Back to Journals » Infection and Drug Resistance » Volume 17
The First Case Report of Inactive Nontuberculous Mycobacterial Pulmonary Disease (NTM-PD) in a Pneumoconiosis Patient Caused by Mycobacterium europaeum in China
Authors Zhou J, Xu H, Du W, Peng L
Received 17 November 2023
Accepted for publication 29 March 2024
Published 16 April 2024 Volume 2024:17 Pages 1515—1521
DOI https://doi.org/10.2147/IDR.S448805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jiaqing Zhou,1 Huan Xu,2 Wen Du,1 Lijun Peng1
1Department of Respiratory Medicine, West China Fourth Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Department of Scientific Affairs, Vision Medicals for Infectious Diseases, Guangzhou, People’s Republic of China
Correspondence: Lijun Peng, Department of Respiratory Medicine, West China Fourth Hospital, Sichuan University, No. 17, Section 3, Renmin South Road, Wuhou District, Chengdu, 610041, People’s Republic of China, Tel +86 13208119408, Email [email protected]
Abstract: We reported a 51-year-old male electric welder with stage I pneumoconiosis, who had no significant cough, sputum, fever, chest pain, or other discomfort. However, regular physical examination at our hospital revealed bilateral pulmonary nodules with cavity formation. Blood routine, liver or kidney function, and infection-related biomarkers, including interleukin-6 (IL-6), high-sensitivity C-reactive protein (hs-CRP), and procalcitonin (PCT), were normal. Sputum and alveolar lavage fluid (BALF) acid-fast bacilli (AFB) smears, BALF Mycobacterium tuberculosis (TB) PCR, and T-SPOT.TB were negative. The nucleic acid sequence of Mycobacterium europaeum was detected by BALF metagenomic next-generation sequencing (mNGS), which was confirmed by the subsequent positive culture for NTM. Considering stable conditions, no significant discomfort, and no significant changes in the lung lesion, the patient was diagnosed with inactive nontuberculous mycobacterial pulmonary disease (NTM-PD).
Keywords: pneumoconiosis, nontuberculous mycobacterial pulmonary disease, NTM-PD, Mycobacterium europaeum, metagenomic next-generation sequencing, mNGS
Introduction
Pneumoconiosis is a group of occupational lung diseases caused by long-term inhalation of different pathogenic production dust and retention in the lungs, the lung tissue mainly presented with diffuse fibrosis in the lung tissue, and the clinical manifestations were cough, phlegm, chest pain, and dyspnea.1 Due to long-term exposure to production dust, the respiratory defenses of pneumoconiosis patients are impaired. Thus, pneumoconiosis patients often experience complications such as pulmonary tuberculosis, bronchitis, pneumonia, emphysema, spontaneous pneumothorax, etc.1 Pneumoconiosis is also one of the most common host factors for nontuberculous mycobacterial pulmonary disease (NTM-PD),2 especially for those in South Africa.3
According to the Chinese guidelines,2 the diagnosis of NTM-PD could be made based on the clinical symptoms, radiologic imaging, and microbiological tests. Patients with respiratory symptoms and/or systemic symptoms, chest imaging presented with nodular or cavitary opacities or bronchiectasis with multiple small nodules, microbiological tests found NTM evidence, and appropriate exclusion of other diagnoses, the NTM-PD could be diagnosed. However, until recently, there are no guidelines to give an exact definition for distinguishing active and in active NTM-PD. Some studies used other methods such as the inhibitory titer of anti-IFN-gamma auto-antibody to differentiate patients with active from inactive NTM infection.4 Mycobacterium europaeum, a slow-growing non-tuberculous mycobacterium, belongs to the Mycobacterium simiae complex, which can be isolated from the respiratory samples of immunodeficient patients with influenza5 or from the sputum of cystic fibrosis patients.6 Cases of M. europaeum have also been reported in the lungs of patients without underlying disease.7 Approximately 75% to 94% NTM-PD patients present with pulmonary lesions, which are often misdiagnosed as pulmonary tuberculosis due to their resemblant clinical manifestations and lung imagings.8 Therefore, rapid and accurate pathogenic diagnosis is essential to improve the prognosis of NTM-PD. With the development of molecular diagnostic technology, metagenomic next-generation sequencing (mNGS) has also been gradually and widely used in clinical practice. In this paper, we applied mNGS to confirm the diagnosis of the first case of inactive NTM-PD caused by M. europaeum in China.
