Back to Journals » Research and Reports in Tropical Medicine » Volume 8
The first report of two cases of cystic echinococcosis in the lung by Echinococcus ortleppi infection, in Vietnam
Received 10 September 2016
Accepted for publication 8 December 2016
Published 27 March 2017 Volume 2017:8 Pages 45—51
DOI https://doi.org/10.2147/RRTM.S122014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Unnasch

Nguyen Van De,1 Duyet Le Van2
1Department of Parasitology, Hanoi Medical University of Vietnam, 2Clinical Laboratories, National Hospital of Tropical Diseases, Hanoi, Vietnam
Abstract: In 2013, two cases of infection by Echinococcus that caused cystic echinococcosis in the lungs were reported. In the first case, there was a cyst of 6 x 7 cm in diameter, and in the second case, there were four cysts of 5 x 6 cm, 4 x 4 cm, 3.5 x3 cm and 2.5 x 2 cm in diameter, respectively. In both cases, Echinococcus larvae were collected from the cysts. The larvae were identified as Echinococcus ortleppi by morphology and a molecular method (using reduced nicotinamide adenine dinucleotide hydrogenase [NADH] with 99%–100% homology compared with E. ortleppi in GenBank). This is the first time that this species has been found in humans in Vietnam.
Keywords: E. ortleppi, hydatid, Vietnam
Introduction
Human echinococcosis is a zoonotic infection caused by larval forms (metacestodes) of tapeworms of the genus Echinococcus. The adult stage develops in the small intestine of caniids, and the larval stage develops in the viscera of a variety of mammalian species, including humans.1 At present, nine valid species of the genus Echinococcus have been identified in the world, including E. granulosus sensu stricto (G1 [sheep strain], G2 [Tasmanian sheep strain], G3 [buffalo strain]), E. equinus (G4), E. ortleppi (G5), E. canadensis (G6–G10), E. multilocularis, E. vogeli, E. oligarthrus, E. felidis, and E. shiquicus.2–5 Two of these species are of special medical importance. These are E. granulosus and E. multilocularis, causing cystic echinococcosis and alveolar echinococcosis in humans, respectively.6 The first report of E. ortleppi (genotype G5) was in Italy.7 The adult worm is 3–6 mm in length, including three to four proglottids, and is a common parasite in the intestine of the canine family. Humans are considered aberrant intermediate hosts that acquire the infection through accidental ingestion of parasite eggs that may develop into the larval stage of the parasite (metacestode) in suitable internal organs, mainly the liver and lungs. Growing cysts may also damage surrounding tissues and blood vessels. Any associated clinical problems will be dependent on the number of cysts, their size, location, and rate of growth. The organs most frequently affected are the liver and the lungs in approximately 65% and 25% of cases, respectively.8 The kidneys, spleen, brain, heart, skeletal system, and musculature tissues can also be affected.6 In Italy, the chest X-rays and lung images in 24/28 (85.7%) patients found a total of 149 cysts, mostly with hepatic localization (96%).9 The lifespan of hydatid cysts of E. granulosus can be as long as 16 years in horses10 and 53 years in humans.13
Reports of echinococcosis in humans have been reviewed by Alvarez Rojas et al,12 in livestock by Cardona and Carmena,13 and in domestic dogs and wild carnivores by Carmena and Cardona.14,15
Echinococcosis is distributed almost worldwide and is especially common in such areas as Australia, Tasmania, New Zealand, Southern and Northern Africa, and South America.6 In Asia, echinococcosis has been reported in Japan, China, Korea, Mongolia, Thailand, Indonesia, Bangladesh, and India,16 but not in Vietnam.
In 2013, two patients were detected with cystic echinococcosis at the National Hospital of Tuberculosis and Lung Diseases in Hanoi, Vietnam. The main clinical and para-clinical symptoms of the patients were described. In the first patient, samples were collected during surgery for removal of the cyst from the lung, and in the second patient, samples were collected by extracting fluids from the cyst in the lung. Echinococcus antigen in an enzyme-linked immunosorbent assay (ELISA) kit was used for detection of Echinococcus-specific antibodies with cutoff OD <0.3. The ELISA test and morphological identification of species were carried out in the Department of Parasitology, Hanoi Medical University, and the molecular methods were performed in the Molecular Diagnostic Laboratory of the National Hospital of Tropical Diseases. The two patients were followed-up for 2 years after their treatments. These patients are the first reported cases of echinococcosis in Vietnam. Both patients provided written consent to have their data and images published.
