Metrnl in the pathogenesis and prevention of orthopedic conditions, particularly elbow stiffness. While the pathogenesis of elbow stiffness remains elusive, current literature suggests that Metrnl likely exerts a pivotal role in its development. However, the existing body of research remains insufficient to fully elucidate this phenomenon. The function and mechanism of Metrnl in the orthopedic diseases still need more investigation. More preclinical studies are essential to elucidate its molecular mechanisms, providing a stronger scientific foundation for its potential medical applications in the future.
Funding
This work was partially supported by the Natural Science Foundation of Shandong Province (No. ZR2021QH307; No. ZR2023QH498; No. ZR2021MH013), the Shandong Province Major Scientific and Technical Innovation Project (No. 2021SFGC0502), and the Jinan Clinical Medical Science and Technology Innovation Plan (NO. 202328065). The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Metzger CE, Narayanan SA. The role of osteocytes in inflammatory bone loss. Front Endocrinol. 2019;10:285. doi:10.3389/fendo.2019.00285
2. Mbalaviele G, Novack DV, Schett G, Teitelbaum SL. Inflammatory osteolysis: a conspiracy against bone. J Clin Invest. 2017;127(6):2030–2039. doi:10.1172/JCI93356
3. Liu F, Dong J, Zhou D, Zhang Q. Identification of key candidate genes related to inflammatory osteolysis associated with vitamin E-blended UHMWPE debris of orthopedic implants by integrated bioinformatics analysis and experimental confirmation. J Inflamm Res. 2021;14:3537–3554. doi:10.2147/JIR.S320839
4. Li ZY, Zheng SL, Wang P, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Ther. 2014;20(4):344–354. doi:10.1111/cns.12219
5. Ushach I, Burkhardt AM, Martinez C, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clin Immunol. 2015;156(2):119–127. doi:10.1016/j.clim.2014.11.006
6. Li ZY, Fan MB, Zhang SL, et al. Intestinal Metrnl released into the gut lumen acts as a local regulator for gut antimicrobial peptides. Acta Pharmacol Sin. 2016;37(11):1458–1466. doi:10.1038/aps.2016.70
7. Jorgensen JR, Fransson A, Fjord-Larsen L, et al. Cometin is a novel neurotrophic factor that promotes neurite outgrowth and neuroblast migration in vitro and supports survival of spiral ganglion neurons in vivo. Exp Neurol. 2012;233(1):172–181. doi:10.1016/j.expneurol.2011.09.027
8. Hong C, Wang Z, Zheng SL, et al. Metrnl regulates cognitive dysfunction and hippocampal BDNF levels in D-galactose-induced aging mice. Acta Pharmacol Sin. 2022. doi:10.1038/s41401-022-01009-y
9. Sekerci G, Erden Y, Tekin S. Effects of meteorin-like hormone on endocrine function of hypothalamo-hypophysial system and peripheral uncoupling proteins in rats. Mol Biol Rep. 2022;49(7):5919–5925. doi:10.1007/s11033-022-07374-5
10. Li ZY, Song J, Zheng SL, et al. Adipocyte metrnl antagonizes insulin resistance through PPARgamma signaling. Diabetes. 2015;64(12):4011–4022. doi:10.2337/db15-0274
11. Baht GS, Bareja A, Lee DE, et al. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab. 2020;2(3):278–289. doi:10.1038/s42255-020-0184-y
12. Ruperez C, Ferrer-Curriu G, Cervera-Barea A, et al. Meteorin-like/Meteorin-beta protects heart against cardiac dysfunction. J Exp Med. 2021;218(5). doi:10.1084/jem.20201206
13. Lu QB, Ding Y, Liu Y, et al. Metrnl ameliorates diabetic cardiomyopathy via inactivation of cGAS/STING signaling dependent on LKB1/AMPK/ULK1-mediated autophagy. J Adv Res. 2022. doi:10.1016/j.jare.2022.10.014
14. Cai J, Wang QM, Li JW, et al. Serum Meteorin-like is associated with weight loss in the elderly patients with chronic heart failure. J Cachexia, Sarcopenia Muscle. 2022;13(1):409–417. doi:10.1002/jcsm.12865