Case Presentation
On November 16, 2022, a 51-year-old male patient was admitted to our hospital, complaining of recurrent tightness of breath for more than 2 years. He was previously engaged in welding work for 10 years. He was diagnosed with occupational welding pneumoconiosis stage I and had been off the dust 10 months. Two years ago, the main symptoms of the patients were dyspnea and occasional mild coughing. But the dyspnea could be relieved by deep inspiration, so the patient did not pay more attention to his discomfort.
The patient had received several physical examinations in our hospital. On July 4, 2017, chest CT showed scattered large nodular shadows in the upper lobes of both lungs, thickening and calcification of the left pleural apex, which cannot exclude the possibility of tuberculosis (Figures 1 and 2). On May 14, 2018, chest X-ray showed extensive interstitial changes in both lungs, suggesting possible interstitial fibrosis (Figure 3). On November 9, 2022, the patient was re-examined in our hospital. Chest CT showed nodules in both lungs and cavity formation in the posterior segment of the upper lobe of the left lung (Figure 2), indicating possible secondary pulmonary infection with Mycobacterium tuberculosis (TB). However, both sputum acid-fast bacilli (AFB) smears and T-SPOT.TB were negative. Then, he was admitted for further etiological diagnosis.
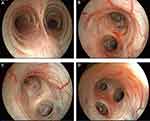
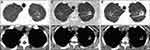 |
Figure 2 Serial chest computed tomography (CT) scans of the patient. (A and B) July 4, 2017. (C and D) November 9, 2022. (E and F) March 2, 2023. |
 |
Figure 3 Chest X-ray of the patient on May 14, 2018. Extensive interstitial changes in both lungs: posteroanterior view (A) and lateral view (B). |
Upon admission (day 1), a physical examination showed a body temperature of 36.6°C, pulse rate of 72 beats/min, respiration of 20 breaths/min, blood pressure of 129/89 mmHg, and weight of 58 Kg. No dry and wet rales were heard in both lungs, the heart rhythm was uniform, and no murmur was heard in all valve areas. White blood cell (WBC, 6.43×109/L), neutrophil count (3.50×109/L), lymphocyte count (2.36×109/L), high-sensitive C-reaction protein (hs-CRP, 0.78 mg/L) were normal. Serum carcinoembryonic antigen and neuron-specific enolase were normal, and only non-small cell lung cancer antigen (3.50 ng/mL) was elevated.
To further clarify the cause of the pulmonary cavity, a fiberoptic bronchoscopy was performed on day 3. The scattered mucous sputum in the lumen can be seen (Figure 4), suggesting the possibility of infectious lesion. In combination with chest CT, bronchoalveolar lavage (BALF) was harvested from the apical segment of the upper lobe of the left lung and sent for pathogenic and pathological examination. After that, the patient was temporarily given cephalexin and bromoxynil for anti-infection and expectoration, respectively. On the same day, BALF Mycobacterium tuberculosis (TB) PCR was negative. Sputum culture showed a small amount of α-hemolytic streptococcus growth. Tests for cryptococcal polysaccharide antigen (CrAg), β-D-glucan (BDG), glactomannan (GM), and Aspergillus antigen were all negative. On day 6, pathological examination showed more ciliated columnar epithelial cells in the erythrocyte and no definite malignant cells. To further clarify the diagnosis, we communicated with the patient and sent BALF for mNGS assay. mNGS results reported Mycobacterium europaeum (6757 reads) (Figure 5), Moraxella catarrhalis (64 reads), and human herpesvirus type 4 (1598 reads). Considering his new cavity formation and high sequence reads of M. europaeum, nontuberculous mycobacterial pulmonary disease (NTM-PD) was considered. To confirm the etiological diagnosis, we performed a second fiberoptic bronchoscopy with the patient’s consent on day 8. BALF was acquired again at the same site and sent to culture, which returned positive results for Mycobacterium spp. (Figure 5).
As the condition was relatively stable, with no obvious cough, sputum, fever, chest pain, and other discomfort, no obvious dry and wet rales in both lungs, and no swelling in both lower extremities, the patient was diagnosed with inactive NTM-PD. He was discharged and treated with cefdinir capsules and acetylcysteine granule. On March 2, 2023, the patient was reviewed again and the chest CT illustrated increased texture diffuse and nodular shadow in both lungs with symmetrical distribution, and cavitation shadow in the posterior segment of the upper lobe of the left lung showing no significant change compared before (Figure 2).