Case report
Description of the first case
The first patient was a 42-year old male residing in the Thach Dong commune, Thach Thanh District, Thanh Hoa Province, in the mountainous region of northern Vietnam. He is a farmer and had never left the country. He felt chest pains on his right side in January 2013, and this symptom increased for 2 months; at the end of February 2013, he visited the Provincial Hospital and then the National Hospital of Lung Diseases (Hanoi, Vietnam). The main clinical symptom was pain in the right chest, absence of fever, and no cough or other symptoms. Paraclinical symptoms were a big nodular shadow circle form of equal density in the right lobe of the lungs, detected by chest X-ray examination, of 6 × 7 cm size (Figure 1). The leukocyte count was 9,600,000, including neutrophils 57.4%, lymphocytes 20.6%, eosinophils 12.8%, monocytes 8.3%, and basophils 0.9%; positive serodiagnosis was obtained by ELISA test with Echinococcus antigen. This patient was treated by surgery combined with use of albendazole 800 mg/day for 30 days (three dosages), and he was free of symptoms during the follow-up period of 3 months.
  | Figure 1 A hydatid cyst (6 × 7 cm in diameter, arrow) in the lung of the first patient, detected by X-ray. |
Description of the second case
The second patient was a 48-year old female residing in Phu Yen town of the Phu Yen District, Son La Province, also in the mountainous north region of Vietnam. She is a medical technician and had also never left the country. She felt chest pains, along with cough and bloody sputum in June 2013; these symptoms increased for 2 months, and in the middle of August 2013, she visited the Provincial Hospital and then the National Hospital of Lung Diseases (Hanoi, Vietnam). The main clinical symptoms were pain in the chest, dyspnea, and cough with blood, in addition to absence of fever. Paraclinical symptoms were four big nodular shadow circles formed of equal density in both lobes of the lungs, detected by chest X-ray examination, with sizes of 5 × 6 cm, 4 × 4 cm, 3.5 × 3 cm, and 2.5 × 2 cm (Figure 2). The leukocyte count was 8,520,000, including neutrophils 72.0%, lymphocytes 12.9%, eosinophils 8.3%, monocytes 6.7%, and basophils 0.1%; positive serodiagnosis was obtained by ELISA test with Echinococcus antigen. A microscopic examination of the fluid, extracted from the biggest cyst using a disposable syringe, was performed. The results showed the presence of a large number of Echinococcus larvae. Surgery was not done on this patient because the cysts were found in both lungs. However, she was treated with albendazole 800 mg/day for 30 days (six dosages). She was free of chest pain during the follow-up period of 3 months and free of cough after 12 months; however, the cysts had decreased in size (the remaining two cysts only: 3.5 × 2 cm and 2.5 × 1.5 cm in the lungs).
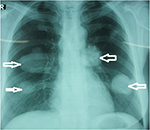  | Figure 2 The hydatid cysts (four cysts: 5 × 6 cm, 4 × 4 cm, 3.5 × 3 cm, and 2.5 × 2 cm in diameter, arrows) in the lungs of the second patient, detected by X-ray. |
Description of the parasite
In the cysts (cystic echinococcosis) of both patients were many protoscoleces, each of which had many hooks (Figure 3).
  | Figure 3 The protoscoleces and the hooklets of Vietnamese Echinococcus. Notes: (A) The larvae observed by microscopy (10×); (B) the larvae observed at 40×; (C) the hooks of the larvae observed at 90×. |
The protoscolex is a spherical body of approximately 0.15 ¥ 0.14 mm in diameter, in which an invaginated scolex with 30 hooks and four suckers is contained, and each hook is 22 µm in diameter length (Figure 3).
These protoscoleces were analyzed by a molecular method using the gene of NADH hydrogenasa. The result identified them as E. ortleppi with 99–100% homology compared with E. ortleppi in GenBank, but only homology with E. granulosus was 93–94% (Tables 1 and 2). The phylogenetic tree of E. ortleppi Vietnam and other strains derived from part of the NADH dehydrogenase nucleotide sequence and the COX1 gene, determined by neighbor-joining (NJ) method using MEGA5.1,6 showed that the strain of Vietnamese E. ortleppi belongs to the same group as strains of E. ortleppi in GenBank (Figures 4 and 5).