15. Huang R, Balu AR, Molitoris KH, et al. The role of Meteorin-like in skeletal development and bone fracture healing. J Orthop Res. 2022;40(11):2510–2521. doi:10.1002/jor.25286
16. Cherian P, Al-Khairi I, Jamal M, et al. Association between factors involved in bone remodeling (Osteoactivin and OPG) with plasma levels of irisin and meteorin-like protein in people with T2D and obesity. Front Endocrinol. 2021;12:752892. doi:10.3389/fendo.2021.752892
17. Gong W, Liu Y, Wu Z, et al. Meteorin-like shows unique expression pattern in bone and its overexpression inhibits osteoblast differentiation. PLoS One. 2016;11(10):e0164446. doi:10.1371/journal.pone.0164446
18. Zhang S, Lei Y, Sun T, et al. Elevated levels of Metrnl in rheumatoid arthritis: association with disease activity. Cytokine. 2022;159:156026. doi:10.1016/j.cyto.2022.156026
19. Sobieh BH, Kassem DH, Zakaria ZM, El-Mesallamy HO. Potential emerging roles of the novel adipokines adipolin/CTRP12 and meteorin-like/METRNL in obesity-osteoarthritis interplay. Cytokine. 2021;138:155368. doi:10.1016/j.cyto.2020.155368
20. Bridgewood C, Russell T, Weedon H, et al. The novel cytokine Metrnl/IL-41 is elevated in Psoriatic Arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clin Immunol. 2019;208:108253. doi:10.1016/j.clim.2019.108253
21. Wang W, Liu Y, Hao J, et al. Comparative analysis of gene expression profiles of Hip articular cartilage between non-traumatic necrosis and osteoarthritis. Gene. 2016;591(1):43–47. doi:10.1016/j.gene.2016.06.058
22. Terkawi MA, Kadoya K, Takahashi D, et al. Identification of IL-27 as potent regulator of inflammatory osteolysis associated with vitamin E-blended ultra-high molecular weight polyethylene debris of orthopedic implants. Acta Biomater. 2019;89:242–251. doi:10.1016/j.actbio.2019.03.028
23. Gao X, Leung TF, Wong GW, et al. Meteorin-beta/Meteorin like/IL-41 attenuates airway inflammation in house dust mite-induced allergic asthma. Cell Mol Immunol. 2022;19(2):245–259. doi:10.1038/s41423-021-00803-8
24. Rao RR, Long JZ, White JP, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–1291. doi:10.1016/j.cell.2014.03.065
25. Javaid HMA, Sahar NE, ZhuGe DL, Huh JY. Exercise inhibits NLRP3 inflammasome activation in obese mice via the anti-inflammatory effect of meteorin-like. Cells. 2021;10(12). doi:10.3390/cells10123480
26. Chen Z, Li J, Peng H, et al. Meteorin-like/Meteorin-beta protects LPS-induced acute lung injury by activating SIRT1-P53-SLC7A11 mediated ferroptosis pathway. Mol Med. 2023;29(1):144. doi:10.1186/s10020-023-00714-6
27. Akkus G, Koyuturk LC, Yilmaz M, et al. Asprosin and meteorin-like protein immunoreactivity in invasive ductal breast carcinoma stages. Tissue Cell. 2022;77:101855. doi:10.1016/j.tice.2022.101855
28. Xu X, Zhang C, Xia Y, Yu J. Over expression of METRN predicts poor clinical prognosis in colorectal cancer. Mol Genet Genomic Med. 2020;8(3):e1102. doi:10.1002/mgg3.1102
29. Kocaman N, Artas G. Can novel adipokines, asprosin and meteorin-like, be biomarkers for malignant mesothelioma? Biotech Histochem. 2020;95(3):171–175. doi:10.1080/10520295.2019.1656344
30. Hu J, He A, Yue X, Zhou M, Zhou Y. METRNL reduced inflammation in sepsis-induced renal injury via PPARδ-dependent pathways. Food Science and Technology. 2022;42. doi:10.1590/fst.61821
31. Chen X, Chen X, Yang Y, et al. Protective role of the novel cytokine Metrnl/ interleukin-41 in host immunity defense during sepsis by promoting macrophage recruitment and modulating Treg/Th17 immune cell balance. Clin Immunol. 2023;254:109690. doi:10.1016/j.clim.2023.109690
32. Jung TW, Pyun DH, Kim TJ, et al. Meteorin-like protein (METRNL)/IL-41 improves LPS-induced inflammatory responses via AMPK or PPARdelta-mediated signaling pathways. Adv Med Sci. 2021;66(1):155–161. doi:10.1016/j.advms.2021.01.007