Discussion
Pneumoconiosis is still the most serious and common occupational disease in China. Pneumoconiosis patients have varying degrees of oxidative stress, altered inflammatory status and immune dysfunction, impairing respiratory defenses. In addition, pneumoconiosis is a chronic, progressive, long-term disease with various respiratory complications, such as TB, NTM, and aspergillus infection. Complications/comorbidities have an important impact on the treatment, progression, and prognosis of rehabilitation of pneumoconiosis. An epidemiologic survey showed that the mortality rate of pneumoconiosis patients due to respiratory complications/comorbidities was 51.8%. Therefore, timely and correct diagnosis and treatment of various complications/comorbidities are of great importance to save patients’ lives, improve prognosis, and enhance their quality of life.1,9
NTM refers to mycobacteria other than Mycobacterium tuberculosis complex and Mycobacterium leprae. NTM is ubiquitous in the environment, only a few of which are pathogenic to human beings, and usually as conditional pathogenic.2,10,11 To date, more than 200 species or subspecies of NTM have been identified, and new species or subspecies are constantly being discovered. More than 50 species are pathogenic, with M. avium complex (MAC), M. kansasii, and M. abscessus as the main clinical agents.12,13 MAC mainly consists of M. intracellulare and M. avium. MAC is the main isolate in NTM in most countries. M. europaeum belongs to the MAC, which is a slow-growing type in group I. In solid medium, the colonies of this group are yellowish when exposed to light, and become yellow or orange after light. To date, there is no report of M. europaeum pulmonary infection in China.
NTM disease mainly affected lung tissue, but all organs and systems of the body can be affected. In recent years, NTM disease has been rapidly increasing and has become one of the most important public health problems threatening human health. According to available data, the incidence and prevalence of NTM disease are increasing in some countries and regions, even surpassing those of tuberculosis.13–16 A recent study showed that the incidence of NTM-PD in the United States increased from 3.13/100,000 (95% CI 2.88–3.40) in 2008 to 4.73/100,000 (95% CI 4.43–5.05) in 2015, and the prevalence increased from 6.78/100,000 (95% CI 6.45–7.14) in 2008 to 11.70/100,000 (95% CI 11.26–12.16) in 2015.15 Epidemiological survey data on NTM disease in large samples are not yet available in China, but epidemiological survey from a tertiary hospital17 showed that the detection rate of NTM increased from 15.6% in 2013 to 46.1% in 2018 among Mycobacterial species, indicating a significant upward trend in NTM disease in China.
NTM-PD is common in those with underlying lung disease or under immune compromised status, such as chronic obstructive pulmonary disease (COPD), bronchiectasis, cystic fibrosis (CF), pneumoconiosis, pulmonary tuberculosis, emphysema and alveolar protein deposition, organ transplant recipients, and long-term immunosuppressant users. However, there also had NTM-PD reports in those with normal immune function.2 The clinical manifestations, pathological features, and CT imaging of NTM-PD are very similar to those of pulmonary tuberculosis. Thus, NTM-PD are often misdiagnosed as tuberculosis, which not only delays the disease but also leads to the development of drug-resistant strains of NTM, posing a serious threat to human health.
NTM has a commonality with M. tuberculosis in bacterial composition and antigen. Compared with M. tuberculosis, the virulence is weaker, the degree of lesion milder, caseous necrosis less, and fibrosis and cavity-like lesions commoner. Pneumoconiosis patients whose CT images presented cavitary lesions with positive acid-fast bacilli (AFB) smears and negative Xpert or TB-DNA but without obvious clinical symptoms of tuberculosis, NTM infection should be highly alerted. The mycobacterial culture, PCR, and mNGS should be further performed to define the strain. Since the positive rate of sputum AFB smears is relatively low, BALF is more satisfactory for test. In addition to conventional microbiological tests, mNGS assay can be used to obtain rapid and accurate pathogenic results.
In this case, the patient had underlying pneumoconiosis with a chronic course. The main clinical manifestation was dyspnea on deep inspiration, CT images suggested new cavity formation, bronchoscopy revealed scattered mucus sputum in the lumen, pathological examination did not find malignant tumor cells, infectious lesions were not ruled out, but routine pathogenic examination had no positive findings, suggesting the possibility of non-clinically common bacterial infections and considering pathogens with chronic infections, such as TB, NTM, Nocardia, Cryptococcus, Talaromyces marneffei, Histoplasma capsulatum. The detection rate of those pathogens by conventional methods is relatively low. While mNGS has an advantage for the nuclei acid testing. Therefore, in addition to conventional pathological and microbiological tests, the patient’s BALF could be sent for mNGS simultaneously. M. europaeum was detected by mNGS, which was confirmed by the culture. Thus, NTM-PD was finally diagnosed. However, the patient’s dynamic follow-up showed no significant worsening of pulmonary symptoms and little change in pulmonary cavitation, so it was determined as an inactive M. europaeum infection.