Discussion
The two cases of echinococcosis in Vietnam were clearly diagnosed because larvae were detected in the cysts from both patients. X-ray diagnosis clearly showed one cyst of 6 × 7 cm in the first patient and four cysts with 2 × 3 cm, 3.5 × 4 cm, 5 × 6.5 cm, and 6 × 6.5 cm in the second patient. In Vietnam, in 2009, De and Khue reported a case, in which a 3 × 3.5 cm cyst in the lung was suspected as echinococcosis and which yielded a positive ELISA test with Echinococcus antigen.18 However, no larvae were collected from the cyst. In recent years, some suspected cases of water cysts in the liver were diagnosed as echinococcosis by ultrasonography, but again no larvae or hooks were collected in the fluid from the cysts. In 1967, Le-Van-Hoa and Vu-Ngoc-Tan19 reported on dogs that were infected with E. granulosus in southern Vietnam and that served as definitive hosts, which could transmit the disease to humans.
Echinococcus ortleppi is a cattle strain (genotype G5) of E. granulosus occurring in Europe,4,28 which is infective to humans. Genotype G5 of E. ortleppi was detected for the first time in Italy in 2008 by Casulli et al,7 and cystic echinococcosis caused by its larval stage was found to be endemic in southern Brazil by Balbinotti et al in 2012.3 In 2011 and 2012, liver infections caused by E. ortleppi tapeworms were diagnosed in two humans in France, and in 2012, a nationwide slaughterhouse survey identified E. ortleppi infections in cattle.20 Echinococcosis in livestock caused by E. ortleppi was described in Kenya, Sudan, India, Italy, Argentina, and Brazil.13,14 More recent studies have reported the presence of this Echinococcus spp. in Ethiopia,30 Egypt,31 and France.7
Conclusion
In 2013, two cases of infection by E. ortleppi that caused cystic echinococcosis in the lungs were reported in Vietnam. This was the first time that this species was detected in humans in Vietnam.
Acknowledgments
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-YS.05-2014.08, in collaboration with the National Hospital of Tuberculosis and Lung Diseases, Hanoi, Vietnam.
Disclosure
The authors report no conflicts of interest in this work.
References
De NV, Khue PV. Zoonotic Parasites. Scientific Book. Vietnam: Education Published House; 2009:116–117. | ||
De NV, Le TH. Taeniasis/Cysticercosis and Molecular Amplication. Scientific Book Medical Published House; 2010:163–165. | ||
Balbinotti H, Santos GB, Badaraco J, et al. Echinococcus ortleppi (G5) and echinococcus granulosus sensu stricto (G1) loads in cattle from Southern Brazil. Vet Parasitol. 2012;188(3–4):255–260. | ||
Nakao M, Lavikainen A, Yanagida T, Ito A. Phylogenetic systematics of the genus Echinococcus (Cestoda: Taeniidae). Int J Parasitol. 2013;43(12–13):1017–1029. | ||
Saarma U, Jõgisalu I, Moks E, et al. A novel phylogeny for the genus Echinococcus, based on nuclear data, challenges relationships based on mitochondrial evidence. Parasitology. 2009;136(3):317–328. | ||
Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 2004;101(30):11030–11035. | ||
Miyazaki I. Helminthic zoonoses. International Medical Foundation of Japan. Tokyo; 1991:247–258. | ||
Casulli A, Manfredi MT, La Rosa G, Cerbo AR, Genchi C, Pozio E. Echinococcus ortleppi and E. granulosus G1, G2 and G3 genotypes in Italian bovines. Vet Parasitol. 2008;155(1–2):168–172. | ||
de la Rue ML, Takano K, Brochado JF, et al. Infection of humans and animals with Echinococcus granulosus (G1 and G3 strains) and E. ortleppi in Southern Brazil. Vet Parasitol. 2011;177(1–2):97–103. | ||