33. Xu L, Cai Y, Wang Y, Xu C. Meteorin-Like (METRNL) Attenuates Myocardial Ischemia/Reperfusion Injury-Induced Cardiomyocytes Apoptosis by Alleviating Endoplasmic Reticulum Stress via Activation of AMPK-PAK2 Signaling in H9C2 Cells. Med Sci Monit. 2020;26:e924564. doi:10.12659/MSM.924564
34. Liu ZX, Ji HH, Yao MP, et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J Cell Mol Med. 2019;23(1):271–280. doi:10.1111/jcmm.13915
35. Dadmanesh M, Aghajani H, Fadaei R, Ghorban K. Lower serum levels of Meteorin-like/Subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PLoS One. 2018;13(9):e0204180. doi:10.1371/journal.pone.0204180
36. Liu J, Diao L, Xia W, et al. Meteorin-like protein elevation post-exercise improved vascular inflammation among coronary artery disease patients by downregulating NLRP3 inflammasome activity. Aging. 2023;15. doi:10.18632/aging.205268
37. Ushach I, Arrevillaga-Boni G, Heller GN, et al. Meteorin-like/meteorin-beta is a novel immunoregulatory cytokine associated with inflammation. J Immunol. 2018;201(12):3669–3676. doi:10.4049/jimmunol.1800435
38. Zhang Q, Lu S, Zhou D, Dong J, Liu F. PTGS2 identified as a biomarker of glucocorticoid-induced osteonecrosis of the femoral head and an enhancer of osteogenesis. Genes Dis. 2023;10(1):14–17. doi:10.1016/j.gendis.2022.01.005
39. Zhang Q, Sun W, Li T, Liu F. Polarization behavior of bone macrophage as well as associated osteoimmunity in glucocorticoid-induced osteonecrosis of the femoral head. J Inflamm Res. 2023;16:879–894. doi:10.2147/jir.s401968
40. Liu N, Dong J, Li L, Liu F. Osteoimmune Interactions and therapeutic potential of macrophage-derived small extracellular vesicles in bone-related diseases. Int J Nanomed. 2023;18:2163–2180. doi:10.2147/IJN.S403192
41. Zhang Q, Dong J, Zhang P, Zhou D, Liu F. Dynamics of transcription factors in three early phases of osteogenic, adipogenic, and chondrogenic differentiation determining the fate of bone marrow mesenchymal stem cells in rats. Front Cell Dev Biol. 2021;9:768316. doi:10.3389/fcell.2021.768316
42. Yan SG, Chevalier Y, Liu F, et al. Metaphyseal anchoring short stem Hip arthroplasty provides a more physiological load transfer: a comparative finite element analysis study. J Orthop Surg Res. 2020;15(1):498. doi:10.1186/s13018-020-02027-4
43. Wang B, Li Q, Dong J, Zhou D, Liu F. Comparisons of the surface micromotions of cementless femoral prosthesis in the horizontal and vertical levels: a network analysis of biomechanical studies. J Orthop Surg Res. 2020;15(1):293. doi:10.1186/s13018-020-01794-4
44. Jung TW, Lee SH, Kim HC, et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARdelta-dependent pathways in skeletal muscle of mice. Exp Mol Med. 2018;50(9):1–11. doi:10.1038/s12276-018-0147-5
45. Gries KJ, Zysik VS, Jobe TK, et al. Muscle-derived factors influencing bone metabolism. Semin Cell Dev Biol. 2022;123:57–63. doi:10.1016/j.semcdb.2021.10.009
46. Chen Y, Hu W, Wang Y, et al. A selected small molecule prevents inflammatory osteolysis through restraining osteoclastogenesis by modulating PTEN activity. Clin Transl Med. 2020;10(8):e240. doi:10.1002/ctm2.240
47. van Kuijk AW, Tak PP. Synovitis in psoriatic arthritis: immunohistochemistry, comparisons with rheumatoid arthritis, and effects of therapy. Curr Rheumatol Rep. 2011;13(4):353–359. doi:10.1007/s11926-011-0181-y
48. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. 2017;376(10):957–970. doi:10.1056/NEJMra1505557
49. Liu F, Dong J, Zhang P, Zhou D, Zhang Q. Transcriptome sequencing reveals key genes in three early phases of osteogenic, adipogenic, and chondrogenic differentiation of bone marrow mesenchymal stem cells in rats. Front Mol Biosci. 2021;8:782054. doi:10.3389/fmolb.2021.782054
50. Maurel DB, Jahn K, Lara-Castillo N. Muscle-bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines. 2017;5(4). doi:10.3390/biomedicines5040062