There are some limitations in our case report, too. On the one hand, due to lack of inactive NTM-PD definition, we only made the diagnosis based on the evidence of progressive. On the other hand, the follow-up time is relatively shorter with only 3 months, and more long-term follow-up is required.
Ethical Approval
This case was approved by the Ethics Committee of West China Fourth Hospital (Grant No. HXSY-EC-2024022) for publication. Patient provided written informed consent to publish details of this case.
Consent to Publish
All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication. The patient gave consent to publication.
Funding
Project supported by the Natural Science Foundation of Sichuan Province, China (Grant No. 2023NSFSC0649).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Occupational Lung Disease Group of Labor Hygiene and Occupational Diseases Branch of Chinese Preventive Medicine Association. Consensus of Chinese experts on pneumoconiosis treatment. J Environ Occup Med. 2018;35(8):677–689. doi:10.13213/j.cnki.jeom.2018.18437
2. Chinese Society of Tuberculosis, Chinese Medical Association. Guidelines for diagnosis and treatment of non-tuberculous mycobacteria disease (2020 edition). Zhonghua Jie He he Hu Xi Za Zhi. 2020;43(11):918–946. doi:10.3760/cma.j.cn112147-20200508-00570
3. Corbett EL, Churchyard GJ, Clayton T, et al. Risk factors for pulmonary mycobacterial disease in South African gold miners. A case-control study. Am J Respir Crit Care Med. 1999;159(1):94–99. doi:10.1164/ajrccm.159.1.9803048
4. Nithichanon A, Chetchotisakd P, Matsumura T, et al. Diagnosis of NTM active infection in lymphadenopathy patients with anti-interferon-gamma auto-antibody using inhibitory ELISA vs. indirect ELISA. Sci Rep. 2020;10(1):8968. doi:10.1038/s41598-020-65933-x
5. Phelippeau M, Delord M, Drancourt M, Brouqui P. Respiratory tract isolation of Mycobacterium europaeum following influenza infection in an immunocompromised patient: a case report. J Med Case Rep. 2014;8(1):463. doi:10.1186/1752-1947-8-463
6. Pourahmad F, Shojaei H, Heidarieh P, Khosravi A, Hashemi A. Report of two cases of Mycobacterium europaeum from Iran. Jpn J Infect Dis. 2012;65(6):539–541. doi:10.7883/yoken.65.539
7. Fujiwara K, Furuuchi K, Aono A, et al. Mycobacterium europaeum lung disease in an immunocompetent patient without underlying lung disease. J Infect Chemother. 2021;27(1):107–109. doi:10.1016/j.jiac.2020.09.012
8. Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12):e124–9. doi:10.1086/648443
9. Cadena J, Rathinavelu S, Lopez-Alvarenga JC, Restrepo BI. The re-emerging association between tuberculosis and diabetes: lessons from past centuries. Tuberculosis. 2019;1166S:S89–S97. doi:10.1016/j.tube.2019.04.015
10. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. doi:10.1183/13993003.00535-2020
11. Haworth CS, Banks J, Capstick T, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017;72(Suppl 2):ii1–ii64. doi:10.1136/thoraxjnl-2017-210927
12. Kwon YS, Koh WJ. Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease. J Korean Med Sci. 2016;31(5):649–659. doi:10.3346/jkms.2016.31.5.649
13. Furuuchi K, Morimoto K, Yoshiyama T, et al. Interrelational changes in the epidemiology and clinical features of nontuberculous mycobacterial pulmonary disease and tuberculosis in a referral hospital in Japan. Respir Med. 2019;152:74–80. doi:10.1016/j.rmed.2019.05.001
14. Smith GS, Ghio AJ, Stout JE, et al. Epidemiology of nontuberculous mycobacteria isolations among central North Carolina residents, 2006-2010. J Infect. 2016;72(6):678–686. doi:10.1016/j.jinf.2016.03.008
15. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008-2015. Ann Am Thorac Soc. 2020;17(2):178–185. doi:10.1513/AnnalsATS.201804-236OC
16. Santin M, Barrabeig I, Malchair P, et al. Pulmonary Infections with Nontuberculous Mycobacteria, Catalonia, Spain, 1994-2014. Emerg Infect Dis. 2018;24(6):1091–1094. doi:10.3201/eid2406.172095
17. Huang JJ, Li YX, Zhao Y, et al. Prevalence of nontuberculous mycobacteria in a tertiary hospital in Beijing, China, January 2013 to December 2018. BMC Microbiol. 2020;20(1):158. doi:10.1186/s12866-020-01840-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.