Schwabe CW. Current status of hydatid disease: a zoonosis of increasing importance. In: Thompson RCA, editors. The Biology of Echinococcus and Hydatid Disease, London: George Allen & Unwin; 1986:81–113. | ||
Linda P, Gilda C, Lidia C, et al. Cystic echinococcosis i n a single tertiary care center in Rome, Italy. Bio Med Res Int. 2013; Article ID 978146, 9 pages. | ||
Roneus O, Christensson D, Nilsson NG. The longevity of hydatid cysts in horses. Vet. Parasitol. 1982;11(2–3):149–154. | ||
Spruance SL. Latent period of 53 years in a case of hydatid cyst disease. Arch Intern Med. 1974;134(4):741–742. | ||
Alvarez Rojas CA, Romig T, Lightowlers MW. Echinococcus granulosus sensu lato genotypes infecting humans–review of current knowledge. Int J Parasitol. 2014;44(1):9–18. | ||
Cardona GA, Carmena D. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet Parasitol. 2013;192(1–3):10–32. | ||
Carmena D, Cardona GA. Canine echinococcosis: global epidemiology and genotypic diversity. Acta Trop. 2013;128(3):441–460. | ||
Carmena D, Cardona GA. Echinococcosis in wild carnivorous species: epidemiology, genotypic diversity, and implications for veterinary public health. Vet Parasitol. 2014;202(3–4):69–94. | ||
Ito A, Wen H, Yamazaki H. Taeniasis/cysticercosis and echinococcosis in Asia. Asian Pasasitology. 2005;2:155–234. | ||
Ahmed ME, Eltom KH, Musa NO, et al. First report on cerculation of echinococcus ortleppi in the one humped camel (camelus dromedaries), Sudan. BMC Vet Res. 2013;9:127. | ||
Bowles J, McManus DP. NADH dehydrogennase 1 gene sequences compared for species and strains of the genus Echinococcus. Int J Parasitol. 1993;23(7):969–972. | ||
Dybiez M, Gierczak A, Dabrwoska J, RdzanckI, Michalowicz B. Molecular diagnosis of cystic echinococcosis in human from central Poland. Prasitol Int. 2013;62(4):364–367. | ||
Fasihi MH, Budke CM, Rostami S. The monetary burden of cystic echinococcosis in Iran. PLoS Negl Trop Dis. 2012;6(11):e1915. | ||
Nakao M, McManus PD, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genom. Parasitology. 2007;134(Pt 5):713–722. | ||
Lavikainen A, Lehtinen MJ, Laaksonen S, Agren E, Oksanen A, Meri S. Molecular characherization of Echinococcus isolates of cervid origin from Finland and Sweden. Parsitology. 2006;133(Pt 5):713–722. | ||
Lavikainen A, Lehtinen MJ, Meri T, Hirvenla-KoskiV, Meri S. Molecular genetic characherization of Fennoscandial cervid strain, a new genotypic group(G10) of Echinococcus granulosus. Parsitology. 2003;127(Pt 5):207–215. | ||
Obwaller A, Schneider R, Walochnik J, et al. Echinococcus granulosus strain differentiation based on sequence heterogeneity in mitochondrial genesof cytocrome c oxydaza-1 and NADH dehydrogenase 1. Parsitology. 2004;128(Pt 5):569–575. | ||
Hoa LV, Tan VN. Concerning the pesence of cestodes, Echinococcus granulosus (Batsch, 1786), in a wild dog, Cyon primaerus (Hodgs) in South Vietnam. Bull Soc Pathol Exot Filiales. 1967;60(1):64–71. French. | ||
Thompson RCA. The taxonomy, phylogeny and transmission of Echinococcus. Exp Parasitol. 2008;119(4):439–446. | ||
Grenouillet F, Umhang G, Arbez-Gindre F, et al. Echinococcus ortleppi infections in humans and cattle, France. Emerg Infect Dis. 2014;20(12):2100–2102. | ||
Tigre W, Deresa B, Haile A, et al. Molecular characterization of Echinococcus granulosus s.l. cysts from cattle, camels, goats and pigs in Ethiopia. Vet Parasitol. 2016;215:17–21. | ||
Amer S, Helal IB, Kamau E, Xiao L. Molecular characterization of Echinococcus sensu lato from farm animals in Egypt. PLoS One. 2015;10(3):e0118509. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