51. Muruganandan S, Govindarajan R, Sinal CJ. Bone marrow adipose tissue and skeletal health. Curr Osteoporos Rep. 2018;16(4):434–442. doi:10.1007/s11914-018-0451-y
52. Loffler D, Landgraf K, Rockstroh D, et al. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int J Obes. 2017;41(1):112–119. doi:10.1038/ijo.2016.180
53. Lee K, Seo I, Choi MH, Jeong D. Roles of mitogen-activated protein kinases in osteoclast biology. Int J Mol Sci. 2018;19(10). doi:10.3390/ijms19103004
54. Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35(1):10–14. doi:10.1016/j.jaut.2009.12.009
55. Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30(1):10–16. doi:10.1016/j.joca.2021.05.007
56. Dobson GP, Letson HL, Grant A, et al. Defining the osteoarthritis patient: back to the future. Osteoarthritis Cartilage. 2018;26(8):1003–1007. doi:10.1016/j.joca.2018.04.018
57. Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38–50. doi:10.1016/j.cytogfr.2018.10.002
58. Zhang H, Wang L, Cui J, et al. Maintaining hypoxia environment of subchondral bone alleviates osteoarthritis progression. Sci Adv. 2023;9(14):eabo7868. doi:10.1126/sciadv.abo7868
59. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147. doi:10.1016/j.bcp.2020.114147
60. Li J, Zhang W, Liu X, et al. Endothelial Stat3 activation promotes osteoarthritis development. Cell Prolif. 2023;56(12):e13518. doi:10.1111/cpr.13518
61. Tamaddon M, Gilja H, Wang L, et al. Osteochondral scaffolds for early treatment of cartilage defects in osteoarthritic joints: from bench to clinic. Biomater Transl. 2020;1(1):3–17. doi:10.3877/cma.j.issn.2096-112X.2020.01.002
62. Sobieh BH, El-Mesallamy HO, Kassem DH. Beyond mechanical loading: the metabolic contribution of obesity in osteoarthritis unveils novel therapeutic targets. Heliyon. 2023;9(5):e15700. doi:10.1016/j.heliyon.2023.e15700
63. Fahmi H, Martel-Pelletier J, Pelletier JP, Kapoor M. Peroxisome proliferator-activated receptor gamma in osteoarthritis. Mod Rheumatol. 2011;21(1):1–9. doi:10.1007/s10165-010-0347-x
64. Liu J, Jia S, Yang Y, et al. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-kappaB and NLRP3/caspase-1/GSDMD signaling. Biomed Pharmacother. 2023;158:114118. doi:10.1016/j.biopha.2022.114118
65. Ucar IMB, Sargin G, Tuzcu A, Cildag S, Senturk T. Correlation of serum subfatin, cthrc1, ctrp3, ctrp6 levels with disease indices in patients with axial spondyloarthritis. BMC Rheumatol. 2023;7(1):29. doi:10.1186/s41927-023-00356-5
66. Guglielmetti CLB, Gracitelli MEC, Assuncao JH, et al. Randomized trial for the treatment of post-traumatic elbow stiffness: surgical release vs. rehabilitation. J Shoulder Elbow Surg. 2020;29(8):1522–1529. doi:10.1016/j.jse.2020.03.023
67. Born CT, Gil JA, Goodman AD. Joint contractures resulting from prolonged immobilization: etiology, prevention, and management. J Am Acad Orthop Surg. 2017;25(2):110–116. doi:10.5435/JAAOS-D-15-00697
68. Tu B, Yu B, Wang W, et al. Inhibition of IL-17 prevents the progression of traumatic heterotopic ossification. J Cell Mol Med. 2021;25(16):7709–7719. doi:10.1111/jcmm.16617
69. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total Hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(Suppl 3):144–151. doi:10.2106/JBJS.G.00587
70. Mitchell W, Bridget Matthews J, Stone MH, Fisher J, Ingham E. Comparison of the response of human peripheral blood mononuclear cells to challenge with particles of three bone cements in vitro. Biomaterials. 2003;24(5):737–748. doi:10.1016/s0142-9612(02)00405-2
71. Martinez BP, Batista AK, Gomes IB, et al. Frequency of sarcopenia and associated factors among hospitalized elderly patients. BMC Musculoskelet Disord. 2015;16:108. doi:10.1186/s12891-015-0570-x
72. Kharraz Y, Guerra J, Mann CJ, Serrano AL, Munoz-Canoves P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013;2013:491497. doi:10.1155/2013/491497
73. Lee DE, McKay LK, Bareja A, et al. Meteorin-like is an injectable peptide that can enhance regeneration in aged muscle through immune-driven fibro/adipogenic progenitor signaling. Nat Commun. 2022;13(1):7613. doi:10.1038/s41467-022-35390-3
74. Wu H, Ballantyne CM. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest. 2017;127(1):43–54. doi:10.1172/JCI88880
75. Lee JO, Byun WS, Kang MJ, et al. The myokine meteorin-like (metrnl) improves glucose tolerance in both skeletal muscle cells and mice by targeting AMPKalpha2. FEBS J. 2020;287(10):2087–2104. doi:10.1111/febs.15301
76. Zomer HD, Trentin AG. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci. 2018;90(1):3–12. doi:10.1016/j.jdermsci.2017.12.009
77. McDermott K, Fang M, Boulton AJM, Selvin E, Hicks CW. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care. 2023;46(1):209–221. doi:10.2337/dci22-0043
78. Hu Y, Xiong Y, Tao R, et al. Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing. Biomater Transl. 2022;3(3):188–200. doi:10.12336/biomatertransl.2022.03.003
79. Xu TY, Qing SL, Zhao JX, et al. Metrnl deficiency retards skin wound healing in mice by inhibiting AKT/eNOS signaling and angiogenesis. Acta Pharmacol Sin. 2023;44(9):1790–1800. doi:10.1038/s41401-023-01090-x
80. Zheng S, Li Z, Song J, et al. Endothelial METRNL determines circulating METRNL level and maintains endothelial function against atherosclerosis. Acta Pharm Sin B. 2023;13(4):1568–1587. doi:10.1016/j.apsb.2022.12.008
81. Song L, Chang X, Hu L, et al. Accelerating wound closure with metrnl in normal and diabetic mouse skin. Diabetes. 2023;72(11):1692–1706. doi:10.2337/db23-0173
82. Zheng SL, Li ZY, Song J, Liu JM, Miao CY. Metrnl: a secreted protein with new emerging functions. Acta Pharmacol Sin. 2016;37(5):571–579. doi:10.1038/aps.2016.9
83. Yu M, Liu W, Li J, et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11(1):350. doi:10.1186/s13287-020-01824-2
84. Arra M, Swarnkar G, Ke K, et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat Commun. 2020;11(1):3427. doi:10.1038/s41467-020-17242-0
85. Hou J, Chen J, Fan J, et al. Inhibition of NF-kappaB signaling-mediated crosstalk between macrophages and preosteoblasts by metformin alleviates trauma-induced heterotopic ossification. Inflammation. 2023;46(4):1414–1429. doi:10.1007/s10753-023-01817-2
86. Zheng W, Li X, Li J, et al. Mechanical loading mitigates osteoarthritis symptoms by regulating the inflammatory microenvironment in a mouse model. Ann N Y Acad Sci. 2022;1512(1):141–153. doi:10.1111/nyas.14760
87. Fu XL, Duan W, Su CY, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. 2017;66(12):1597–1608. doi:10.1007/s00262-017-2052-5
88. Qiao Z, Tang J, Wu W, Tang J, Liu M. Acteoside inhibits inflammatory response via JAK/STAT signaling pathway in osteoarthritic rats. BMC Complement Altern Med. 2019;19(1):264. doi:10.1186/s12906-019-2673-7
89. Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89(7):667–676. doi:10.1007/s00109-011-0748-0
90. Ding G, Cheng L, Qin Q, Frontin S, Yang Q. PPARdelta modulates lipopolysaccharide-induced TNFalpha inflammation signaling in cultured cardiomyocytes. J Mol Cell Cardiol. 2006;40(6):821–828. doi:10.1016/j.yjmcc.2006.03.422
91. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–469. doi:10.1038/nature07206
92. Akbal A, Dernst A, Lovotti M, et al. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell Mol Immunol. 2022;19(11):1201–1214. doi:10.1038/s41423-022-00922-w
93. Traves PG, de Atauri P, Marin S, et al. Relevance of the MEK/ERK signaling pathway in the metabolism of activated macrophages: a metabolomic approach. J Immunol. 2012;188(3):1402–1410. doi:10.4049/jimmunol.1101781
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.